Features of changes in the monocyte-macrophage cells in the placenta during preeclampsia
Objective: To determine the features of changes in the cells of the monocytic-macrophage link in the placenta with preeclampsia of varying severity. Materials and methods: The study included 52 patients. Women were divided into the main group with preeclampsia (n=26) and the comparison group – physiological pregnancy (n=26). The main group was divided into two subgroups with mild (n=12) and severe (n=14) preeclampsia. The content of CD68+ cells inside the placental villi was studied. The study was carried out using immunohistochemistry, which was confirmed by Western blotting. Results: It was found that the content of CD68+ cells inside the placental villi was significantly higher in the groups of mild –7,5% (5.6; 8.3) and severe – 9,1% (6.5; 10.8) preeclampsia relative to the comparison group – 5,1% (2.5; 5.3) (p<0.001). The relative level of expression of the CD68 protein, assessed by the Western blot method, was also statistically significantly increased in PE. Conclusion: When studying CD68+ cells in placental villi, a correlation was found between an increase in their content and the severity of preeclampsia. Thus, the results obtained may indicate a potential role of the placenta in the pathogenesis of PE as an agent activating cytotoxic properties in maternal blood monocytes.Boris D.A., Tyutyunnik V.L., Kan N.E., Shchegolev A.I., Sinitsyna V.A., Sadekova A.A., Krasnyi A.M.
Keywords
Hypertensive disorders during pregnancy (chronic and gestational hypertension, preeclampsia) are a unique problem, since this pathology and its therapeutic treatment affect both mother and fetus, sometimes putting their well-being at risk. In particular, preeclampsia is a pregnancy-specific syndrome which is the main cause of maternal and perinatal morbidity and mortality, as well as preterm birth [1, 2]. Hypertensive disorders account for about 10% of pregnancies, with preeclampsia affecting up to 6–8% of pregnancies [3]. The most important clinical parameters for the diagnosis of preeclampsia are arterial hypertension and proteinuria after the 20th week of pregnancy. However, preeclampsia can also be masked by other diseases and complications, such as placental insufficiency, fetal growth retardation, neurological or hematological disorders, hepatic and renal insufficiency, etc. Such diseases occur when there is no generally accepted clinical picture, therefore diagnosing is significantly complicated [4, 5].
Preeclampsia is commonly called the “disease of theories”. Its concept has transformed over many decades from a kidney-specific disease leading to chronic nephritis to a state of toxemia caused by circulating toxins. Since then, the understanding of the pathogenesis of this disorder has broadened significantly. There are some theories about the connection of preeclampsia with genetically determined disorders, hormonal disorders, endothelial pathology, imbalance of angiogenic and antiangiogenic factors, changes in blood coagulation properties, volemic and metabolic disorders [7–10]. Despite the variety of existing theories, most scientists agree that the pathogenetic component of preeclampsia is associated with an increased systemic inflammatory reaction which may result from endogenous activation of innate immune cells, such as monocytes and granulocytes, as well as excessive production of proinflammatory cytokines [7, 11–14].
The placenta is known to be a key factor in the etiology of preeclampsia, and its removal is necessary for the reduction of symptoms of this complication. A few studies indicate that it is the placenta that takes part in the activation of monocyte-macrophage cells. Blood monocytes perform important immunological functions, such as phagocytosis, antigen presentation, cytokine secretion, and regulation of innate and adaptive immune response [15–17]. Previously, we showed that blood monocytes during pregnancy cause apoptosis of a semi-allogenic trophoblast which is accompanied by the appearance of fetal DNA in the maternal blood; this process was more severe in preeclampsia [11, 18–20]. This factor may be of key importance in the development of preeclampsia, however, the mechanisms of activation of monocytes, which ultimately lead to the destruction of the placenta in this complication of pregnancy, remain unclear. Two variants of preeclampsia development can be assumed: in the first case, monocytes are activated directly in the bloodstream under the influence of fetal antigens and placental proinflammatory factors; in the second case, monocytes and antigens contact on the surface of the placental cells [7, 11, 14, 21].
In this work, we tried to confirm the hypothesis that monocytes can be activated by interacting with placental cells. Therefore, we identified the content of monocyte-macrophage cells in the placenta of patients with various degrees of preeclampsia.
The aim of the study was to determine the characteristics of the cells of the monocyte-macrophage lineage in the placenta of patients with various degrees of preeclampsia.
Materials and methods
A total of 52 patients were included in the study. The women were divided into two groups: the main group included the patients with preeclampsia (n=26) and the comparison group consisted of patients with normal pregnancy (n=26). The main group was divided into two subgroups with mild (n=12) and severe (n=14) preeclampsia [11].
This study was approved by the local ethics committee. All women were aware of the objectives of the study and signed an informed consent to participate in the study. There were the following criteria for inclusion in the study: single pregnancy from 22 to 40 weeks’ gestation, age from 18 to 45 years, absence of severe extragenital pathology, presence of mild or severe preeclampsia (for the main group). The exclusion criteria are multiple pregnancy, acute infectious, autoimmune and oncological diseases, pregnancy resulting from assisted reproductive technologies, severe extragenital pathology [11, 21].
Macroscopic and microscopic evaluation of afterbirth of 52 patients was carried out; fetal membranes, umbilical cord and placenta were analyzed in order to verify the diagnosis of preeclampsia which was made after a clinical and laboratory examination. Placenta samples obtained no later than 5–10 minutes after delivery were used for this purpose. Small fragments of placental tissue (1.5×1.0×0.3 cm in size) from the paracentral zone (including the chorionic villi, basal and chorial plates) were examined. The obtained pieces were fixed in a 10% solution of buffered neutral formalin (BioVitrum, Russia) for 24 hours, then they were placed in paraffin, according to the standard procedure; sections 4 microns thick were subsequently made from them on the Accu-Cut SRM 200 Rotary Microtome (Sakura, Japan). The histological sections obtained separately for immunohistochemical examination were applied to highly adhesive glasses and dried vertically in a thermostat at a temperature of 55–56°C for 10 hours. Deparaffinization, restoring of antigenic activity and all stages of the immunohistochemical reaction, as well as staining with hematoxylin were carried out in the Roche Ventana BenchMark ULTRA staining system (Switzerland). The following reagents were used in the staining system: Prep Concentrate (×10); EZ Prep Concentrate (×10); ULTRA LCS (pre-diluted liquid cover-slipping oil); SSC buffer, concentrate (×10); Reaction buffer Concentrate (×10); ULTRA CC1 (TRIS/Borat/EDTA buffer (CC1) for antigen retrieval, diluted); Hematoxylin II (histological staining agent Hematoxylin II ≤ 60%), dispenser for 250 tests or equivalent; bluing reagent. UltraView Universal DAB Detection Kit was used as a primary antibody detection system. Rabbit monoclonal antibodies against the monocyte/macrophage marker CD68 (Abcam, USA) were used to determine monocytes in placental villi. Therefore, cells were counted using 10 high power fields from each section. The content of CD68+ cells in placental villi was estimated as a percentage (%) of the total number of villous cells using the ImageJ (USA) program [11, 19, 21].
Western blotting was used to confirm the results of immunohistochemical analysis. This method included gel electrophoresis for separating denatured proteins along the length of polypeptide with subsequent electrophoretic transfer to the membrane (PVDF) and immuno-staining procedures for visualizing the protein on the blotting membrane. In order to denature the proteins, the protein samples were heated prior to electrophoresis. It ensured the separation of proteins by size and prevented the degradation of samples by proteases. After electrophoretic separation, the proteins were transferred to the membrane, where they were blocked by milk for the prevention of nonspecific binding of antibodies; then the proteins were stained with antibodies against the CD68 protein. Next, the membrane was stained with a secondary antibody, which recognized the first antibody staining, and was subsequently used for detection. Antibodies against CD68 (Abcam, USA) were used in the study [11, 19, 21].
Statistical analysis
The results are presented as median, upper and lower quartiles Me (Q1;Q3). The figures are presented in the form of a box and whisker plot (5%; Q1; Me; Q3; 95%). The statistical significance of the differences was determined using the nonparametric Mann–Whitney test. To compare the three independent groups, the Kruskal–Wallis test was used. The comparison of groups according to qualitative characteristics was carried out using Fisher’s exact criterion. The differences were considered statistically significant at p<0.05. When three groups were compared, multiple comparisons correction was applied. Statistical processing of the results and plotting were carried out using the programs AtteStat (Russia), Statistica 10 and OriginPro 8.5 (USA) [11, 19, 21].
Results
The analysis of the age of pregnant women showed that older patients experienced severe preeclampsia more frequently in comparison with the control group (p=0.012). The body mass index (BMI) was higher in the group of the patients with severe preeclampsia compared to the patients with the normal pregnancy, namely 28.2 (24.9; 31.2) versus 25.1 (24.5; 27.3), (p<0.001). In terms of the somatic and gynecological diseases, statistically significant differences could not be identified. Gestational age at delivery in a group of patients with mild and severe preeclampsia was statistically significantly lower compared to the control group (p<0.001), which is probably due to the deterioration of the condition of the pregnant woman and/or fetus in case of preeclampsia. The comparative analysis revealed a higher frequency of abdominal delivery in the groups with preeclampsia, but statistically significant differences could not be identified. The clinical characteristics of the pregnant women are presented in Table.
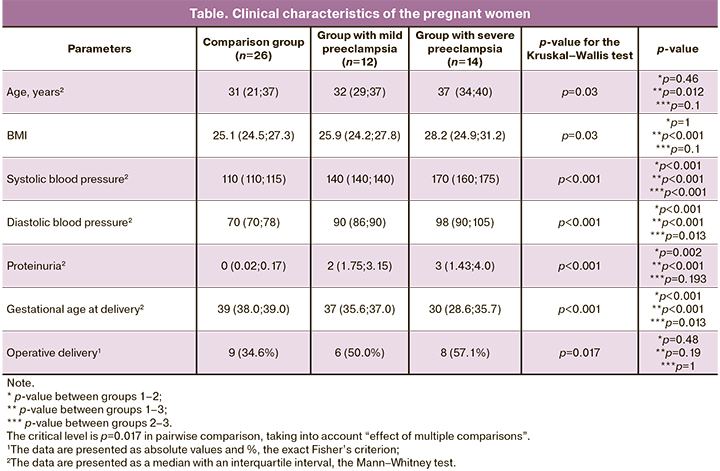
The content of CD68+ cells in placental villi was studied using the immunohistochemical method. The stained CD68+ cells inside the placenta villi are presented in Figures 1–3.
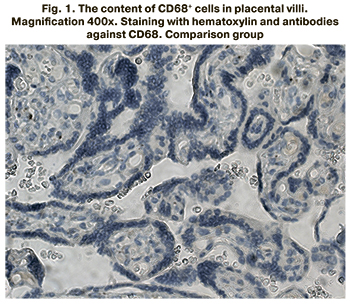
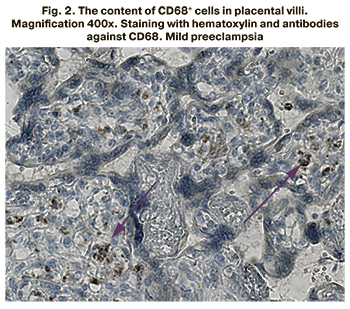
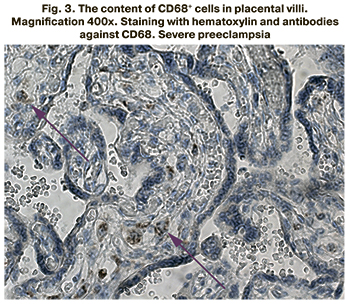
The content of CD68+ cells inside the placental villi was found to be significantly higher in the groups of patients with mild and severe preeclampsia compared to the control group (p<0.001 for all groups). The content of CD68+ cells was 7.5% (5.6;8.3) in patients with mild preeclampsia, 9.1% (6.5;10.8) in severe preeclampsia, 5.1% (2.5; 5.3) in the comparison group. The results are presented in Figure 4.
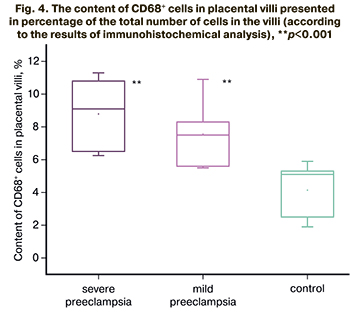
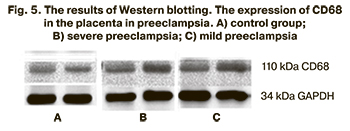
The results of immunohistochemical analysis were confirmed by Western blotting. The results are presented in Figure 5.
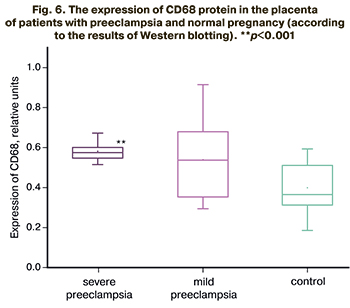
The comparative analysis of the patients with normal pregnancy revealed statistically significant differences in the level of CD68 protein expression in severe preeclampsia (p<0.001) and close to statistically significant differences in mild preeclampsia (p=0.056). The relative level of CD68 protein expression in the group of patients with mild preeclampsia was 0.52 (0.35–0.67) relative units; it was 0.57 (0.55–0.60) relative units in patients with severe preeclampsia and 0.36 (0.30–0.50) relative units in the comparison group. The results are presented in Figure 6.
Thus, the number of CD68+ cells inside the placental villi has been shown to increase with the severity of preeclampsia. The results of the study may indicate the potential role of the placenta as a factor of activation of monocyte-macrophage cells.
Discussion
Cluster of differentiation 68 (CD68) is a member of the lysosomal/endosomal-associated membrane glycoprotein (LAMP) family, which is expressed on the surface of monocytes and macrophages and used as their marker [20]. According to the results of immunohistochemical analysis, the number of CD68+ cells in the placental villi increased with the progression of the severity of preeclampsia. The reliability of the obtained results was also confirmed by the Western blotting method, which revealed statistically significant differences in the expression of the CD68 protein between the study groups with preeclampsia and the comparison group.
The analysis of modern literature and foreign studies showed controversial results. For example, M.R. Bürk at al. noted a decrease in CD68+ cells in the placenta [22]. Kim et al. were unable to detect any changes in the composition of these cells in the placenta [23]. At the same time, C.J. Lockwood et al. [24] and L.A. Al-Khafaji et al. [25] could describe an increase in CD68+ cells in the placental villi; the findings of their study are consistent with our results.
The results of our study suggest that maternal blood monocytes are present in greater numbers in the chorionic area in patients with preeclampsia than in normal pregnancy [26, 27]. CD68+ resident macrophages (Hofbauer cells) are also known to be present in the placenta [15, 17]. However, an increase in CD68+ cells is most likely to occur in preeclampsia due to maternal monocytes that invade the placental villi, since the distinguishing feature of preeclampsia is increased apoptosis of the placental villi [18], and Hofbauer cells are of fetal origin and are immunotolerant to the fetal part of the placenta and are not involved in damage to the villi. In this study, a correlation was found between the number of CD68+ cells in the placental villi and the severity of preeclampsia. Apoptosis of placental villi and the level of cytotoxic markers of maternal blood monocytes are known to be correlated with the severity of preeclampsia [11, 18, 20, 28, 29]. The results of the study also indirectly confirm the hypothesis that maternal blood monocytes are able to activate in the placenta.
Conclusion
The analysis of CD68+ cells in the placental villi revealed a correlation between an increase in their content and the severity of preeclampsia. Thus, the obtained results may indicate a potential role of the placenta in the pathogenesis of preeclampsia as an agent activating cytotoxic properties in maternal blood monocytes.
References
- Барановская Е.И. Преэклампсия в современных условиях. Акушерство и гинекология. 2018; 11: 5-9. [Baranovskaya А.I. Preeclampsia in modern conditions Akusherstvo i ginekologiya / Obstetrics and Gynecology. 2018; 11: 5-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.11.5-9.
- Волков В.Г., Гранатович Н.Н., Сурвилло Е.В., Черепенко О.В. Ретроспективный анализ материнской смертности от преэклампсии и эклампсии. Российский вестник акушера-гинеколога. 2017; 17(3): 4-8. [Volkov V.G., Granatovich N.N., Survillo E.V., Cherepenko O.V. Retrospective analysis of maternal mortality in preeclampsia and eclampsia Rossiyskiy vestnik akushera-ginekologa / The Russian bulletin of the obstetrician-gynecologist. 2017; 17(3): 4-8. (in Russian)]. https://doi.org/10.17116/rosakush20171734-8.
- Сидорова И.С., Никитина Н.А., Унанян А.Л. Преэклампсия и снижение материнской смертности в России. Акушерство и гинекология. 2018; 1: 107-12. [Sidorova I.S., Nikitina N.A., Unanyan A.L. Preeclampsia and lower maternal mortality in Russia Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2018; 1: 107-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.1.107-112.
- ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet. Gynecol. 2019; 133(1): 1. https://dx.doi.org/10.1097/AOG.0000000000003018.
- ACOG Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet. Gynecol. 2002; 99(1): 159-67. https://dx.doi.org/10.1016/s0029-7844(01)01747-1.
- Воднева Д.Н., Романова В.В., Дубова Е.А., Павлов К.А., Шмаков Р.Г., Щеголев А.И. Клинико-морфологические особенности ранней и поздней преэклампсии. Акушерство и гинекология. 2014; 2: 35-40. [Vodneva D.N., Romanova V.V., Dubova E.A., Pavlov K.A., Shmakov R.G., Shchegolev A.I. The clinical and morphological features of earlyand late-onset preeclampsia. Akusherstvo i ginekologiya/ Obstetrics and Gynecology, 2014; 2: 35-40. (in Russian)].
- Redman C.W., Sargent I.L. Pre-eclampsia, the placenta and the maternal systemic inflammatory response: a review. Placenta. 2003; 24(Suppl. A): S21-7. https://dx.doi.org/10.1053/plac.2002.0930.
- Yockey L.J., Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018; 49(3): 397-412. https://dx.doi.org/10.1016/j.immuni.2018.07.017.
- Павлов К.А., Дубова Е.А., Щеголев А.И. Фетоплацентарный ангиогенез при нормальной беременности: роль плацентарного фактора роста и ангиопоэтинов. Акушерство и гинекология. 2010: 6: 10-5. [Pavlov K.A., Dubova Ye.A., Shchegolev A.I. Fetoplacental angiogenesis during normal pregnancy: A role of placental growth factor and angiopoietins. Akusherstvo i ginekologiya / Obstetrics and Gynecology, 2010; 6: 10-5. (in Russian)].
- Dubova E.A., Pavlov K.A., Lyapin V.M., Shchyogolev A.I., Sukhikh G.T. Vascular endothelial growth factor and its receptors in the placental villi of pregnant patients with pre-eclampsia. Bull. Exp. Biol. Med. 2013; 154(6): 792-5. https://dx.doi.org/10.1007/s10517-013-2058-8.
- Борис Д.А., Волгина Н.Е., Красный А.М., Тютюнник В.Л., Кан Н.Е. Прогнозирование преэклампсии по содержанию CD16-негативных моноцитов. Акушерство и гинекология. 2019; 7: 49-55. [Boris D.A., Volgina N.E., Krasnyi A.M., Tyutyunnik V.L., Kan N.E. Prediction of preeclampsia on the couts of CD-16 negative monocytes Akusherstvo i ginekologiya / Obstetrics and Gynecology, 2019; 7: 49-55. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.7.49-55.
- Aggarwal R., Jain A.K., Mittal P., Kohli M., Jawanjal P., Rath G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019; 33(4): e22834. https://dx.doi.org/10.1002/jcla.22834.
- Tranquilli A.L., Dekker G., Magee L., Roberts J., Sibai B.M., Steyn W. et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014; 4(2): 97-104. https://dx.doi.org/10.1016/j.preghy.2014.02.001.
- Chen W., Qian L., Wu F., Li M., Wang H. Significance of toll-like receptor 4 signaling in peripheral blood monocytes of pre-eclamptic patients. Hypertens. Pregnancy. 2015; 34(4): 486-94. https://dx.doi.org/10.3109/10641955.2015.1077860.
- Malyshev I., Malyshev Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage (switch) phenotype. Biomed. Res. Int. 2015; 2015: 341308. https://dx.doi.org/10.1155/2015/341308.
- Matias M.L., Gomes V.J., Romao-Veiga M., Ribeiro V.R., Nunes P.R., Romagnoli G.G. et al. Silibinin downregulates the NF-κB pathway and NLRP1/NLRP3 inflammasomes in monocytes from pregnant women with preeclampsia. Molecules. 2019; 24(8): 1548. https://dx.doi.org/10.3390/molecules24081548.
- Cubro H., Kashyap S., Nath M.C., Ackerman A.W., Garovic V.D. The role of interleukin-10 in the pathophysiology of preeclampsia. Curr. Hypertens. Rep. 2018; 20(4): 36. https://dx.doi.org/10.1007/s11906-018-0833-7.
- Сухих Г.Т., Красный А.М., Кан Н.Е., Майорова Т.Д., Тютюнник В.Л., Ховхаева П.А., Сергунина О.А., Тютюнник Н.В., Грачева М.И., Вавина О.В., Озернюк Н.Д., Борис Д.А. Апоптоз и экспрессия ферментов антиоксидантной защиты в плаценте при преэклампсии. Акушерство и гинекология. 2015; 3: 11-5. [Sukhikh G.T., Krasnyi A.M., Kan N.E., Maiorova T.D., Tyutyunnik V.L., Khovkhaeva P.A., Sergunina O.A., Tyutyunnik N.V., Gracheva M.I., Vavina O.V., Ozernyuk N.D., Boris D.A. Placental apoptosis and antioxidant defense enzyme gene expression in preeclampsia Akusherstvo i ginekologiya / Obstetrics and Gynecology, 2015; 3: 11-5. (in Russian)].
- Красный А.М., Грачева М.И., Садекова А.А., Вторушина В.В., Балашов И.С., Кан Н.Е., Тютюнник В.Л. Комбинированное исследование общей, фетальной ДНК, цитокинов в плазме крови матери при преэклампсии. Бюллетень экспериментальной биологии и медицины. 2017; 164(12): 686-91. [Krasnyi A.M., Gracheva M.I., Sadekova A.A., Vtorushina V.V., Balashov I.S., Kan N.E., Tyutyunnik V.L. Complex analysis of total and fetal DNA and cytokines in blood plasma of pregnant women with preeclampsia Bulleten ekperementalnoy biologii i medicini / Bulletin of Experimental Biology and Medicine. 2017; 164(12): 686-91. (in Russian)].
- Chistiakov D.A., Killingsworth M.C., Myasoedova V.A., Orekhov A.N., Bobryshev Y.V. CD68/macrosialin: not just a histochemical marker. Lab. Invest. 2017; 97(1): 4-13. https://dx.doi.org/10.1038/labinvest.2016.116.
- Щеголев А.И., Туманова У.Н., Ляпин В.М., Серов В.Н. Синцитиотрофобласт ворсин плаценты в норме и при преэклампсии. Акушерство и гинекология. 2020; 6: 21-8. [Shchegolev A.I., Tumanova U.S., Lyapin V.M., Serov V.N. The syncytiotrophoblast of the placental villi in health and in preeclampsia Akusherstvo i ginekologiya / Obstetrics and Gynecology. 2020; 6: 21-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.6.21-28.
- Bürk M.R., Troeger C., Brinkhaus R., Holzgreve W., Hahn S. Severely reduced presence of tissue macrophages in the basal plate of pre-eclamptic placentae. Placenta. 2001; 22(4): 309-16. https://dx.doi.org/10.1053/plac.2001.0624.
- Kim J.S., Romero R., Kim M.R., Kim Y.M., Friel L., Espinoza J. et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008; 52(4): 457-64. https://dx.doi.org/10.1111/j.1365-2559.2008.02964.x.
- Lockwood C.J., Matta P., Krikun G., Koopman L.A., Masch R., Toti P. et al. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am. J. Pathol. 2006; 168(2): 445-52. https://dx.doi.org/10.2353/ajpath.2006.050082.
- Al-Khafaji L.A., Al-Yawer M.A. Localization and counting of CD68-labelled macrophages in placentas of normal and preeclamptic women. In: AIP Conference Proceedings. 2017; 1888: 020012. https://doi.org/10.1063/1.5004289.
- Romão-Veiga M., Bannwart-Castro C.F., Borges V.T.M., Golim M.A., Peraçoli J.C., Peraçoli M.T.S. Increased TLR4 pathway activation and cytokine imbalance led to lipopolysaccharide tolerance in monocytes from preeclamptic women. Pregnancy Hypertens. 2020; 21: 159-65. https://dx.doi.org/10.1016/j.preghy.2020.06.002.
- Wu Z.M., Yang H., Li M., Yeh C.C., Schatz F., Lockwood C.J. et al. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012; 33(3): 188-94. https://dx.doi.org/10.1016/j.placenta.2011.12.007.
- Shchyogolev A.I., Dubova E.A., Pavlov K.A., Lyapin V.M., Kulikova G.V., Shmakov R.G. Morphometric characteristics of terminal villi of the placenta in pre-eclampsia. Bull. Exp. Biol. Med. 2012; 154(1): 92-5. https://dx.doi.org/10.1007/s10517-012-1883-5.
- Gomes V.J., Nunes P.R., Matias M.L., Ribeiro V.R., Devides A.C., Bannwart-Castro C.F. et al. Silibinin induces in vitro M2-like phenotype polarization in monocytes from preeclamptic women. Int. Immunopharmacol. 2020; 89 (Pt A): 107062. https://dx.doi.org/10.1016/j.intimp.2020.107062.
Received 24.05.2021
Accepted 22.09.2021
About the Authors
Daiana A. Boris, PhD, Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(915)081-89-97, dayana_boris@mail.ru, https://orcid.org/0000-0002-0387-4040, 117997, Russia, Moscow, Ac. Oparina str., 4.Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher of Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015,
SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN: 5378-8437,
Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksandr I. Shchegolev, Dr. Med. Sci., Professor, Head of the Department of Morbid Anatomy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)531-44-44, ashegolev@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Veronica A. Sinitsyna, senior laboratory assistant, Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-28-92, v_sinitsyna@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alsu A. Sadekova, PhD, Scientific researcher of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation, +7(495)438-22-72, a_sadekova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Healthcare of Russian Federation, +7(495)438-22-72, alexred@list.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions: Boris D.A., Tyutyunnik V.L., Kan N.E., Shchegolev A.I., Sinitsyna V.A., Sadekova A.A., Krasnyi A.M. – developing of research design, obtaining data for analysis, reviewing publications on the topic of the article, statistical analysis of the obtained data, article writing.
Conflicts of interest: Authors declare no conflicts of interest.
Funding: The study was conducted without sponsorship.
Patient Consent for Publication: All patients provided informed consent for the publication of their data and associated images.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Boris D.A., Tyutyunnik V.L., Kan N.E., Shchegolev A.I., Sinitsyna V.A., Sadekova A.A., Krasnyi A.M. Features of changes in the monocyte-macrophage cells
in the placenta during preeclampsia.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2021; 10: 48-54 (in Russian)
http://dx.doi.org/10.18565/aig.2021.10.48-54



