Experience in random-start ovarian stimulation for preserving reproductive material of cancer patients
Aim. To compare the effectiveness of ovarian stimulation during the luteal and follicular phases in patients referred by an oncologist for retrieval and cryopreservation of reproductive material.Nazarenko T.A., Martirosyan Ya.O., Biryukova A.M., Dzhanashvili L.G., Ivanets T.Yu., Sukhova Yu.V.
Materials and methods. Patients requiring gonadotoxic treatment for cancer underwent ovarian stimulation according to the standard protocol with the gonadotrophin-releasing hormone (GnRH) antagonist in the follicular phase (FF) and random-start protocol in the luteal phase (LF). The comparative analysis included daily and total gonadotropin doses, duration of stimulation, outcomes of stimulation (number of mature oocytes), and features of steroidogenesis dynamics.
Results. All patients included in the study were comparable in age and serum levels of anti-Müllerian hormone (AMH). The mean age of women in groups 1 and 2 was 33.38 (3.73) and 33.3 (5.47) years, respectively. There was no statistically significant difference in the daily and total gonadotropin doses between the two groups. The findings of this study demonstrated that the administration of exogenous gonadotropins in LF induced follicular growth leading to comparable results in the number of obtained and mature oocytes.
Conclusion. The findings of the present study showed the feasibility of ovarian stimulation in any phase of the menstrual cycle. This opportunity is essential for cancer patients given the limited time frame in which reproductive material must be obtained before gonadotoxic therapy can begin. Further, more extensive studies are needed to investigate folliculogenesis providing ovarian stimulation in any phase of the menstrual cycle.
Keywords
Despite decades of research on the physiology and function of the female reproductive system, relatively little is understood about the nuances of ovarian follicular development both in the gonadotropin-independent phase and during the menstrual cycle. Early studies on the mechanisms regulating the biology of folliculogenesis were based on indirect histological and/or endocrinological observations aimed at monitoring follicular growth by determining the parameters of steroidogenesis and ovarian biopsy [1, 2]. More recently, the use of ultrasound has provided new data on the physiological aspects of ovarian activity, in particular, on follicular growth during the luteal phase (LP) of the menstrual cycle [3, 4]. Some researchers reported several major and minor follicular waves during the menstrual cycle, thus challenging the traditional theory that a single cohort of antral follicles grows only during the follicular phase of the menstrual cycle [4].
The possibility of starting gonadotrophins for ovarian stimulation in both the follicular and luteal phases allowed the use of innovative assisted reproductive technologies (ART) protocols, including random-start protocols [5–7] and double stimulation protocols (DuoStim) aimed at obtaining oocytes regardless of the cycle day [8].
Random-start protocols are particularly useful for female cancer patients seeking fertility preservation by cryopreservation of reproductive material before starting cancer treatment. The choice of a protocol for ovarian stimulation in cancer patients is based on a balance between the limited time frame before starting gonadotoxic therapy, and the need to obtain a sufficient number of oocytes and embryos for cryopreservation. The flexibility of random-start protocols suggests their advantage in the context of oncofertility.
Most studies investigating the use of classical gonadotrophin-releasing hormone (GnRH) antagonist protocol in the FP and random-start protocol reported comparable efficacy of the two methods [5, 9]. Some studies have even shown that oocytes obtained in the LP exhibit a higher in vitro fertilization rate [10, 11].
The lack of available research regarding comparative effectiveness and safety of traditional and random-start protocols served as a major rationale for this study.
This study aimed to investigate the effectiveness of ovarian stimulation protocol in the LP for retrieval and cryopreservation of reproductive material in cancer patients.
Materials and methods
This retrospective clinical study was conducted at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The study included female cancer patients seeking retrieval and cryopreservation of oocytes and/ or embryos before cancer treatment.
The study design is presented in Fig. 1.
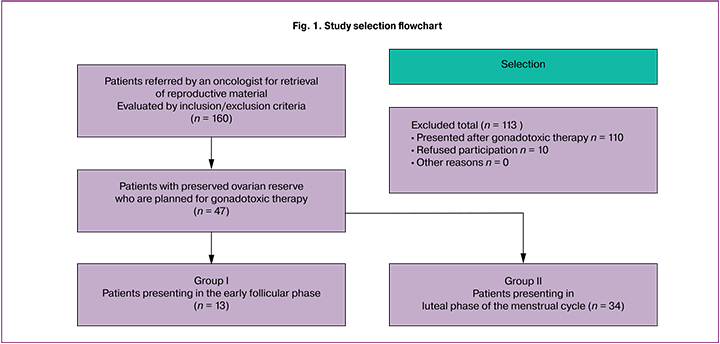
A total of 160 patients were consulted at the F. Paulsen Research and Educational Center for ART from March to December 2019. Of them, 47 women who were referred by an oncologist for retrieval and cryopreservation of reproductive material before gonadotoxic therapy (chemotherapy, radiation therapy) were selected for the study. Before taking part in the study, all patients signed an informed consent form approved by the Ethics Committee.
Inclusion criteria were age from 18 to 42, serum level of anti-Müllerian hormone (AMH) not less than 0.75ng/ml, and planned gonadotoxic therapy (chemo, radiation therapy). Exclusion criteria included extremely low ovarian reserve, oocyte retrieval failure, recurrent cancer, distant metastases, a history of gonadotoxic treatment, and contraindications to ovarian stimulation.
Patients were randomly assigned to one of two groups based on the day of the presentation. Patients in the FP (group 1) underwent ovarian stimulation according to the standard protocol with the gonadotrophinreleasing hormone (GnRH) antagonist on days 2-3 of the menstrual cycle. Recombinant FSH (rFSH), human menopausal gonadotropin (HMG) preparations, or a combination thereof, were used as gonadotropins. Two women with breast cancer and overexpression of estrogen receptors received 2.5 mg aromatase inhibitors (Letrozole) for ovarian stimulation according to international clinical guidelines.
Patients in the LP (group 2) underwent ovarian stimulation according to the random-start protocol with rFSH and HMG gonadotropins (on day 3 after the ovulation, which corresponded to days 16–18 of the cycle). Subgroup 2a (n = 8) and subgroup 2b (n = 5) did not receive and received additional GnRH antagonist, respectively. In this group, three patients with breast cancer and overexpression of estrogen receptors received aromatase inhibitors for ovarian stimulation.
During stimulation, ultrasound examination was used to count the number of antral, growing, and preovulatory follicles. The parameters of steroidogenesis were evaluated on days of enrolment into the in vitro fertilization (IVF) program, GnRH antagonist administration, and final oocyte maturation trigger injection.
The transvaginal ovarian puncture was performed 36 hours after administration of human chorionic gonadotropin (hCG) at a dose of 10,000 IU and 38 hours after administration of diphereline at a dose of 0.2 mg. After the transvaginal puncture, oocyte maturation was identified and evaluated. All mature oocytes (MII) were cryopreserved or fertilized. On day 5 of culture, the embryos were cryopreserved.
A total of 47 ovarian stimulation programs were carried out, followed by cryopreservation of reproductive material. Of these, 13 and 34 programs were performed according to the random-start protocol and standard GnRH antagonist-based protocol in FP, respectively. Oocyte cryopreservation, embryo cryopreservation, and combined cryopreservation of oocytes and embryos was performed in 78.7% (n = 37), 17% (n = 8), and 4.3% (n = 2) of patients, respectively.
The number of retrieved oocytes, mature oocyte counts, daily and total gonadotropin doses, and the duration of stimulation was evaluated.
Statistical analysis was performed using Microsoft Excel 2010 and SPSSVV22.0. Quantitative variables were expressed as means, standard deviation, standard error of the mean, median, and 95% confidence interval (CI). Qualitative variables were summarized as counts and percentages. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov or the Shapiro – Wilk test, depending on the sample size. Quantitative variables showing normal distribution were compared using parametric statistical methods; otherwiseparametric statistical methods (the Kruskal – Wallis test) were used to compare several groups with subsequent Mann – Whitney test with Bonferroni correction. The differences between the related groups showing normal distribution were tested using repeated-measures ANOVA; otherwise, the nonparametric Friedman method was used. Tests were considered statistically significant at p < 0.05. Correction for multiple comparisons of normally distributed samples was done with a Student’s t-test.
Results and discussion
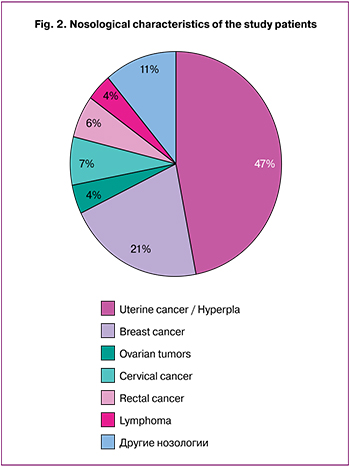 Nosological entities of oncological diseases in patients referred for retrieval and cryopreservation of reproductive material were analyzed. The results of the analysis are presented in Fig. 2. The most common nosological entities were stage I uterine cancer and atypical endometrial hyperplasia (n = 22) and breast cancer (n = 10). Other malignancies (n = 15) included ovarian borderline tumors (after surgery and histological diagnosis verification), cervical cancer, colorectal cancer, lymphoma, and thyroid cancer.
Nosological entities of oncological diseases in patients referred for retrieval and cryopreservation of reproductive material were analyzed. The results of the analysis are presented in Fig. 2. The most common nosological entities were stage I uterine cancer and atypical endometrial hyperplasia (n = 22) and breast cancer (n = 10). Other malignancies (n = 15) included ovarian borderline tumors (after surgery and histological diagnosis verification), cervical cancer, colorectal cancer, lymphoma, and thyroid cancer.
Clinical and laboratory characteristics of the study patients regarding age, body mass index (BMI), AMH level, and antral follicle count (AFC) are presented in Table 1.
All study patients were comparable in age, BMI, and serum AMH levels. The mean age in women in groups 1 and 2 was 33.38 (3.73) and 33.3 (5.47) years, respectively; BMI was 23.2 (2.2) and 21.2 (1.6) kg/m2, respectively. AFC was statistically significantly lower in group 2 than in group 1. It should be noted that this indicator can be considered very subjective due to the presence of the corpus luteum in one of the ovaries.
Thus, the analysis of the clinical and laboratory data of the study patients showed no statistically significant intergroup differences. At the time of enrollment into the study, patients of the two groups had similar indicators of ovarian reserve, confirmed by hormone assays.
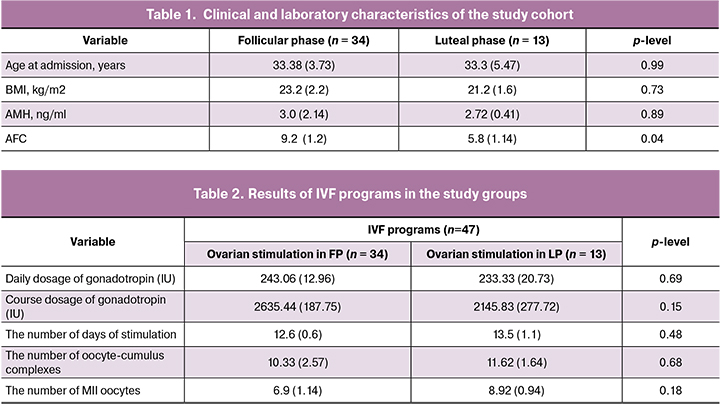
There was no statistically significant difference in the daily and total gonadotropin doses between the two groups. No difference was found in numbers of retrieved oocyte-cumulus complexes and mature oocytes, even though during a preliminary assessment, fewer follicles were visualized in the LF cycle, which was explained by the presence of a corpus luteum in one of the ovaries (Table 2).
On day 5 of culture, embryos of grade ≥3BB were cryopreserved. The number of embryos suitable for cryopreservation was comparable in both groups [4.1 (1.3) and 5.1 (0.2)].
Particular attention was given to gaining insight into steroidogenesis during ovarian stimulation and a functioning corpus luteum. At the start of stimulation, the levels of estradiol (E2) and progesterone (P) in the LF cycle were statistically significantly higher than those in the early FF (E2 was 211.62 (40.19) pmol/L and 693.47 (151.04) pmol/L, p = 0.01; P was 1.13 (0.1) nmol/L and 22.44 (9.97) nmol/L, p = 0.04), which is quite natural (Tab. 3).
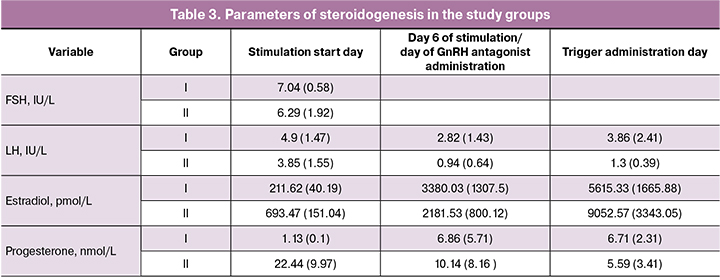
On day 6 of stimulation and on the day of triggering final oocyte maturation, the E2 levels in both groups were statistically comparable. We suggested that on day 6 of stimulation in the LF of the menstrual cycle, the corpus luteum becomes reduced. In the present study, progesterone levels on day 6 of stimulation in LF seemed to be much higher at first glance. Still, an analysis of the obtained data showed a wide scatter of values and the absence of a statistically significant difference with stimulation in FF. Corpus luteum regression was evidenced by comparable levels of progesterone in both groups at the 3rd point of steroidogenesis monitoring: 6.71 (2.31) nmol/L and 5.59 (3.41) nmol/L (p = 0.27).
At the time of administration of the GnRH antagonist, no increase in LH levels was observed in FF and LF in any stimulation cycle. However, this does not exclude the possibility of premature ovulation during stimulation without GnRH analogs. It should be noted that during stimulation in LF in 38.5% of the cycles, we prescribed a GnRH antagonist; in other cases, the stimulation was performed without adding GnRH analogs. It had been suggested that during stimulation in LF, the protective effect of endogenous progesterone prevents parasitic peaks of LH. However, this assumption is inconsistent with our hypothesis on the corpus luteum regression starting from the 6th day of stimulation. These observations indicate the need for further research [12-14].
Therefore, current research evidence on folliculogenesis suggests the possibility of new approaches for controlled ovarian stimulation in ART programs, not only from the point of view of the day of starting ovarian stimulation, but also form completely new protocols for controlled ovarian stimulation. Nevertheless, in two clinical cases of stimulation without a GnRH antagonist in the LF cycle in women with reduced ovarian reserve, we registered premature ovulation.
The study findings suggest that for the safe use of the stimulation protocol in the second phase of the cycle without the simultaneous use of GnRH antagonists, there should be steady growth of at least 7-8 follicles. Uneven growth of 2-3 follicles can lead to premature ovulation, and in these cases, the administration of GnRH antagonist is necessary.
Even though the majority of international clinical guidelines recommend aromatase inhibitors and antiestrogens for ovarian stimulation in breast cancer patients with high estrogen receptor expression, we, unlike other researchers, did not find a decrease in preovulatory values of E2 in cycles with letrozole compared with stimulation without aromatase inhibitors [15, 16]. Perhaps an insignificant reduction in the peak level of E2 is associated with a small number of patients who underwent aromatase inhibitor-based stimulation. Nevertheless, we suggest that the peak level of E2 is primarily determined by the number of preovulatory follicles, while the administration of aromatase inhibitors has a minor effect on its value.
Conclusion
- Summing up the results of the study, it can be concluded that the effectiveness of ovarian stimulation in FF and LF of the menstrual cycle is comparable.
- According to our findings, the regression of corpus luteum functional activity starts from day 6 of ovarian stimulation in the LF cycle, which is evidenced in a decrease in endogenous progesterone concentration.
- Premature ovulation was observed only in cycles with uneven growth of 2-3 follicles.
- There was no significant decrease in preovulatory estradiol levels during ovarian stimulation with aromatase inhibitors.
References
- McNatty K.P. Hormonal correlates of follicular development in the human ovary. Aust. J. Biol. Sci. 1981; 34(3): 249-68. https://dx.doi.org/10.1071/bi9810249.
- Sherman B.M., Korenman S.G. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J. Clin. Invest. 1975; 55(4): 699-706. https://dx.doi.org/10.1172/jci107979.
- Baerwald A.R., Adams G.P., Pierson R.A. A new model for ovarian follicular development during the human menstrual cycle. Fertil. Steril. 2003; 80(1): 116-22. https://dx.doi.org/10.1016/s0015-0282(03)00544-2.
- Baerwald A.R., Adams G.P., Pierson R.A. Characterization of ovarian follicular wave dynamics in women. Biol. Reprod. 2003; 69(3): 1023-31. https://dx.doi.org/10.1095/biolreprod.103.017772.
- Cakmak H., Katz A., Cedars M.I., Rosen M.P. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil. Steril. 2013; 100(6): 1673-80. https://dx.doi.org/10.1016/j.fertnstert.2013.07.1992.
- von Wolff M., Thaler C.J., Frambach T., Zeeb C., Lawrenz B., Popovici R.M., Strowitzki T. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil. Steril. 2009; 92(4): 1360-5. https://dx.doi. org/10.1016/j.fertnstert.2008.08.011.
- Kim J.H., Kim S.K., Lee H.J., Lee J.R., Jee B.C., Suh C.S., Kim SH. Efficacy of random-start controlled ovarian stimulation in cancer patients. J. Korean Med. Sci. 2015; 30(3): 290-5. https://dx.doi.org/10.3346/jkms.2015.30.3.290.
- Kuang Y., Chen Q., Hong Q., Lyu Q., Ai A., Fu Y., Shoham Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod. Biomed. Online. 2014; 29(6): 684-91. https://dx.doi.org/10.1016/j.rbmo.2014.08.009.
- Muteshi C., Child T., Ohuma E., Fatum M. Ovarian response and follow-up outcomes in women diagnosed with cancer having fertility preservation: Comparison of random start and early follicular phase stimulation - cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 230: 10-4. https://dx.doi.org/10.1016/j.ejogrb.2018.09.007.
- Jochum F., Sananès N., Teletin M., Lichtblau I., Rongières C., Pirrello O. Luteal phase stimulation, the future of fertility preservation? Retrospective cohort study of luteal phase versus follicular phase stimulation. J. Gynecol. Obstet. Hum. Reprod. 2019; 48(2): 91-4. https://dx.doi.org/10.1016/j.jogoh.2018.11.003.
- Wei L.H., Ma W.H., Tang N., Wei J.H. Luteal-phase ovarian stimulation is a feasible method for poor ovarian responders undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer treatment compared to a GnRH antagonist protocol: A retrospective study. Taiwan. J. Obstet. Gynecol. 2016; 55(1): 50-4. https://dx.doi.org/10.1016/j.tjog.2015.07.001.
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum. Reprod. 1986; 1(2): 81-7. https://dx.doi.org/10.1093/oxfordjournals.humrep.a136365.
- Kuang Y., Chen Q., Fu Y., Wang Y., Hong Q., Lyu Q. et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 2015; 104(1): 62-70. e63. https://dx.doi.org/10.1016/j.fertnstert.2015.03.022.
- Beguería R., García D., Vassena R., Rodríguez A. Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. Hum. Reprod. 2019; 34(5): 872-80. https://dx.doi.org/10.1093/humrep/dez034.
- Yasmin E., Balachandren N., Davies M.C., Jones G.L., Lane S., Mathur R. et al. Fertility preservation for medical reasons in girls and women: British fertility society policy and practice guideline. Hum. Fertil.(Camb.). 2018; 21(1): 3-26. https://dx.doi.org/10.1080/14647273.2017.1422297.
- Oktay K., Harvey B.E., Loren A.W. Fertility preservation in patients with cancer: ASCO clinical practice guideline update summary. J. Oncol. Pract. 2018; 14(6): 381-5. https://dx.doi.org/10.1200/jop.18.00160.
Received 29.01.2020
Accepted 07.02.2020
About the Authors
Tatiana A. Nazarenko, Dr.Med.Sci., Professor, Head of the Institute of Reproductive Medicine, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Tel.: +7(915)3220879. E-mail:t.nazarenko@mail.ru https://orcid.org/0000-0002-5823-1667
4, Oparina str., Moscow, 117997, Russian Federation.
Yana O. Martirosyan, Clinical Resident at the Department of Obstetrics, Gynecology, Perinatology and Reproductive Medicine of the Medical Faculty, I.M. Sechenov
First MSMU of Minzdrav of Russia (Sechenov University). Tel.: +7 (925)1249999. E-mail: marti-yana@index.ru. https://orcid.org/0000-0002-9304-4410
4, Oparina str., Moscow, 117997, Russian Federation.
Almina M. Biryukova, Ph.D., Clinical Care Supervisor at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov
NMRC for OG&P of Minzdrav of Russia. 4, Oparina str., Moscow, 117997, Russian Federation.
Lana G. Dzhanashvili, Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel.: +79629185619.
E-mail: lana.janashvili@gmail.com. https://orcid.org/0000-0002-2891-39744, Oparina str., Moscow, 117997, Russian Federation.
Tatiana Yu. Ivanets, Dr.Med.Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7 (495)438-25-66. E-mail: t_ivanets@oparina4.ru; ORCHID 0000-0002-7990-0276
4, Oparina str., Moscow, 117997, Russian Federation.
Yuliya V. Sukhova, MD, Clinical Pathologist at the Clinical Diagnostic Laboratory,V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(495)438-25-77. E-mail: j_bezzubenko@mail.ru. https://orcid.org/0000-0001-9657- 5375
4, Oparina str., Moscow, 117997, Russian Federation
For citation: Nazarenko T.A., Martirosyan Ya.O., Biryukova A.M., Dzhanashvili L.G., Ivanets T.Yu., Sukhova Yu.V. Experience in random-start ovarian stimulation for preserving reproductive material of cancer patients.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 52-58. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.52-58



