За последние несколько лет наблюдается растущий интерес к сохранению фертильности онкологических больных, что связано с улучшением выживаемости при раке, повышением внимания к качеству жизни онкологических больных в ремиссии, постоянным прогрессом в области вспомогательных репродуктивных технологий, а также с большей осведомленностью врачей и больных о возможностях сохранения репродуктивного материала.
К сожалению, растет число заболевших раком, в том числе молодых людей. По данным Всемирной организации здравоохранения и International Agency for Research on Cancer, в 2018 г. в мире зарегистрировано более 18 млн новых случаев заболевания раком. К 2030 г. ожидается увеличение числа заболевших раком до 23,6 млн человек в год [1].
Из 100 000 случаев гинекологических злокачественных новообразований, диагностируемых каждый год, 15–20% обнаруживаются у женщин до 40 лет. Раннее выявление и улучшение протоколов лечения значительно увеличили выживаемость; так, 5-летняя выживаемость при раке молочной железы (РМЖ) у женщин в репродуктивном и пубертатном периодах составляет 90% [2].
Вместе с тем в современном обществе существует явная тенденция к сдвигу репродуктивного возраста в сторону его поздних границ, что уже приводит к тому, что у значительной части женщин рак диагностируется раньше, чем рождается первый ребенок. К сожалению, вероятнее всего, такая ситуация в дальнейшем может стать привычной.
В этой связи сохранение репродуктивного материала молодых женщин для отсроченного материнства является актуальной и крайне сложной проблемой современной медицины.
За последнее десятилетие накоплен определенный международный опыт, позволяющий делать обоснованные выводы и рекомендации для клинического применения. Тем не менее вопросов, требующих решения, больше, чем однозначных ответов, что обусловливает необходимость продолжения и активизации исследований в этом направлении.
Во-первых, необходимо решить, кому показано проведение программ, направленных на сохранение репродуктивного материала. Решение этого вопроса лежит в плоскости взаимодействия онкологов и репродуктологов, совместно определяющих целесообразность сохранения репродуктивного материала. Онкологи оценивают перспективы долгосрочного выживания после лечения основного заболевания, что зависит, естественно, от стадии онкологического процесса. Важной и не вполне решенной проблемой является степень риска потери репродуктивной функции при проведении терапии онкологического заболевания. В этой связи существует необходимость выделения группы больных, у которых лечение онкологического заболевания не приводит к снижению репродуктивной функции. Это в основном те случаи, когда не проводится химио-/лучевая терапия или пациентке не грозит удаление репродуктивных органов. Примером могут служить органосохраняющие операции при раке шейки матки (РШМ) 1А стадии, раке щитовидной железы и ряде других ситуаций [3]. Но периоды лечения, реабилитации, в том числе и психологической, требуют времени. Если женщина изначально имеет снижение репродуктивных возможностей в силу возраста, низких показателей овариального резерва, наличия гинекологических заболеваний, бесплодия в анамнезе, то это время может быть фатальным для ее дальнейших репродуктивных намерений, что еще раз подчеркивает необходимость совместного определения возможностей каждой пациентки для отсроченного деторождения. Недаром американским обществом клинической онкологии (ASCO) в 2006 г. было декларировано, что все медицинские работники должны учитывать возможность бесплодия, вызванного лечением рака, и направлять пациентов, которые просят сохранить фертильность, к врачам-репродуктологам как можно скорее, до начала лечения [4]. Ключевыми словами в этом заявлении являются «как можно скорее» и «до начала лечения», так как сегодня мы не можем абсолютно точно предсказать степень гонадотоксичности проводимой терапии и влияние времени лечения на репродуктивные возможности пациентки. Следует заметить, что это утверждение относится не только к доказанно токсичным видам лечения, но и к использованию длительной терапии гормональными препаратами – антиэстрогенами, агонистами гонадотропин-рилизинг-гормона (ГнРГ), прогестинами [5].
Большие надежды специалисты возлагали на предварительную защиту яичников при проведении гонадотоксичной терапии. В качестве способа сохранения функции яичников использовали препараты агонистов ГнРГ, которые обязательно назначали больным. Оправдались ли эти надежды?
Эффект агониста ГнРГ основан на гипотезе о том, что подавление гипофиза и «инактивация» активности яичников приведет к снижению чувствительности к цитотоксическим средствам. Однако, по мнению специалистов, активация первичных и вторичных фолликулов не зависит от гонадотропинов, поэтому защитное влияние агонистов ГнРГ не может быть объяснено таким образом [6]. Следует признать, что возможное защитное действие агонистов ГнРГ и способ этого действия в настоящее время неясны.
Тем не менее большинство метаанализов с 2011 г. показалои более низкую частоту возникновения преждевременной недостаточности яичников после введения агониста ГнРГ при проведении химиотерапии. Однако не удалось достоверно доказать, связано ли это с назначением агониста ГнРГ или с другими факторами – дозами препаратов, возрастом пациенток и исходным состоянием овариального резерва. Привлекает внимание работа Demeestere et al. [7], которые опубликовали первое исследование по долгосрочному действию агонистов ГнРГ и не смогли продемонстрировать преимущества со средним периодом наблюдения 5,3 года после химиотерапии. В настоящее время все еще существуют потребность в пояснениях и необходимость дополнительных проспективных рандомизированных исследований по использованию агонистов ГнРГ.
В качестве новых возможных методик защиты яичников рассматривается применение иматиниба – конкурентного ингибитора тирозинкиназы, сфингозин-1-фосфата (S1P) – ингибитора апоптотического пути, колониестимулирующего фактора роста гранулоцитов (G-CSF), антимюллерова гормона (АМГ), AS101 и, возможно, других веществ [8]. Сегодня проводится поиск новых способов защиты яичников, и, к сожалению, нельзя гарантировать сохранение репродуктивной функции, назначая агонисты ГнРГ. Стоит ли тогда применять с этой целью столь дорогостоящее лечение, имеющее выраженные побочные эффекты?
Транспозиция яичников – метод, давно известный и широко применяемый в клинической практике [9]. По мнению специалистов, транспозицию яичников следует рекомендовать при проведении целевой лучевой терапии на область малого таза, но она не подходит для пациентов, которые подвергаются общему облучению тела. Результаты транспозиции яичников перед лучевой терапией зависят от различных факторов: хирургического метода [10], характера облучения, исходного состояния яичников. Данные метаанализа, состоящего из 24 публикаций с общим количеством 892 пациентов, демонстрируют, что показатель успешности в смысле сохраненной функции яичников достигает 90% [11]. Однако даже эти оптимистичные результаты не позволили специалистам сделать однозначные выводы вследствие неоднородности случаев и отсутствия проспективных рандомизированных исследований.
Резюмируя сказанное, вывод о приоритетности методов предварительного забора и консервации репродуктивного материала не подлежит сомнению, в чем единодушны все специалисты [12]. Значимыми при принятии конкретных решений являются ответы на вопросы кому, когда, какой материал и как консервировать. Если с точки зрения онкологии имеют бесспорное значение характер и выраженность онкологического процесса, определяющие долгосрочные перспективы выживаемости больных, то с точки зрения репродуктивной медицины важными параметрами являются возраст пациентки и состояние ее овариального резерва. Нельзя гарантировать получение яйцеклеток и полноценных эмбрионов у женщин позднего репродуктивного возраста, о чем свидетельствуют многочисленные исследования. Так, по консолидированному мнению специалистов, частота наступления беременности в программах ЭКО у женщин в возрасте 38–40 лет не превышает 20% на попытку лечения, 40–42 года – не больше 12%, после 43 лет – стремится к нулю; и это без учета неразвивающихся беременностей, частота которых у женщин позднего возраста достигает 25% [13]. В этой связи целесообразность органосохраняющего лечения и сохранения репродуктивного материала у женщин старшего возраста вызывает большие сомнения. Справедливым можно считать высказывание Y. Yoshimura [14], что целью онкофертильности является не только сохранение фертильности, но и переосмысление рождения детей, включая вариант жизни без детей. Подтверждением этого являются работы, оценивающие частоту использования пациентками ранее консервированного материала. Так, исследование Garcia-Velasco и соавт. (2018 г.) [15] включало в себя самое большое зафиксированное на данный момент количество циклов ЭКО, выполненных у пациенток с ранее криоконсервированными ооцитами. В нем отмечается, что показатели возвращения пациенток для реализации репродуктивной функции низкие, составляя 12,1% в группе женщин, консервировавших ооциты по социальным показаниям, и 7,4% – в группе пациенток с онкологическими заболеваниями. Это связано в первую очередь с изменением психологических и социальных установок пациенток, а не с рецидивом заболевания или смертью. Все сказанное подчеркивает значимость психологического консультирования больных.
В ранге реальных методов, обеспечивающих сохранение репродуктивного материала пациентки, несомненно, первое место занимает предварительная консервация эмбрионов [16]. Наличие 4 консервированных бластоцист практически обеспечивает наступление беременности и рождение ребенка [17]. Однако возникают определенные юридические и этические проблемы [18], так как консервированные эмбрионы в равной степени принадлежат обоим партнерам, что создает трудности в их использовании при разрыве отношений. Поэтому целесообразно проведение криоконсервации некоторого количества ооцитов в неоплодотворенном состоянии, даже в случае наличия стабильного партнера. Показатели рождаемости у женщин после переноса замороженных до химио-/лучевой терапии эмбрионов сопоставимы с контрольными группами, подобранными по возрасту, однако наблюдаются низкие показатели использования сохраненных эмбрионов [19].
Эффективной методикой, но уступающей консервации эмбрионов, является предварительная консервация ооцитов. Американское общество репродуктивной медицины (ASRM) декларирует, что криоконсервация ооцитов – эффективный метод онкофертильности, основанный на соответствующем консультировании пациентов [20]. По мнению некоторых специалистов, успешное размораживание ооцитов достигает 92,3%, оплодотворение происходит в 76,6% [21]. Клиническая беременность после переноса эмбрионов на размороженный ооцит составляет от 4,5 до 33,3% [22]. Существует вполне обоснованное мнение, что возможность наступления беременности зависит от количества криоконсервированных ооцитов и возраста женщины на момент их забора [23]. По результатам многоцентрового исследования женщин разного возраста (1468 женщин), которым была произведена витрификация ооцитов, наблюдается увеличение кумулятивного показателя живорождения после размораживания ооцитов в зависимости от их числа, достигнув максимального значения – 85,2%, если консервировано 15 ооцитов [24]. Используя в своих расчетах количество ооцитов, полученных до начала химиотерапии, и количество успешно оплодотворенных яйцеклеток, исследование Ferti-PROTEKT [25] рассчитало теоретическую рождаемость в зависимости от возраста женщины. В группе пациенток моложе 26 лет предполагаемая частота наступления беременности составила 40%, у пациенток от 26 до 30 лет – 35%, от 31 до 35 лет – 30%, от 36 до 40 – 25%. В исследовании Alvarez et al. [26] из 306 женщин с онкологическими заболеваниями репродуктивной системы, крови, желудочно-кишечного тракта, которым было произведено сохранение генетического материала (ооциты/эмбрионы), 22 был произведен перенос эмбрионов; при этом частота живорождения составила 22,72%. Исследование c участием A. Cobo et al. [15] включает в себя самую большую на сегодняшний день выборку пациенток: более 6000 женщин, прошедших более 8000 программ сохранения репродуктивного материала, из которых около 700 вернулись для последующей беременности и получили 162 и 25 здоровых детей, рожденных в группе женщин, сохраняющих ооциты по желанию, и в группе онкофертильности соответственно. Целью исследования было изучение возможности функционирования программ по онкофертильности в клинической практике. Также стремились определить влияние заболевания на исход ЭКО у больных раком, у которых витрифицировали ооциты, путем сравнения с результатами, достигнутыми у женщин, которым проводилась плановая криоконсервация ооцитов по социальным показаниям. Никаких различий в количестве полученных ооцитов между двумя группами не наблюдалось. Тем не менее меньшее количество ооцитов MII было витрифицировано у онкологических больных, стимулированных с помощью летрозола, по сравнению с пациентами, у которых использовали традиционную стимуляцию гонадотропинами в протоколе с антагонистами ГнРГ [27].
Представленные данные и ряд других исследований однозначно подтверждают положение о том, что для реального сохранения репродуктивной функции онкологических больных необходима предварительная консервация достаточного количества ооцитов/эмбрионов, для чего, в свою очередь, необходимо провести стимуляцию яичников до назначения гонадотоксичной терапии. Количество полученных ооцитов зависит не только от возраста пациентки, но и от овариального резерва, который значительно варьирует у каждой женщины. По данным реестра FertiPROTEKT [28], включающего в себя сведения о 4060 женщинах, которым проводилось сохранение генетического материала с 2007 по 2013 гг., среднее количество ооцитов, собранных в зависимости от возраста, составило: в группе пациенток ≤30 лет – 12,9, в группе 31–35 лет – 12,3, в группе 36–40 лет – 9,0 и у женщин в возрасте >40 лет – 5,7. Пациенткам, имеющим не гормональнозависимый рак, стимуляцию следует проводить по общим принципам, используя протокол с антагонистами ГнРГ, как более короткий и менее нагрузочный, вводя гонадотропины с начала фолликулярной фазы. Сразу же после забора ооцитов можно начать лечение онкологического заболевания. В среднем необходимо 12–14 дней для выполнения этой процедуры, и здесь крайне важны предварительные договоренности онкологов и репродуктологов, что не всегда осуществляется на практике. Пациентки обращаются в любой день менструального цикла, а онкологи определяют время до начала лечения 10–14 днями. В связи с этим репродуктологами разработаны так называемые Random start-протоколы, когда стимуляция яичников может быть начата в любой день менструального цикла [29]. Исследователи отмечают, что частота беременности после начала стимуляции в поздней фолликулярной или лютеиновой фазе так же высока, как и после начала стимуляции в ранней фолликулярной фазе, а частота встречаемости пороков развития не изменяется [30]. Согласно исследованиям, проведенным на сегодняшний день, стимуляция, начавшаяся в лютеиновую фазу, занимает на 1–2 дня дольше и требует немного более высокой дозы гонадотропинов, нежели при начале стимуляции в раннюю фолликулярную фазу [28]. В зависимости от фазы цикла, стимуляция может осуществляться следующим образом:
- начало стимуляции в раннюю и среднюю фолликулярную фазу: традиционный протокол с антагонистом ГнРГ, гонадотропины – человеческий менопаузальный гонадотропин (ЧМГ) или рекомбинантный ФСГ (рФСГ), добавление антагониста ГнРГ при диаметре доминантного фолликула ≥14 мм, введение агониста ГнРГ в качестве триггера овуляции (трипторелин 0,2 мг) при наличии не менее 3 фолликулов ≥17 мм;
- в конце фолликулярной фазы, при наличии преовуляторного фолликула вводят агонист ГнРГ 0,2 мг в качестве триггера овуляции, фолликул можно пунктировать и продолжать стимуляцию в лютеиновую фазу;
- начало стимуляции в лютеиновую фазу: гонадотропины – ЧМГ или рФСГ, агонист ГнРГ (трипторелин 0,2 мг) в качестве триггера овуляции. При такой стимуляции доза гонадотропинов несколько выше, чем при начале стимуляции в раннюю фолликулярную фазу;
- двойная стимуляция. При двойной стимуляции [31] первоначально проводится стимуляция по классическому протоколу с антагонистами ГнРГ, в качестве триггера овуляции используют агонист ГнРГ. Можно предположить, что первая стимуляция также может быть начата в любой фазе цикла (случайное начало стимуляции). Мелкие фолликулы не аспирируют. Вторая стимуляция начинается примерно через 5 дней. Время, необходимое для двойной стимуляции, составляет около 30 дней.
Было проведено несколько исследований для обнаружения корреляции между онкологическим заболеванием и предполагаемым ответом на стимуляцию яичников. В одних исследованиях сделали выводы о более низком ожидаемом ответе на стимуляцию, по сравнению с контрольными группами [27, 32], в то время как другие специалисты опровергли эти выводы [33–36]. В работе Turan et al. [37] в метаанализ было включено 10 ретроспективных когортных исследований по типу случай-контроль, сравнивавших ответ яичников на стимуляцию у женщин, больных раком, и у здоровых женщин соответствующего возраста (контрольная группа). Авторы не установили снижения количества полученных ооцитов у женщин с РМЖ. Ученые собираются проводить дальнейшие расширенные исследования, в которых будет учитываться наличие мутации гена BRCA, что предоставит более полную информацию об общем влиянии РМЖ на предполагаемую реакцию яичников на стимуляцию. И все-таки большинство специалистов считают, что разница в числе получаемых зрелых ооцитов у онкологических больных и других пациенток программ ЭКО объясняется не влиянием заболевания на ответ яичников, а более мягкими и ненагрузочными протоколами лечения, которые используют у больных раком. Прежде всего, это относится к гормональнозависимым заболеваниям, а среди них РМЖ встречается наиболее часто у молодых женщин [38]. Можем ли мы стимулировать яичники у больных РМЖ, когда и как? Несомненно, до начала химиотерапии. Наши исследования продемонстрировали тактику ведения больных РМЖ, когда четкая согласованность действий онколога и репродуктолога позволила консервировать ооциты/эмбрионы в промежуток времени между операцией и химиотерапией. При этом использовались разные протоколы стимуляции, и сразу после забора ооцитов пациентки получали первый курс химиотерапии [39]. Главная задача при проведении стимуляции яичников у этой группы больных – не допустить сверхвысоких уровней эстрогенов в преовуляторном периоде и в то же время получить достаточное количество зрелых ооцитов. Большинство исследователей рассматривают использование ингибиторов ароматазы – с одной стороны, как индуктора фолликулогенеза, с другой – как протективное антиэстрогенное средство при эстрогензависимых заболеваниях. Тем не менее работы, оценивающие показатели эффективности и безопасности, достаточно противоречивы [40]. По данным Oktay K. et al. [41], в циклах стимуляции с летрозолом антральное пространство формируется раньше, и критерии введения триггера должны быть расширены на 2–3 мм, с целью достижения тех же показателей зрелости ооцитов, что и при стандартных протоколах. Использованием стандартных критериев можно объяснить низкий ответ на стимуляцию, о котором сообщалось в некоторых исследованиях. Никаких различий в количестве полученных ооцитов между двумя группами не наблюдалось, тем не менее, меньшее количество ооцитов MII было витрифицировано у онкологических больных, стимулированных с помощью летрозола, по сравнению с пациентами, у которых использовались стандартные протоколы стимуляции [27]. Вероятно, более низкие дозы гонадотропинов, применяемые у этих пациентов, объясняют меньшее количество зрелых ооцитов. Недавнее исследование показало, что в протоколах с использованием летрозола получали сравнимое количество ооцитов, но зрелых среди них было меньше [36]. Тем не менее преовуляторные уровни эстрадиола у онкологических больных при использовании специальных протоколов были как минимум в два раза ниже, чем у пациенток, применяющих стандартную стимуляцию, что, несомненно, – главный итог в контексте рассматриваемой проблемы. С этой целью в большинстве исследований рекомендуется применять ингибиторы ароматазы, например, летрозол 5 мг (2,5 мг каждое утро и вечер с первого дня стимуляции) [42]. Очевидно, что существует необходимость в обобщении имеющегося опыта и разработке протоколов стимуляции, объединяющих показатели безопасности и эффективности лечения. Внушают оптимизм последние исследования, подтверждающие безопасность проведения стимуляции отсутствием значительного увеличения долгосрочного риска РМЖ у женщин после ЭКО [43, 44].
Получение незрелых ооцитов без стимуляции яичников и доращивание их in vitro до зрелого состояния (in vitro maturation, IVM) – направление, которое известно больше 30 лет [45]. Несмотря на первоначальный интерес, методика дозревания ооцитов не стала широко использоваться в клинической практике вследствие низкой частоты беременностей, по сравнению с традиционным ЭКО. Ученые объясняют это низким качеством дозревших ооцитов в результате асинхронного созревания ядра и интрацитоплазматических структур клетки в условиях in vitro [46]. Новый интерес к указанному направлению возник в контексте сохранения репродуктивного материала у онкологических больных. Действительно, в ряде случаев нельзя и нет времени проводить стимуляцию и забирать ооциты у пациентки. Прежде всего, это касается опухолей яичников, когда используют методики дозревания и последующей консервации ооцитов, полученных из удаленного яичника. Исследования в этом направлении интенсивно проводятся, результаты пока не очевидны. Отмечается, что после дополнительной криоконсервации ооцитов, которая необходима в качестве меры по сохранению фертильности, показатели успешности IVM снижаются [47]. Cao et al. [48] сообщили, что только 13% созревших, криоконсервированных и оплодотворенных ооцитов развивались в эмбрионы, по сравнению с 33% – без предварительной криоконсервации. Roesner и соавт. [49] сообщили только о 2 родах после 32 циклов оттаивания в 61 случае криоконсервирования in vitro созревших ооцитов. Авторы делают вывод, что процедура IVM не очень эффективна и должна проводиться только у женщин с большим количеством антральных фолликулов, и только если 2 недели, необходимые для стимуляции яичников, недоступны. Huang et al. [50] сообщают о результатах IVM у 4 женщин в возрасте 21–38 лет. У 3 женщин была выполнена частичная овариэктомия и у 1 – полная овариэктомия. В общей сложности из ткани от 4 женщин было получено 11 ооцитов, из которых только 8 ооцитов смогли созреть, в дальнейшем была проведена их витрификация. Это и другие более поздние сообщения показывают, что из удаленной ткани яичника может быть собрано очень мало ооцитов. Кроме того, если учесть низкие темпы развития бластоцисты и низкую рождаемость, очевидно, что этот метод не столь эффективен, что, по всей видимости, послужило основанием для разработки еще одной экспериментальной методики – технологии ex vivo получения и дальнейшего in vitro дозревания ооцитов. Источником незрелых ооцитов, способных к дозреванию в условиях in vitro, служит мозговой слой яичника [51]. Сегодня существует значительный интерес исследователей к этому направлению, разрабатываются способы эффективного дозревания ооцитов, полученных из мозгового слоя яичников и, что особенно важно, методов тестирования качества эмбрионов при их оплодотворении.
Нельзя не упомянуть о продолжающихся исследованиях, направленных на получение половых клеток из стволовых. В настоящее время эмбриональные стволовые клетки и индуцированные плюрипотентные стволовые клетки, обнаруженные в кортексе яичника, были дифференцированы в ооциты. Кульминацией исследований стало успешное рождение потомства у мышей. Имеются данные экспериментальных исследований об успешно завершенных программах IVM, проведенных на клетках человека [52, 53].
Криоконсервация и трансплантация овариальной ткани – направление, которое обсуждается довольно давно. Согласно обновленным клиническим рекомендациям Американской Ассоциации Клинических Онкологов, на данный момент трансплантация криоконсервированной овариальной ткани все еще остается экспериментальной методикой, но может рассматриваться в качестве метода выбора у онкологических больных препубертатного возраста. Ожидается пересмотр отношения сообщества к данному направлению и более широкое его внедрение в клиническую практику [54].
Поскольку объем криоконсервированной ткани невелик, а некоторые из фолликулов дегенерируют во время криоконсервации и трансплантации, трансплантаты активны только в течение нескольких лет; трансплантат не может долгосрочно восстановить функцию яичников и выступить в роли заместительной терапии, а также отсрочить менопаузу, на чем настаивали ряд авторов [55]. Исходя из чего, трансплантацию предварительно консервированной ткани яичника можно рассматривать только в контексте сохранения и восстановления репродуктивной функции [56]. Специалисты указывают на риски метода, связанные с повторными хирургическими вмешательствами, потенциальной возможностью метастазов в яичнике и рядом других [57]. Тем не менее это направление интенсивно разрабатывается. На данный момент предлагается большое количество экспериментальных методик для повышения эффективности криоконсервации овариальной ткани. В исследовании Man L. et al. [58] с целью уменьшения ишемии и, таким образом, увеличения частоты наступления беременности у пациенток, подвергающихся аутотрансплантации, описывается метод совместной трансплантации экзогенных эндотелиальных клеток (ExECs) с криоконсервированной тканью яичника на мышиной модели ксенотрансплантата для ускорения перфузии ткани яичника. Совместная трансплантация с ExEC увеличивала объем и качество фолликулов, а ExEC, экспрессирующие AMГ, способствуют сохранению покоящихся первичных фолликулов. Эта комбинированная стратегия может быть полезным инструментом для уменьшения ишемии и модуляции фолликулярной активации в контексте сохранения фертильности и/или лечения бесплодия в целом. Новая двухэтапная процедура трансплантации овариальной ткани совместно со стволовыми клетками, полученными из жировой ткани, направленная на повышение выживаемости фолликулов за счет усиления реваскуляризации в ксенотрансплантированной ткани яичника (ASC), была недавно предложена Manavella et al. [59]. При использовании этого подхода наблюдаются более высокие показатели выживаемости трансплантированной ткани. Кроме того, были продемонстрированы значительно более низкие показатели роста фолликулов и апоптоза.
Учитывая ургентность ситуации, многие специалисты не без основания настаивают на необходимости использования всех доступных методик. Интересной может быть обобщенная тактика, представленная в работе японских ученых [14], определяющая место тех или иных способов сохранения репродуктивного материала при ряде онкологических заболеваний (таблица).
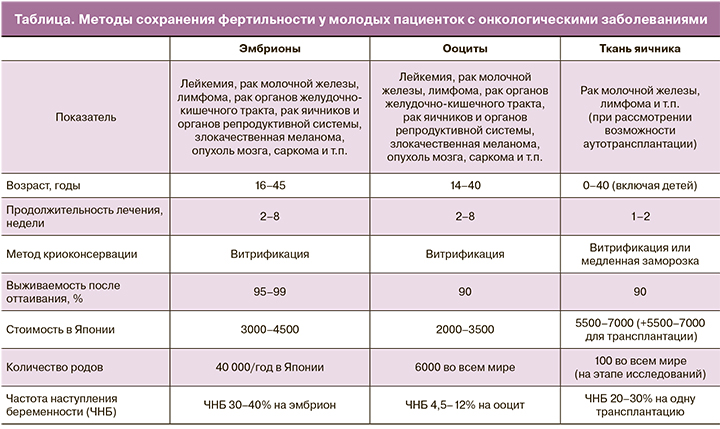
Оптимизм внушает интенсивное развитие методик, которые сейчас считаются экспериментальными, но через непродолжительное время, несомненно, станут широко использоваться в клинической практике.
Заключение
Резюмируя представленные данные, можно с уверенностью сказать, что, несмотря на все сложности, в медицине реально существует направление сохранения репродуктивного материала у онкологических больных, востребованность которого повышается. Залогом успеха являются быстрые и координированные действия онкологов и репродуктологов, а клиническими способами для сохранения репродуктивного материала сегодня могут быть консервация ооцитов и эмбрионов, полученных при стимуляции яичников.



