Вирусные инфекции, передаваемые половым путем – аногенитальная герпесвирусная инфекция и аногенитальные (венерические) бородавки – являются одними из наиболее распространенных заболеваний в структуре сексуально трансмиссивных инфекций. По оценкам Всемирной организации здравоохранения, около 417 миллионов человек в мире инфицированы вирусом простого герпеса второго типа (ВПГ-2) и около 291 миллиона женщин – вирусом папилломы человека (ВПЧ) [1]. При этом, по данным российской официальной статистики, заболеваемость вирусными инфекциями, передаваемыми половым путем (ИППП), в последние годы снижается. Так, уровень заболеваемости аногенитальными бородавками в Российской Федерации в период с 2006 по 2016 годы снизился на 38,4% (с 33,6 до 20,7 случаев на 100 тысяч населения), а аногенитальной герпетической инфекцией – на 46,8 % (с 23,7 до 12,6 случаев на 100 тысяч населения). Безусловно, данные показатели не отражают истинной заболеваемости вирусными ИППП, а являются следствием неполной регистрации новых случаев заболеваний.
Инфекционный процесс в аногенитальной области, вызванный вирусом простого герпеса, может проявляться, как клинически (типичными и атипичными формами), так и субклинически. Классическая манифестная клиническая картина заболевания, характеризующаяся полиморфизмом высыпаний и выраженными субъективными проявлениями, как правило, не вызывает затруднений в отношении диагностики, однако атипичные формы генитального герпеса (одиночная, безболезненная язва/эрозия/везикула, перианальные, мошоночные или вульварные трещины, эритема без образования пузырьков, пустулезные элементы, геморрагические везикулы, изолированная дизурия и другие) нередко требуют проведения подтверждающих лабораторных тестов [2].
Клинические проявления папилломавирусной инфекции (ПВИ) гениталий также достаточно вариабельны. В настоящее время условно выделяют экзофитные (остроконечный кондиломы, поражения в виде папул и др.) и эндофитные формы заболевания, которые могут сочетаться между собой и в большинстве наблюдений бывают обусловлены различными генотипами вирусов папилломы человека [3]. Современными исследователями установлена достоверная зависимость частоты выявления манифестных и субклинических форм ПВИ от возраста инфицированных лиц (в возрасте до 25 лет преобладают манифестные формы, в более старшем возрасте – субклинические и латентные формы инфекции) и их гендерной принадлежности (у женщин инфицирование ВПЧ высокого онкогенного риска чаще сопровождается манифестными проявлениями, а у мужчин – протекает в латентной форме). По данным авторов, существует взаимосвязь между моно- и микст-инфицированием ВПЧ высокого онкогенного риска и особенностями клинического течения заболевания: инфицирование двумя и более генотипами вирусов папилломы человека высокого онкогенного риска ассоциируется с манифестными формами и персистирующим течением ПВИ, в то время как инфицирование одним генотипом ВПЧ – с субклиническими / латентными формами инфекции и ее транзиторным течением [4].
Терапия вирусных ИППП до настоящего времени остается актуальной проблемой. В связи с рецидивирующим характером аногенитальной герпетической инфекции, обусловленным пожизненной персистенцией вируса, основными задачами лечения заболевания являются уменьшение тяжести и продолжительности клинических проявлений инфекционного процесса и максимально возможное удлинение периода ремиссии. Согласно общемировой практике, при рецидивировании заболевания не чаще 6 раз в течение года, проводится эпизодическая терапия аналогами нуклеозидов (ацикловиром, валацикловиром, фамцикловиром) в момент конкретного обострения, при более частом рецидивировании – супрессивная терапия продолжительностью не менее 6 месяцев. Поскольку любое обострение аногенитальной герпетической инфекции связано с неблагоприятными изменениями иммунного статуса, при лечении рецидивирующих форм заболевания оправдано применение иммунотерапии, включающей интерфероны или стимуляторы их эндогенного образования [2].
В настоящее время определено, что ВПГ индуцирует интерферон-независимый клеточный противовирусный ответ, который впоследствии исчезает во время начала экспрессии вирусных генов [5]. В дополнение к противовирусным эффектам, проявляющимся на уровне клетки, интерфероны (ИФН-альфа, -бета, -гамма) модулируют множество иммунорегуляторных функций, включающих взаимодействия между клетками, например, натуральных киллеров (NК-клеток) и Th-клеток с клетками, зараженными вирусом. При этом на уровне отдельных клеток именно ИФН-гамма обладает прямой активностью против ВПГ, ингибируя экспрессию его ранних генов и в целом репликацию вируса. Эта активность проявляется на различных типах клеток и может превосходить антивирусное действие других интерферонов [6].
В последние годы активно изучаются особенности иммунного ответа организма человека и при инфицировании вирусами папилломы человека. Известно, что антиген-презентирующие клетки не инфицируются ВПЧ, избегая тем самым прямого пути активации иммунитета. Ранние вирусные белки ВПЧ локализуются в основном в ядре инфицированных клеток, и у больных с индуцированной ВПЧ дисплазией регистрируется очень слабый иммунный ответ на эти белки. Поздние гены ВПЧ содержат кодоны, которые очень редко используются клетками человека. За счет этого синтез капсидных белков ВПЧ протекает медленно и в малых количествах, тормозя развитие противовирусного иммунитета. Таким способом вирусная инфекция защищается от системного воздействия иммунитета хозяина на молекулярном уровне [7].
Согласно отечественным и зарубежным рекомендациям, основным направлением в лечении папилломавирусной инфекции является деструкция клинических проявлений заболевания. Однако существующие на сегодняшний день методы терапии не всегда достаточно эффективны и не предотвращают развитие рецидивов, возникающих не только в связи с реинфицированием, но и вследствие реактивации процессов репликации вируса, выщепления его генома из хромосомы человека и перехода в активное состояние. Одним из наиболее значимых факторов риска рецидивирования ПВИ является снижение иммунной защиты организма. У лиц, инфицированных ВПЧ, отмечается снижение показателей Т-клеточного звена иммунитета, иммунорегуляторного индекса (CD4/CD8), количества клеток Лангерганса и иммунного ответа цервикальных лимфоцитов. Также возможно снижение функциональной активности NK-клеток и уровня основных сывороточных иммуноглобулинов. Настоящий дисбаланс иммунной системы обосновывает использование в комплексной терапии папилломавирусной инфекции иммунотропных препаратов.
Интерферон-гамма (иммунный интерферон) является важнейшим провоспалительным цитокином, продуцентами которого в организме человека являются естественные киллерные клетки, CD4 Тh1 клетки и CD8 цитотоксические супрессорные клетки. Рецепторы к интерферону-гамма имеют макрофаги, нейтрофилы, естественные киллерные клетки, цитотоксические Т-лимфоциты. ИНФ-гамма активирует эффекторные функции этих клеток (микробицидность, цитотоксичность, продукцию цитокинов, супероксидных и нитрооксидных радикалов), а также обладает уникальными иммунорегулирующими действиями, которые являются особенно важными во врожденном ответе хозяина на внедрение инфекционных агентов [8].
ИНФ-гамма играет ведущую роль в защите макроорганизма против вирусной инфекции, особенно в долговременном ее контроле. Помимо прямого противовирусного действия он может способствовать удалению вируса из организма и через другие механизмы. Так, ИФН-гамма и фактор некроза опухоли синергично активизируют экспрессию гена хемокина RANTES, который играет ключевую роль в хемоаттракции лейкоцитов в очаг воспаления [9]. Привлечение лимфоцитов в ткани включает взаимодействия между адгезивными молекулами на сосудистых эндотелиальных клетках и соответствующих лигандах на поверхности лимфоцитов. Синтез ИФН-гамма индуцируется митогенными или антигенными стимулами и только в некоторых клетках иммунной системы, что нередко обусловливает его дефицит в организме, инфицированном вирусами.
Препарат Ингарон (ИНФ-гамма) представляет собой рекомбинантный интерферон-гамма человека, полученный микробиологическим синтезом в рекомбинантном штамме Escherichia coli и очищенный колоночной хроматографией. Препарат блокирует репликацию вирусных ДНК и РНК, синтез вирусных белков и сборку зрелых вирусных частиц, оказывает цитотоксическое воздействие на вирусинфицированные клетки.
Целью настоящего исследования явилось изучение эффективности и безопасности применения препарата Ингарон (ИНФ-гамма) в терапии вирусных инфекций, передаваемых половым путем (генитальной герпетической инфекции и аногенитальных (венерических) бородавок).
Материалы и методы исследования
В исследование было включено 90 пациентов обоего пола в возрасте от 20 до 48 лет, из них 26 пациентов с диагнозом «Аногенитальная герпетическая инфекция» (1 группа) и 64 пациентов с диагнозом «Аногенитальные (венерические) бородавки» (2 группа). У всех пациентов в течение последнего года регистрировалось не менее 4 рецидивов заболевания. Диагнозы были подтверждены результатами исследования методом полимеразной цепной реакции (идентификацией генетического материала инфекционных этиологических агентов). У всех пациентов, включенных в исследование, были исключены другие инфекции, передаваемые половым путем.
Пациенты обеих групп были рандомизированы на подгруппы в зависимости от проводимой терапии: пациентам 1А подгруппы (n=14) проводилась комбинированная терапия препаратами ацикловир и Ингарон; пациентам 1В подгруппы (n=12) – препаратом ацикловир. Пациенты 2А подгруппы (n=17) получали терапию препаратом Ингарон в комбинации с криодеструкцией аногенитальных бородавок; пациентам 2В подгруппы (n=17) проводилась криодеструкция аногенитальных бородавок; пациенты 2С подгруппы (n=15) получали терапию препаратом Ингарон в комбинации с обработкой Солкодермом (комбинация азотной, уксусной, щавелевой, молочной кислот и тригидрата нитрата меди в виде раствора для наружного применения); пациентам 2D (n=15) подгруппы проводилась обработка патологических высыпаний в области половых органов препаратом Солкодерм.
Препарат ацикловир назначался по схеме: 200 мг 5 раз в сутки в течение 5–7 дней, препарат
Ингарон – по схеме: подкожно 500 000 МЕ 1 раз в сутки через день, на курс 5 инъекций. Раствор Солкодерм наносился однократно на аногенитальные бородавки, не затрагивая здоровых тканей 1 раз в неделю до полного исчезновения высыпаний.
Наблюдение за пациентами с целью оценки эффективности и безопасности терапии продолжалось в течение 200 дней (для пациентов 1 группы) и 100 дней (для пациентов 2 группы).
 Результаты исследования
Результаты исследования
Эффективность применения препарата Ингарон в терапии аногенитальной герпетической инфекции
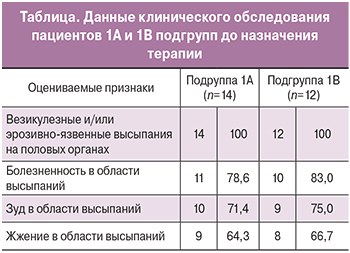
На момент обращения за медицинской помощью длительность заболевания у пациентов 1 группы составляла от одного года до трех лет. В течение последнего года у всех пациентов регистрировалось 4 и более рецидивов генитального герпеса. На момент включения в исследование все пациенты имели клинические проявления аногенитальной герпетической инфекции, при этом обе группы являлись сопоставимыми по степени их выраженности (табл.). Диагноз был подтвержден методом полимеразной цепной реакции: у всех пациентов был выявлен ВПГ 2 типа.
Оценка субъективных и объективных клинических проявлений аногенитальной герпетической инфекции проводилась: на 1 визите – при первом посещении (1 день исследования), на 2 визите – по окончании активной терапии (10 день исследования), на 3 визите – через 40 дней и на 4 визите – через 200 дней после завершения терапии.
Согласно полученным данным, на 2 визите (10 день исследования) клинические проявления заболевания в виде герпетических высыпаний сохранялись у 3 (21,4%) пациентов 1А подгруппы и 3 (25,0%) пациентов 1В подгруппы, из них болезненность в области высыпаний отмечалась– у 1 (7,1%) и 1 (8,3%) пациента соответственно, зуд и жжение в области высыпаний – у 2 (14,3%) пациентов и 1 (8,3%) пациента соответственно. К 3 визиту (через 40 дней с момента обращения) рецидивы аногенитальной герпетической инфекции были зарегистрированы у 1 (7,1%) пациента 1А подгруппы и у 5 (41,7%) пациентов 1В группы. К 4 визиту рецидивы аногенитальной герпетической инфекции повторно отмечались у 3 (25,0%) пациентов 1В подгруппы (рис.1).

Таким образом, за время наблюдения отсутствие рецидивов аногенитальной герпетической инфекции было зарегистрировано у 92,9% пациентов, проводивших комбинированную терапию препаратами ацикловир и Ингарон, и у 58,3% пациентов, проводивших терапию ацикловиром. Обращало на себя внимание, что у 4 (33,3%) пациентов 1В подгруппы на 3 и 4 визите исследования даже при отсутствии клинических проявлений генитального герпеса в отделяемом из половых органов был выявлен вирус простого герпеса. У пациентов 1А подгруппы на протяжении всего периода наблюдения случаев бессимптомного вирусовыделения зарегистрировано не было.
Эффективность применения препарата Ингарон в терапии аногенитальных (венерических) бородавок
При обследовании пациенток была выявлена следующая локализация высыпаний на половых органах: в области вульвы – у 6 (35,3%) пациенток 2А подгруппы, 8 (47,0%) пациенток 2В подгруппы, 8 (53,3%) пациенток 2С подгрупп и 7 (46,7%) пациенток 2D подгруппы; в области задней спайки – у 4 (23,5%), 5 (29,4%), 3 (20%) и 2 (13,3%) пациенток соответственно; на внутренней поверхности больших половых губ – у 3 (17,6%) пациенток 2А подгруппы, у 1 (5,9%) пациентки из 2В подгруппы, у 1 (6,75) пациентки из 2С и 2D подгрупп; в области наружного отверстия уретры – у 1 (5,9%) пациентки из 2А подгруппы и 1 (6,7%) пациентки из 2D подгруппы. Сочетанное поражение слизистой оболочки малых половых губ и задней спайки наблюдалось у 3 (17,6%) пациенток 2А подгруппы и 2 (13,3%) пациенток из 2С и 2D подгрупп; слизистой оболочки малых половых губ и наружной поверхности больших половых губ – у 3 (17,6%) пациенток из 2В подгруппы, 3 (20,0%) пациенток из 2С подгруппы и у 2 (13,3%) пациенток 2D подгруппы.
У всех пациенток, включенных в исследование, диагноз «Аногенитальные (венерические) бородавки» был подтвержден идентификацией ДНК вируса папилломы человека: генотипы ВПЧ 16, 18 были обнаружены у 5 (29,4%) пациенток 2А подгруппы, 6 (35,3%) пациенток 2В подгруппы, 2 (13,3%) пациенток 2С и 2D подгрупп; генотипы ВПЧ 31, 33 – у 2 (11,8%) пациенток 2А подгруппы, 4 (23,5%) из 2В подгруппы, 4 (26,6%) пациенток из 2С подгруппы и у 5 (33,3%) пациенток 2D подгруппы (из них, у 1 пациентки 2С и 2D подгрупп в ассоциации с 35 генотипом ВПЧ); генотипы 6 и 11 – у 9 (52,0%), 7 (41,1%) и 5 (33,3%) пациенток соответственно. У 1 (5,9%) пациентки подгруппы А был идентифицированы ВПЧ 16,18 в ассоциации с ВПЧ 31,33; у 1 (6,7%) пациентки 2С подгруппы ВПЧ 18, 6 в ассоциации с 31, 39, 58 типами; у 1 пациентки 2D подгруппы были выявлены 35, 51, 52, 26 типы ВПЧ.
 После проведенного курса терапии эффективность лечения оценивалась через 10 (Визит 2), 40 (Визит 3) и 100 дней (Визит 4) исследования. Рецидивы аногенитальных бородавок были зарегистрированы у 2 пациенток, проводивших комбинированную терапию: у 1 (5,9%) пациентки 2А подгруппы и у 1 (6,7%) пациентки 2С подгруппы. Во 2В подгруппе на Визитах 3 и 4 были зарегистрированы рецидивы аногенитальных бородавок у 2 (11,8%) и 5 (29,4%) пациенток, а во в 2D подгруппе – у 3 (20%) и 6 (40%) пациенток соответственно. Основной локализацией высыпаний являлась слизистая оболочка малых половых губ (рис. 2).
После проведенного курса терапии эффективность лечения оценивалась через 10 (Визит 2), 40 (Визит 3) и 100 дней (Визит 4) исследования. Рецидивы аногенитальных бородавок были зарегистрированы у 2 пациенток, проводивших комбинированную терапию: у 1 (5,9%) пациентки 2А подгруппы и у 1 (6,7%) пациентки 2С подгруппы. Во 2В подгруппе на Визитах 3 и 4 были зарегистрированы рецидивы аногенитальных бородавок у 2 (11,8%) и 5 (29,4%) пациенток, а во в 2D подгруппе – у 3 (20%) и 6 (40%) пациенток соответственно. Основной локализацией высыпаний являлась слизистая оболочка малых половых губ (рис. 2).
Таким образом, эффективность комбинированной терапии (Ингарон + криодеструкция; Ингарон + Солкодерм) составляла 94,1% и 93,4% соответственно и значительно превышала таковую при использовании только криодеструкции (58,8%) и Солкодерма (40,0%).
При оценке показателей общего анализа крови и биохимических показателей клинически значимых изменений после проведенного лечения не было выявлено ни у одного из пациентов, включенных в исследование.
При проведении терапии препаратом Ингарон у 2 пациенток (1 пациентки 1 группы и 1 пациентки 2 группы) через 12 часов после первой инъекции были зарегистрированы гриппоподобные симптомы (слабость, незначительный озноб), которые разрешились самостоятельно через 24 часа.
Обсуждение
Согласно данным современных исследователей, интерферон-гамма демонстрирует высокую эффективность в терапии вирусных заболеваний. Так, в работе Гайнановой Е.Г. и соавт. (2010) препарат Ингарон в дозе 500 000 МЕ (5 инъекций) применялся в комбинированной терапии у пациентов с варицелла-зостер герпесвирусной инфекцией в сравнении с ацикловиром, применявшимся в дозе 400 мг 5 раз в сутки в течение 10 дней. В группе пациентов, получавших комбинированную терапию, наблюдалось сокращение продолжительности интоксикационного синдрома (повышения температуры, миалгий и артралгий), уменьшалась длительность существования кожных проявлений, регионарного лимфаденита и болевого синдрома. На клиническую эффективность препарата Ингарон указывали в своих работах Ф.И. Ершов, А.Н. Наровлянский и соавторы (2009). В сравнительном анализе различных иммунологических методов в терапии и профилактике рецидивов герпетической инфекции, данные авторы установили, что применение Ингарона позволяет сократить частоту рецидивов и снизить интенсивность их клинических проявлений, а также нормализует параметры интерферонового статуса, повышает количество и функциональную активность клеточного и гуморального иммунитета.
По результатам настоящего исследования также была установлена эффективность гамма-интерферона в терапии герпесвирусной инфекции: отсутствие рецидивов аногенитальной герпетической инфекции было зарегистрировано у 92,9% пациентов, проводивших комбинированную терапию препаратами ацикловир и Ингарон, и у 58,3% пациентов, проводивших терапию ацикловиром.
В комплексной терапии аногенитальных (венерических) бородавок была также установлена достоверно более высокая эффективность комбинированной терапии в сравнении с изолированной деструкцией высыпаний: эффективность комбинированной терапии (Ингарон + криодеструкция; Ингарон + Солкодерм) составляла 94,1% и 93,4% соответственно и превышала таковую при использовании только криодеструкции (58,8%) и Солкодерма (40,0%). Рецидивы заболевания регистрировались у 6,2% больных после комбинированной терапии, у 41,2% пациентов, которым проводилась криодеструкция, и у 60,0% больных, получавших терапию Солкодермом. Корреляционной зависимости частоты рецидивов заболевания от типа вируса папилломы человека выявлено не было.
Серьезных нежелательных явлений терапии, а также клинически значимых изменений в общеклиническом и биохимическом анализах крови зарегистрировано не было, что свидетельствует о высоком профиле переносимости и безопасности терапии препаратом Ингарон.
Заключение
Применение препарата Ингарон в комбинированной терапии вирусных инфекций, передаваемых половым путем, демонстрирует высокий профиль клинической эффективности и безопасности и способствует снижению частоты рецидивов заболеваний у пациентов.



