Specific features of TLR9 expression in placental tissue in preeclampsia and fetal growth restriction
Aim. To evaluate the level of TLR9 expression in placental tissue in cases of preeclampsia and fetal growth restriction.Nizyaeva N.V., Аmiraslanov E.Yu., Lomova N.А., Dolgopolova Е.L., Nagovitsyna М.N., Shmakov R.G.
Material and methods. Histological study through staining with hematoxylin and eosin and immunohistochemical study using Ventana (Roche, UK) immunostainer (with a closed protocol for detection) were performed with the use of primary polyclonal antibodies to TLR9 (1: 300; GenTex) on serial paraffin sections of placenta samples collected from 40 women at 26–39 weeks of gestation participating in the study. Of them, 18 women were diagnosed with severe preeclampsia, 12 – with moderate preeclampsia, 10 women were included in the control group. In 8 cases of severe preeclampsia (PE), intrauterine fetal growth restriction (IUGR) was detected. Comparative assessment of the optical density of staining was carried out using the nonparametric Mann–Whitney test.
Results. In severe PE, including the cases complicated by IUGR, a significant increase in cytoplasmic as well as granular staining of TLR9 in villous cytotrophoblast and syncytiotrophoblast (STB) and in extravillous trophoblast (EVT) was detected. Moreover, the number of TLR9 granules and their size in STB and EVT was associated with the severity of PE. The diameter of stained granules in STB in severe PE complicated by IUGR was significantly greater (1.27+0.41 µm), compared to severe PE without IUGR (1.16+0.33 µm) (p=0.05), as well as compared to moderate PE (1.13+0.41 µm) (p=0.001), and the control group (1.09+0.30 µm) (p=0.001). In severe PE complicated by IUGR, the maximum diameter of granules for EVT was 1.49+0.41 µm, which was significantly greater than in severe PE without IUGR, in moderate PE, and in the control group (p=0.001). Granular staining was specific for cytotrophoblast and STB, as well as for placental macrophages (Kashchenko–Hofbauer cells).
Conclusion. Thus, in severe preeclampsia, including the cases complicated by fetal growth restriction, a significant increase in cytoplasmic as well as granular staining of TLR9 in the villous cytotrophoblast and STB and in EVT was detected. Considering that an increase in TLR9 expression was associated with the proinflammatory response, the possibility of interaction between DNA fragments, including fetal DNA and damage-associated molecular patterns (DAMP), may underlie in the genesis of PE and development of systemic inflammatory response.
Keywords
Placenta is a viral and bacterial barrier mainly due to non-specific resistance, and one of its important links are toll-like receptors (TLRs) [1]. TLR9 recognizes non-methylated CpG-DNA sequences of exogenous and endogenous DNA: bacterial and viral DNA, as well as damaged fragments of host and fetal DNA [2, 3], initiating the signaling cascade that leads to the activation of NF-kB factor and production of pro-inflammatory cytokines [1, 3]. In addition, due to association with the AP-1 protein, TLRs can also regulate not only the level of pro-inflammatory response, but also apoptosis, cell proliferation, and differentiation [4, 5].
Preeclampsia (PE) is a pregnancy specific multisystem disorder, which is detected after 20 weeks of gestation and manifests with high blood pressure and proteinuria, and often edema [6, 7]. Although, the best-studied cause of PE is incomplete spiral arteries transformation due to poor trophoblast invasion [6], a number of studies showed that PE is characterized by higher levels of Th1 and Th17 cytokines, and pro-inflammatory immune responses accordingly [8].
Aim of study: to evaluate the level of TLR9 expression in placental tissue in cases of preeclampsia (PE) and intrauterine fetal growth restriction (FGR).
Materials and methods
The study included 18 puerperas with severe preeclampsia (among them 8 women were with detected FGR; 12 women – with moderate PE and 10 women – with uncomplicated full-term pregnancy in the control group.
Inclusion criteria in the group of women with moderate PE were: arterial hypertension ≥140/90 mm Hg, proteinuria ≥0,3 g per day; in the group of women with severe PE: arterial hypertension ≥160/110 mm HGм, proteinuria ≥5 g per day [6, 7]. Inclusion criteria in the group of women with uncomplicated full-term pregnancy were: normal physiological changes during the course of pregnancy, cesarean section due to abnormal fetal position, uterine scar, as well as a pathology which was not related to obstetric anamnesis (severe myopia, risk for retinal detachment).
Exclusion criteria in all groups were: acute and chronic inflammatory diseases, multiple pregnancies, severe extragenital pathology, medical condition after organ transplantation, the use of assisted reproductive technologies, oncological diseases in anamnesis, congenital malformations in pregnancy, diabetes mellitus, spontaneous delivery.
Histological study on serial paraffin sections stained with hematoxylin and eosin, as well as an immunohistochemical study using Ventana (Roche, UK) immunostainer (with a closed protocol for detection) were performed with the use of primary polyclonal antibodies to TLR9 (1:300; GenTex) on placenta samples collected from women at 26–39 weeks of gestation, who underwent caesarean sections [9]. Assessment of membrane and cytoplasmic staining of TLR9 reaction products in placental structures was made in optical density units with the use of Nikon Imaging Software (NIS-Elements, Czech Republic) platform for controlling Nikon Eclipse microscope. For convenience of the presentation, all data were multiplied by 100. Due to the technical complexity and small diameter of intermediate villous vessels endothelium and mesenchymal stromal cells, only cytoplasmic staining was assessed.
We analyzed at least 20 fields of view (FOV) in each sample at x400 magnification. The diameter of TLR9-stained cytoplasmic granules in syncytiotrophoblast (STB) and in extravillous trophoblast (EVT) was measured in μm using NIS-Elements. Additionally, the number of granules in STB was assessed using 5-point semiquantitative scale: 0 – granules were not detected; 1 – very few granules in individual villi (50-100 in FOV), 2 – a moderate amount of granules in some villi (100-150 in FoV); 3 – a moderate amount of granules in all villi (150–200 in FOV); 4 – many granules in most villi (200–250 in FoV); 5 – many granules in all villi (> 250 in FOV). The calculation was made in FOV size 175х130 μm). Along with this, quantitative assessment of granules in the EVT was made per one cell.
Statistical analysis
Statistical data processing was performed using SPSS Statistics V21 for Windows. The Shapiro-Wilk test was used to check the parameters of a normal distribution. The critical value was at p<0.05. The values of non-normal numerical data distribution were described as median (Me) and quartiles Q1 and Q3 in format of Me (Q1; Q3). The values of normal numerical data distribution were described using the arithmetic mean (M) and the standard deviation (SD) as (M ± SD). Descriptive statistics was used to compare medians and mean values in different groups. To assess the statistical significance of the results, the nonparametric Mann–Whitney test was used with critical value at p<0.05 to measure a distribution different from normal distribution at p <0.05, and Student's test was used with a critical value at p <0.05.
Results
The study detected a significant increase in membrane cytoplasmic staining of TLR9 in STB in the group of patients with severe PE complicated by FGR (membrane staining – 44 (37; 49), cytoplasmic staining – 42 (35;47) compared to the group of patients with severe PE not complicated by FGR (membrane staining – 25 (20;31), cytoplasmic staining – 27 (22;32), as well as in the group of patients with moderate PE (р=0,02). Minimal staining was detected in the control group (membrane staining – 7 (4;17), cytoplasmic staining – 6 (4;16) (Fig. 1 A–H; Fig. 2 A, B).
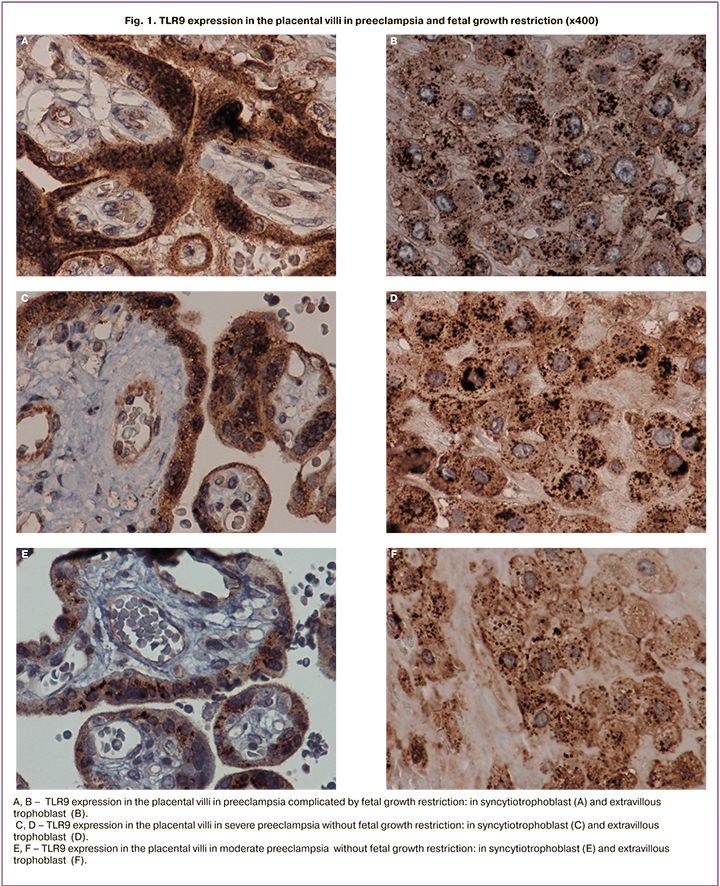
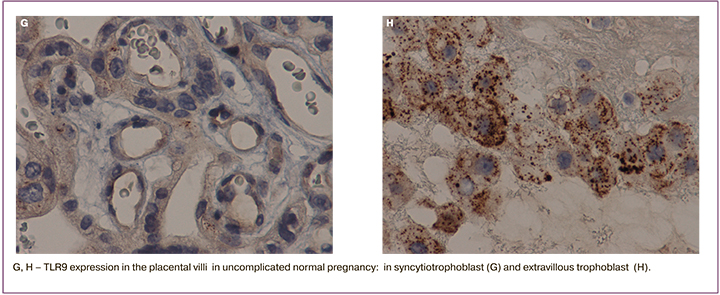
A similar trend was detected, when membrane and cytoplasm in syncytial nodules (the zones of trophoblast proliferation), as well as in EVT were assessed: there was a maximum increase in the group of women with severe PE and FGR compared to the other groups (membrane staining – 45 (30; 60), cytoplasmic staining – 43 (40;58) (р=0,03); (Fig. A – H); Fig. 2 C, D,). Maximal cytoplasmic staining in EVT was in group of women with severe PE, the median was 38 (30;42), and with moderate PE – 19 (16;29), and it was comparable with the control group – 20 (5;26) (Fig. 1 A–H; Fig. 2 E, F). Cytoplasmic staining in the vascular endothelium was maximal in the group with severe PE complicated by FGR – 23 (16;28), and severe PE not complicated by FGR – 22 (18;26), minimal expression in the control group – 5 (3;10) (р=0,01); (Fig. 1 A, B, E; Fig 2 G).
Expression in decidual cells, as well as in mesenchymal stromal cells was significantly higher in the group of women with severe PE not complicated with FGR – 25 (18;29), than in the group of women with severe PE complicated with FGR – 21 (19;30) (р=0,04), minimal expression was in the control group (Fig. 2 H–J). TLR9 expression in membrane and in macrophage cytoplasm in the group of women with sever PE complicated with FGR was 20 (18;25) and not complicated by FGR – 20 (15;25) was higher than in the group with moderate PE, although without significant differences – 15 (10;20) (р=0,78), (Fig. 2 K, L).
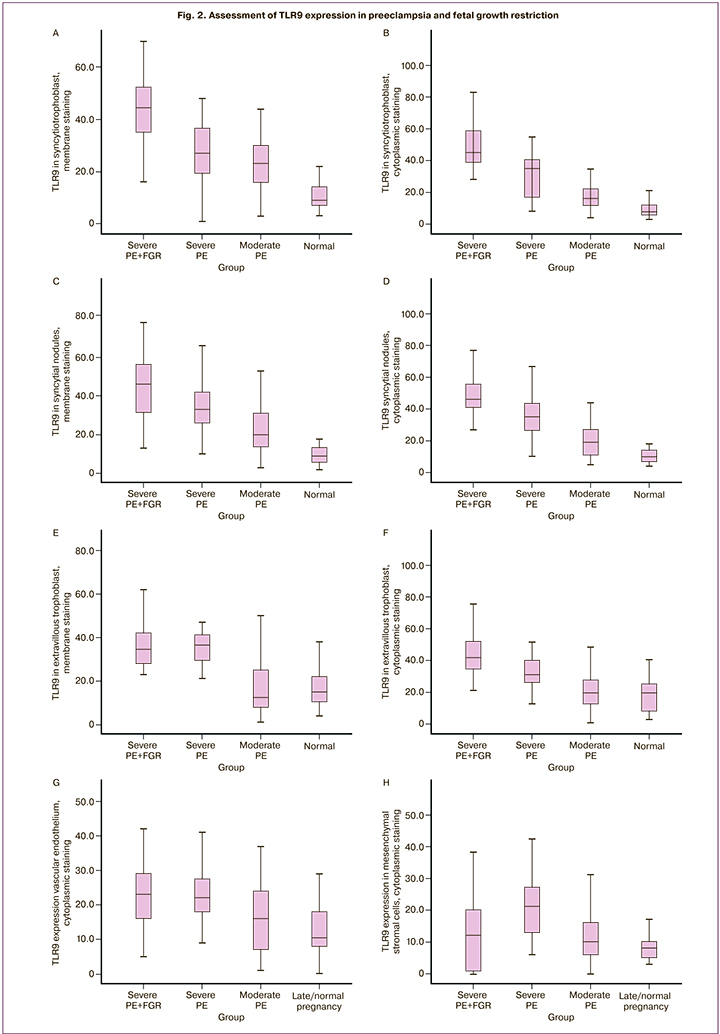
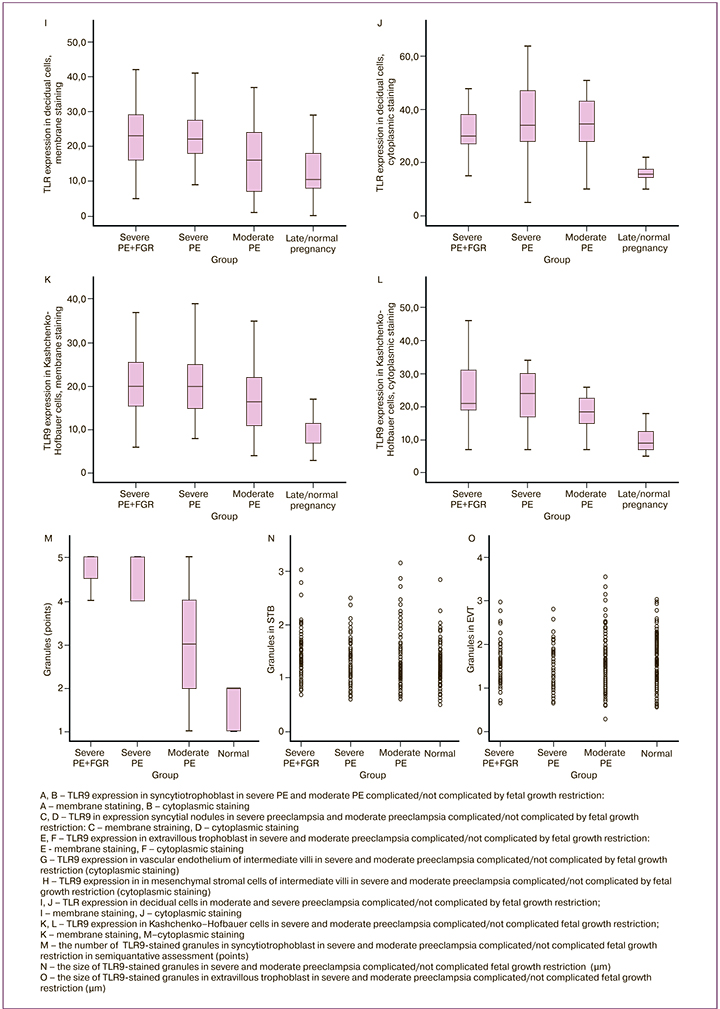
It should be noted that in the cytotrophoblast, STB, as well as in EVT and in macrophage cytoplasm (Kaschenko–Hoffbauer cells), there were granules stained with primary antibodies to TLR9. The number and size of granules in STB and EVT were associated with the severity degree of PE (Fig. 1 A–H; Fig. 2 M–O). In the cytotrophoblast and vilous STB, stained granules were located mainly in the nuclear membrane, as well as in EVT.
The diameter of stained granules in STB in severe PE complicated by FGR was significantly larger (1,27+0,41), compared to severe PE not complicated by FGR (1,16+0,33) (р=0,05), as well as compared to moderate PE (1,13+0,41) (р=0,001) and the control group (1,09+0,30) (р=0,001) (Fig. 1; Fig.2 N, O).
In PE, small granules formed conglomerates, which optically merged and layed on top of each other, therefore, it was difficult to count them (Fig. 1 B, D, F, H). In severe PE complicated by FGR, the granule size 1,49+0,41 in EVT was larger than in the other studied groups (р=0,001) (Fig.1. B, D, F, H; Fig. 2. O). Quntitative count in the group of women with uncomplicated pregnancy showed the presence of 20 granules in EVT, with PE – 20–50 granules; with severe PE and FGR, the number of granules in some cells was 100 (p <0.05).
Discussion
As it is known, TLR9 belongs to the DNA recognition receptors of innate immune system, the ligands of which can be unmethylated CpG sequences in the microbial and non-microbial molecules, as well as DNA fragments, including fetal DNA. Thus, nucleic acids can be considered as regulatory mechanisms of immune responses and the level of proinflammatory response [1–3, 10].
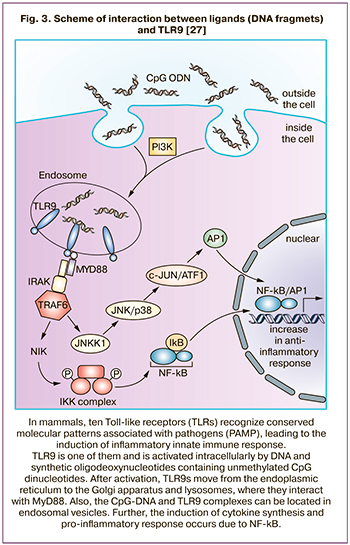 Unlike most TLRs, which are located on the plasma membrane, TLR9s are cytoplasmic detectors, predominantly expressed in the endoplasmic reticulum [11, 12]. After activation, TLR9s move from the endoplasmic reticulum to the Golgi apparatus and lysosomes, where they interact with MyD88 [1, 11, 12]. Also, CpG-DNA and TLR9 complexes can be located in endosomal vesicles (Fig. 3) [13]. It seems that the formation of TLR9 granules in cytoplasm is both associated with endocytosis and the formation of carrier proteins complex for ligand recognition (for example, with granulin, as well as with HMGB-1) [14]. Granulin and other proteins bind to CpG-DNA, thereby forming granulin-DNA-TLR9 complex [14, 15]. In PE, this is confirmed by increased levels of granulin in plasma [16, 17]. The other authors [18] found that endogenously expressed TLR9 persists in the endoplasmic reticulum. In contrast, TLR4 migrates to the cell surface, indicating that retention in the endoplasmic reticulum is not common to all TLRs. Since TLR9 is observed in endocytic vesicles after exposure to synthetic CpG agonist, it can be assumed that there must be a special mechanism for TLR9 translocation into signaling compartments that contain CpG-DNA [18].
Unlike most TLRs, which are located on the plasma membrane, TLR9s are cytoplasmic detectors, predominantly expressed in the endoplasmic reticulum [11, 12]. After activation, TLR9s move from the endoplasmic reticulum to the Golgi apparatus and lysosomes, where they interact with MyD88 [1, 11, 12]. Also, CpG-DNA and TLR9 complexes can be located in endosomal vesicles (Fig. 3) [13]. It seems that the formation of TLR9 granules in cytoplasm is both associated with endocytosis and the formation of carrier proteins complex for ligand recognition (for example, with granulin, as well as with HMGB-1) [14]. Granulin and other proteins bind to CpG-DNA, thereby forming granulin-DNA-TLR9 complex [14, 15]. In PE, this is confirmed by increased levels of granulin in plasma [16, 17]. The other authors [18] found that endogenously expressed TLR9 persists in the endoplasmic reticulum. In contrast, TLR4 migrates to the cell surface, indicating that retention in the endoplasmic reticulum is not common to all TLRs. Since TLR9 is observed in endocytic vesicles after exposure to synthetic CpG agonist, it can be assumed that there must be a special mechanism for TLR9 translocation into signaling compartments that contain CpG-DNA [18].
Interestingly, that TLR9 and TLR8 have common ligands, but membrane staining of TLR9 is less pronounced [19]. TLR9 expression is predominantly represented by cytoplasmic and granular staining; granular staining was present both in villous and extravillous throphoblast, single granules were also found in placental macrophages (Kaschenko – Gofbauer cells). It is important to note that the number and size of stained granules in the trophoblast is related to the severity of PE. It is apparently associated with the severity of inflammatory response (Fig. 3), and an increase in the proinflammatory response is a leading factor in the pathogenesis of PE [8].
Considering that there are few immune cells within placenta compared to other organs (intestines, uterus), and the level of fetal infection protection should be high, TLRs may be an important component of such protection. Along with this, TLR4 granules (protection against bacterial infection) are described in neutrophils being a part of neutrophil extracellular traps) [20, 21]. When activated, they are ejected into extracellular space, activating microbial agents at a distance from the cell [20, 21]. It is possible that placenta has a similar defense mechanism in the form of TLR9, the aim of which is protection against viruses.
In PE complicated by FGR, maintenance of membrane staining in cytotrophoblast and STB probably may be explained by partial preservation of membranes of villous trophoblast, due to the fact that a certain time interval is required for the formation of FGR, since in cases of rapid occurrence of placental insufficiency, there is no time for the formation of FGR, and the condition of a woman with PE requires urgent delivery. Along with increasing proinflammation, Through AP-1, innate immune receptors, including TLRs can regulate apoptosis and proliferation through AP-1. Therefore, for physiological regeneration, an optimal level of their expression is necessary. Significantly increased or decreased expression leads to impaired regeneration of cells and tissues. In placenta, this primarily concerns the invasion ability of trophoblast [22, 23].
The ability of TLR9 to interact with DNA fragments and damage-associated molecular patterns (DAMPs) is essential in the genesis of PE [2, 16, 23, 24], and as was previously shown [25], the level of fetal DNA in maternal plasma was elevated [26]. In PE, granular staining and an increase in the diameter and number of granules in the villous trophoblast and EVT requires further research.
Our previous studies have shown that PE is based on degenerative changes in cytotrophoblast and STB [19]. Damage to membranes, as well as desquamation of cells desquamation on the villous tips leads to dysfunction of the hematoplacental barrier, affecting gas exchange between the mother and the fetus. STB damage of the placental villi results in an increase both in the levels of total and fetal DNA in the maternal bloodstream. DNA fragments, binding to DNA and the receptors that recognize RNA, apparently can play the role of activation trigger of inflammatory signaling pathways, including the formation of endothelial dysfunction both in the placenta and in the mother's body, mediating the formation of a systemic proinflammatory response and PE [25, 26].
Conclusion
Thus, in severe PE, including those complicated by IGR, a significant increase in cytoplasmic and granular TLR9 staining in villous cytotrophoblast, STB and EVT was detected. Granular type of staining was specific only for cytotrophoblast, STB, as well as for placental macrophages (Kaschenko–Gofbauer cells). Possible interaction of DNA fragments, including fetal DNA and the molecules damage associated with TLR9, may lie in the genesis of PE and the development of the systemic proinflammatory response.
References
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124(4): 783-801. https://dx/doi.org/10.1016/j.cell.2006.02.015.
- Goulopoulou S., Matsumoto T., Bomfim G.F., Webb R.C. Toll-like receptor 9 activation: A novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin. Sci. 2012; 123(7): 429-35. https://dx.doi.org/10.1042/CS20120130.
- Martínez-Campos C., Burguete-García A.I., Madrid-Marina V. Role of TLR9 in oncogenic virus-produced cancer. Viral Immunol. 2017; 30(2): 98-105. https://dx.doi.org/10.1089/vim.2016.0103.
- Harju K., Glumoff V., Hallman M. Ontogeny of toll-like receptors Tlr2 and Tlr4 in mice. Pediatr. Res. 2001; 49(1): 81-3. https://dx.doi.org/10.1203/00006450-200101000-00018.
- Amirchaghmaghi E., Taghavi S.A., Shapouri F., Saeidi S., Rezaei A., Aflatoonian R. The role of toll like receptors in pregnancy. Int. J. Fertil. Steril. 2013;7(3): 147-54.
- Адамян Л.В., Артымук Н.В., Башмакова Н.В., Белокринницкая Т.Е., Беломестнов С.Р., Братищев И.В., Вученович Ю.Д., Куликов А.В., Краснопольский В.И., Левит А.Л., Никитина Н.А., Петрухин В.А., Пырегов А.В.,Серов В.Н., Сидорова И.С., Филиппов О.С., Ходжаева З.С., Холин А.М., Шешко Е.Л., Шифман Е.М., Шмаков Р.Г. Гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Преэклампсия. Эклампсия. Клинические рекомендации (Протокол лечения). М.; 2016. [Adamyan L.V., Artymuk N.V., Bashmakova N.V., Belokrinnitskaya T.E., Belomestnov S.R., Bratishchev I.V. et al. Hypertensive disorders during pregnancy, childbirth and the postpartum period. Preeclampsia. Eclampsia. Clinical recommendations (Treatment protocol). Moscow; 2016. (in Russian)].
- Dong X., Gou W., Li C., Wu M., Han Z., Li X. et al. Proteinuria in preeclampsia: Not essential to diagnosis but related to disease severity and fetal outcomes. Pregnancy Hypertens. 2017; 8: 60-4. https://dx.doi.org/10.1016/j.preghy.2017.03.005.
- Cухих Г.Т., Ванько Л.В. Иммунные факторы в этиологии и патогенезе осложнений беременности. Акушерство и гинекология. 2012; 1: 128-36. [Sukhikh G.T., Vanko L.V. Immune factors in the etiology and pathogenesis of pregnancy complications. Obstetrics and Gynecology. 2012; 1:128-36. (in Russian)].
- Низяева Н.В., Волкова Ю.С., Муллабаева С.М., Щеголев А.И. Методические основы изучения ткани плаценты и оптимизация режимов предподготовки материала. Акушерство и гинекология. 2014; 8: 10-8. [Nizyaeva N.V., Volkova Yu.S., Mullabaeva S.M., Shchegolev A.I. The methodical bases for placental tissue examination and the optimization of material pre-preparation regimens. Obstetrics and Gynecology. 2014; 8:10-8. (in Russian)].
- De Lorenzo G., Ferrari S., Cervone F., Okun E. Extracellular DAMPs in plants and mammals: immunity, tissue damage and repair. Trends Immunol. 2018; 39(11): 937-50. https://dx.doi.org/10.1016/j.it.2018.09.006.
- Yu L., Wang L., Chen S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010; 14(11): 2592-603. https://dx.doi.org/10.1111/j.1582-4934.2010.01127.x.
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010; 11(5): 373-84. https://dx.doi.org/10.1038/ni.1863.
- Honda K., Ohba Y., Yanai H., Hegishi H., Mizutani T., Takaoka A. et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005; 434(7036): 1035-40. https://dx.doi.org/10.1038/nature03547.
- Park B., Buti L., Lee S., Matsuwaki T., Spooner E., Brinkmann M.M. et al. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity. 2011; 34(4): 505-13. https://dx.doi.org/10.1016/j.immuni.2011.01.018.
- Ivanov S., Dragoi A.M., Wang X., Dallacosta C., Louten J., Musco G. et al. Anovel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007; 110(6): 1970-81. https://dx.doi.org/10.1182/blood-2006-09-044776.
- Stubert J., Kleber T., Bolz M., Külz T., Dieterich M., Richter D.U. et al. Acute-phase proteins in prediction of preeclampsia in patients with abnormal midtrimester uterine Doppler velocimetry. Arch. Gynecol. Obstet. 2016; 294(6): 1151-60. https://dx.doi.org/10.1007/s00404-016-4138-2.
- Aghaeepour N., Lehallier B., Baca Q., Ganio E.A., Wong R.J., Ghaemi M.S. et al. A proteomic clock of human pregnancy. Am. J. Obstet. Gynecol. 2018; 218(3): 347. e1-347. e14. https://dx.doi.org/10.1016/j.ajog.2017.12.208.
- Leifer C.A., Kennedy M.N., Mazzoni A., Lee C., Kruhlak M.J., Segal D.M. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 2004; 173(2): 1179-83. https://dx.doi.org/10.4049/jimmunol.173.2.1179.
- Низяева Н.В., Амирасланов Э.Ю., Ломова Н.А., Савельева Н.А., Павлович С.В., Наговицына М.Н., Щеголев А.И. Повышение экспрессии TLR8 в ткани плаценты при преэклампсии. Бюллетень экспериментальной биологии и медицины. 2019; 168(9): 371-5. [Nizyaeva N.V., Amiraslanov E.Yu., Lomova N.A., Savelyeva N.A., Pavlovich S.V., Nagovitsyna N.M. et al. Increased expression of TLR8 in placental tissue in preeclampsia. Bulletin of Experimental Biology and Medicine. 2019; 168(9): 371-5. (in Russian)].
- Нестерова И.В., Колесникова Н.В., Чудилова Г.А., Ломтатидзе Л.В., Ковалева С.В., Евглевский А.А., Нгуен Т.З.Л. Новый взгляд на нейтрофильные гранулоциты: переосмысление старых догм. Часть 1. Инфекция и иммунитет. 2017; 7(3): 219-30. [Nesterova I.V., Kolesnikova N.V., Chudilova G.A., Lomtatidze L.V., Kovaleva S.V., Evglevsky A.A. et al. A new look at neutrophil granulocytes: rethinking old dogmas. Part 1. Infection and Immunity. 2017; 7(3): 219-30. (in Russian)].
- Vokalova L., Van Breda S.V., Ye X.L., Huhn E.A., Than N.G., Hasler P. et al. Excessive neutrophil activity in gestational diabetes mellitus: Could it contribute to the development of preeclampsia? Front. Endocrinol. (Lausanne). 2018; 9: 542. https://dx.doi.org/10.3389/fendo.2018.00542.
- Kopeina G.S., Zamarev A. V., Zhivotovsky B.D., Lavric I.N. Programmed necrosis and tissue regeneration. Genes Cells. 2018; 13(2): 35-8. https://dx.doi.org/10.23868/201808017.
- Takaoka A., Wang Z., Choi M.K., Yanai H., Negishi H., Ban T. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007; 448(7152): 501-5. https://dx.doi.org/10.1038/nature06013.
- Takeshita F., Gursel I., Ishii K.J., Suzuki K., Gursel M., Klinman D.M. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin. Immunol. 2004; 16(1): 17-22. https://dx.doi.org/10.1016/j.smim.2003.10.009.
- Баев О.Р., Карапетян А.О., Низяева Н.В., Садекова А.А., Красный А.М. Содержание внеклеточной ДНК плода в материнской крови и экспрессия ДНК-распознающих ZBP-1 рецепторов в структурах плаценты при преэклампсии и преждевременных родах. Клеточные технологии в биологии и медицине. 2019; 3: 179-84. [Baev O.R., Karapetyan A.O., Nizyaeva N.V., Sadekova A.A., Krasny A.M. Content of fetal extracellular DNA in maternal blood and expression of DNA-recognizing ZBP-1 receptors in placental structures in preeclampsia and preterm birth. Cell technologies in biology and medicine. 2019; 3:179-84. (in Russian)].
- Карапетян А.О., Баева М.О., Баев О.Р. Роль внеклеточной ДНК плода в прогнозировании больших акушерских синдромов. Акушерство и гинекология. 2018; 4: 10-5. [Karapetyan A.O., Baeva M.O., Baev O.R. The role of extracellular fetal DNA in predicting the great obstetric syndromes. Obstetrics and Gynecology. 2018; 4:10-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.4.10-15.
- Thierry A.R., El Messaoudi S., Gahan P.B., Anker P., Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016; 35(3): 347-76. https://dx.doi.org/10.1007/s10555-016-9629-x.
Received 22.10.2020
Accepted 30.12.2020
About the Authors
Natalia V. Nizyaeva, PhD., MD, Senior researcher, 2nd Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. Tel.: +7(926)248-28-93. E-mail: niziaeva@gmail.com. ORCID: 0000-0001-5592-5690; Author ID56054058900.117997, Russia, Moscow, Ac. Oparina str., 4.
Elrad Yu. Аmiraslanov, PhD, Head of the Obstetric Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. E-mail: eldis@mail.ru. ORCID: 0000-0001-5601-1241. 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia A. Lomova, PhD, Researcher of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. E-mail: natasha-lomova@yandex.ru. ORCID: 0000-0002-6090-586Х. 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena. L. Dolgopolova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. E-mail: dolgopolovae93@mail.ru. ORCID: 0000-0003-2572-9028. 117997, Russia, Moscow, Ac. Oparina str., 4.
Marina N. Nagovitsyna, Junior researcher, 2nd Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. E-mail: moremore84@mail.ru. ORCID: 0000-0001-8039-6217. 117997, Russia, Moscow, Ac. Oparina str., 4.
Roman G. Shmakov, MD, Professor, Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation. E-mail: r_shmakov@oparina4.ru. ORCID: 0000-0002-2206-1002. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Nizyaeva N.V., Аmiraslanov E.Yu., Lomova N.А., Dolgopolova Е.L., Nagovitsyna М.N., Shmakov R.G. Specific features of TLR9 expression in placental tissue in preeclampsia and fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 70-78 (in Russian)
https://dx.doi.org/10.18565/aig.2021.1.70-78



