Experience in using ERA-test for diagnosis of endometrial receptivity in frozen embryo transfers in patients with endometriosis
Objective: To evaluate the effectiveness of personalized embryo transfer taking into account the results of the ERA-test in cryopreservation protocols in patients with extragenital endometriosis.Maeva N.Kh., Khabarov S.V.
Materials and methods: The prospective study included 20 patients with extragenital endometriosis which was confirmed histologically and 42 patients with tubal and/or male factors of infertility. The time of optimal endometrial receptivity was determined using the ERA-test. We carried out a comparative assessment of the rate of displaced implantation windows in two groups and the rate of clinical pregnancy after personalized embryo transfer taking into account the results of the ERA-test.
Results: The rate of displacement of the implantation window in the patients with a verified diagnosis of extragenital endometriosis was 55.0% compared to 28.5% in the control group (p=0.01). After the personalized embryo transfer with the analysis of individual variations in the timing of the implantation window, the rate of clinical pregnancy in patients with endometriosis was 63.2% and it did not significantly differ from this indicator in the control group (69.4%).
Conclusion: Due to the high rate of displaced implantation windows in patients with extragenital endometriosis, the ERA-test may be recommended as a standard practice for preparing for embryo transfer in a cryopreservation protocol. According to the results of the ERA-test, personalized embryo transfer contributes to an increase in the pregnancy rate in this group of patients.
Keywords
According to numerous studies, endometriosis affects about 5–10% of reproductive-aged women [1, 2] and up to 35–50% of patients with infertility [3–6].
Fertility decline in patients with endometriosis can result from such factors as the formation of adhesions, damage to ovarian tissue caused by developing cysts which can lead to a decrease in the ovarian reserve, a negative impact on the quality of oocytes and embryos, as well as the impaired endometrial receptivity.
According to the report of the Russian Association of Human Reproduction, the infertile patients treated with assisted reproductive technologies (ART) in the Russian Federation in 2019 had an average pregnancy rate of 43.0% after cryopreservation [7]. The high rate of endometriosis in infertile patients makes the clinicians search for new approaches to improve the treatment of such patients.
Most of the reproductive losses are associated with disorders in the peri-implantation period. Since implantation is a unique biological phenomenon, it is a critical stage of the reproductive process. There is only a specific period of time during which implantation is possible and this period is called implantation window. Impaired endometrial receptivity during this period or desynchronized endometrium-embryo interaction is believed to cause most ineffective attempts in assisted reproduction, particularly in vitro fertilization (IVF) [8–10].
There have been some attempts to assess the endometrial receptivity using echographic, morphological, biochemical and/or immunological research methods [11–14]. However, none of the proposed tests proved to be diagnostically and prognostically significant.
As a rule, controlled preparation of the endometrium with exogenous and endogenous progesterone can be achieved after 120 hours.
The development of the endometrial receptive state depends on a change in gene expression under the influence of progesterone, which leads to a complex of morphological and functional changes characteristic of the implantation window. However, the eutopic endometrium in patients with endometriosis can have a normal histological structure and no morphological changes; its functional properties are different from the endometrium of healthy women. Endometriosis-associated infertility can be caused by the relative local hyperproduction of estrogens and progesterone resistance as a result of epigenetic regulation of gene activity [15]. The absence of a regular reaction of the eutopic endometrium to progesterone can be explained by a decrease in the number of progesterone B-receptors in the endometrium and the lack of dynamics of the ratio of isoforms of A- and B- progesterone receptor. According to a number of studies, such dynamics is characteristic of the normal menstrual cycle in healthy women [15, 16]. It was noted that women with a higher content of progesterone receptors have a spontaneous pregnancy after surgical treatment for endometriosis, unlike patients with a low number of receptors [17]. In addition to changes in the endometrium receptor apparatus, the activity of signaling molecules, namely ones of the IHH–COUPTFII-WNT4 pathway, alters in patients with endometriosis. This pathway normally regulates the proliferation and decidualization of the endometrium, as well as the PGR-GATA2-SOX17 signaling pathway. Transcription factor GATA-2 is a modulator of the activity of progesterone-sensitive genes in 97.0% of cases; if the factor is absent, transcription activation does not occur despite the normal level of progesterone. Some studies have shown that GATA-2 activity in patients with endometriosis is suppressed in the eutopic endometrium as a result of methylation; endometrioid heterotopias actively express GATA-6, which makes the eutopic endometrium progesterone-resistant. There is also a connection between a decrease in the FOXO1 concentration and impaired implantation mechanism due to a change in the expression level of genes involved in the activation of cell invasion, molecular transport, apoptosis, β-catenin (CTNNB1) signaling pathway, and an increase in progesterone receptor signaling [18].
The complex of the above-mentioned mechanisms can lead to dysregulation of endometrial receptivity at the genetic, epigenetic and molecular levels.
The active study of endometrium genomics resulted in the creation of a method for assessing gene expression in the endometrium at the time of the opening of the implantation window, ERA-test (Endometrial Receptivity Analysis), Igenomix laboratory, Valencia, Spain. During the test, RNA is isolated from a fragment of endometrial tissue obtained by pipelle biopsy and then placed in a special solution. Further, the expression profile of 248 genes in the endometrium, including the genes of adhesion molecules, growth factors, integrins, signaling molecules, etc., is evaluated using the Next Generation Sequencing (NGS) method. The obtained expression profile can be referred to the receptive, pre-receptive or post-receptive stage.
Due to the introduction of the ERA-test into clinical practice in 2009, it is known that up to 30.0% of women in the population have an earlier or later implantation window [19], which may reduce the effectiveness of ART treatment. Embryo transfer in the IVF program at a time when the endometrium is in the pre-receptive or post-receptive stage is one of the reasons for repeated implantation failures. Thus, J. Tan et al. showed in their work that among patients with the history of unsuccessful transfers of euploid blastocysts, 22.5% of patients had a displaced implantation window according to the results of the ERA-test. A special analysis algorithm makes it possible to predict the time of reaching the receptive state of the endometrium with an accuracy of up to six hours and to carry out embryo transfer to the patient on the basis of the individual opening time of the implantation window, that is, personalized embryo transfer. According to the study results, embryo transfer led to an increase in the implantation rate up to 73.7% and ongoing pregnancy up to 54.2% when the ERA-test was used in comparison with the transfer of frozen thawed embryos carried out using the standard protocol (54.2 and 41.7%, respectively) [20]. Therefore, it is recommended for patients with repeated implantation failures to conduct a test to assess the susceptibility of the endometrium in preparation for embryo transfer in order to increase the effectiveness of infertility treatment.
Few studies have been devoted to the study of endometrial receptivity in patients with extragenital endometriosis and adenomyosis. According to the available data, the rate of repeated implantation failures was 66.6% in patients with adenomyosis compared with the rate of 34.9% in the control group (p<0.001, CI: 15.5–47.9%). The results of the ERA-test in the group of patients with adenomyosis showed that the rate of a displaced implantation window was significantly higher (47.2%) than in the control group (21.6%) (p<0.001, CI: 8.7–42.5%). Pregnancy rate after personalized embryo transfer in the group of patients with adenomyosis was 62.5% and this fact indicates that the displaced implantation window is the cause of unsuccessful embryo transfer in patients with adenomyosis [21].
Some studies showed that there was a significant decrease in the number of pinopodia and the expression of leukemia-inhibiting factor (LIF) in the endometrium during the supposed implantation window, as well as an increase in aromatase activity and a decrease in the concentration of HOXA10 proteins and αVβ3 integrin in patients with extragenital endometriosis [12, 22]. However, the study of Da Broi et al. (2017) [23] did not confirm the presence of statistically significant differences in the severity of pinopodia in the endometrium of patients with and without endometriosis. The results of the studies are ambiguous, so the possibility of using biochemical and morphological markers to determine the endometrial receptivity is still discussed.
The pilot study of J.A. Garcia-Velasco et al. [24] which was devoted to the endometrial transcriptomics in patients with endometriosis did not reveal a significant change in gene activity during the implantation window in such patients in comparison with the control group; however, due to the small number of cases included in the study, it is necessary to continue working in this area to improve the effectiveness of the treatment of endometriosis-associated infertility.
The aim of this study is to evaluate the effectiveness of personalized embryo transfer taking into account the results of the ERA-test in cryopreservation protocols in patients with extragenital endometriosis.
Materials and methods
The prospective study involving 62 patients with infertility was conducted in the period from January 2020 to August 2021 at the VitroKlinik clinic of VITROMED LLC. The study was approved by the Ethical Committee of Tula State University.
During the examination, all patients underwent diagnostic laparoscopy. On the basis of its results, the patients were divided into two groups: the study group included 20 patients with histologically verified diagnosis of II–III stage extragenital endometriosis; the control group included 42 patients with tubal and/or male factors of infertility and the absence of foci of extragentital endometriosis which was confirmed by laparoscopy.
There were the following exclusion criteria: obesity (BMI ≥30 kg/m2), endometrial hypoplasia, intrauterine synechiae, endometrial hyperplasia and polyps, uterine malformations, uterine fibroids with submucous nodules and/or with nodules larger than 4 cm of any localization.
Estrogen/gestagen replacement therapy (HRT) was administered to 12 patients with extragenital endometriosis and 18 patients from the control group. It was given in the biopsy sampling cycle to perform the ERA-test and in the subsequent cycle of frozen thawed embryo transfer using the following scheme: 17β-estradiol 3.0 mg/day was applied as a transdermal gel from the 3rd day of the menstrual cycle for 8 days, followed by the addition of micronized progesterone at a dose of 600 mg/day intravaginally until the thickness of the endometrium reached 7 mm or more. If the endometrial thickness was not sufficient, estradiol was administered for the next 4–6 days.
The ERA-test and transfer of frozen thawed embryos was performed in another way in 8 patients with extragenital endometriosis and 24 patients of the control group: ovulation trigger (recombinant human chorionic gonadotropin, Ovitrel 250 mcg) was administered subcutaneously when the dominant follicle reached a diameter of 17 mm.
Ultrasound monitoring of folliculogenesis and endometrial condition was performed on the Canon Aplio i800 system using a rectovaginal transducer with a central frequency of 11 MHz on the 2nd and 3rd, 8th and 9th days of the menstrual cycle, as well as on the day before the administration of the ovulation trigger or the start of progesterone use.
If pathological changes in the endometrium were visualized including its thickness less than 7 mm on the day of the trigger/progesterone administration, the patient was excluded from the study.
On the day of the administration of the ovulation trigger or vaginal progesterone, all patients were examined for the level of progesterone in the blood serum. If the level of progesterone was less than 1.0 ng/ml, endometrium preparation continued and biopsy for the ERA-test was obtained.
Endometrial biopsy was performed 120 hours after the first administration of progesterone preparations or 156 hours after the administration of the ovulation trigger using the standard pipelle probe. The material was also sent for histological examination to exclude organic changes in the endometrium.
As a primary outcome, the rate of displaced personal implantation window was studied taking into account 120 hours of progesterone effect on the endometrium in each group of patients. The secondary outcome was the assessment of the pregnancy rate after the personalized embryo transfer using the results of the ERA-test.
The onset of pregnancy was assessed after the results of a blood test for human beta-chorionic gonadotropin. Clinical pregnancy was confirmed by the presence of a gestational sac in the uterine cavity during the ultrasound examination on the 21st day after embryo transfer.
Statistical analysis
Statistical processing of the obtained data was carried out using a standard software package for the statistical analysis (Microsoft Excel 2010). To assess the intergroup differences, a two-sided t-test was used. The differences between the compared groups were regarded as statistically significant at a significance level of p≤0.05.
Results
The analysis of the patient’s history and clinical parameters in both groups showed that there were no significant differences between them (Table).
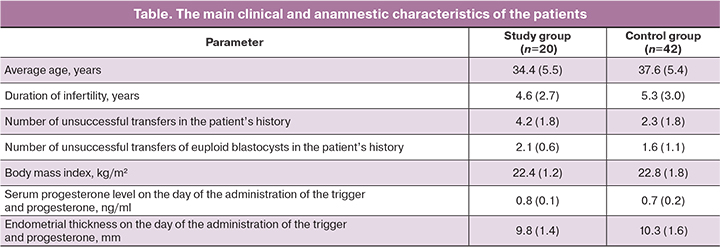
The average age of the patients was 34.4 years (from 31 to 44 years) in the group of patients with extragenital endometriosis and 37.6 years (from 30 to 51 years) in the control group. In the control group, 20/42 (47.6%) patients had tuboperitoneal infertility, 16/42 (38.1%) patients had a male factor, 6/42 (19.5%) patients had a combination of tubal and male factors.
The average duration of infertility in patients of both groups was also comparable: 4.6 (2.7) years in the study group and 5.3 (3.0) years in the control group. The shorter duration of infertility in patients with extragenital endometriosis can probably be explained by more active medical tactics in the management of such patients (a relatively early administration of ART treatment).
Previous embryo transfers were not performed in 10/42 (23.8%) patients from the control group and one unsuccessful embryo transfer was noted in the history of 8/42 (19.0%) patients. On average, the control group patients had a history of 2.3 embryo transfers without implantation, which is significantly less than in patients with endometriosis (p=0.03). All patients from the study group had a history of at least 3 and on average 4.2 unsuccessful embryo transfers. These results confirm the available literature data on the increase in the rate of repeated implantation failures in the group of patients with endometriosis-associated infertility [25].
Previously, all patients had embryos transferred at the blastocyst stage; the quality of embryos was not lower than 2BB. The transfer of euploid embryos after the results of PGT-A was noted in the history of 8/20 (40.0%) patients from the study group (the average number of transferred blastocysts was 1.6) and 18/42 (42.9%) patients from the control group (on average 2.1 unsuccessful cryotransfers). There was no statistically significant difference in the number of attempts and the number of transferred embryos after PGT-A.
The level of progesterone on the day of the administration of the ovulation trigger or vaginal progesterone was less than 1 ng/ml in all patients. The thickness of the endometrium on the day of the administration of the ovulation trigger or vaginal progesterone was at least 8.5 mm and on average it was 9.8 mm in patients with extragenital endometriosis, which is comparable to the thickness of the endometrium in patients of the control group - 10.3 mm. In addition to the thickness of the endometrium, its structure was also evaluated. In all patients included in the study, the endometrium before the administration of an ovulation trigger or progesterone was three-line and homogeneous.
According to the results of our study, the rate of cases of displaced implantation window was 11/20 (55.0%) compared with 11/42 (28.5%) in the control group (p=0.01) among the patients with a verified diagnosis of extragenital endometriosis (Figure). Patients with endometriosis who received HRT in the biopsy cycle had the implantation window which was displaced in 6/12 (50.0%) patients; among the patients who underwent biopsy in a modified menstrual cycle, implantation window was displaced in 6/8 (75.0%) patients. Implantation window was displaced in 8/18 (44.4%) patients of the control group who received HRT and in 3/24 (12.5%) patients during biopsy in the natural cycle.
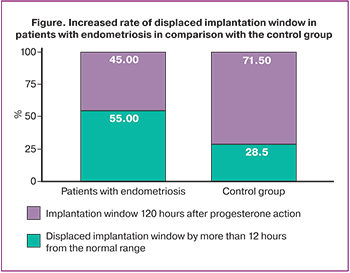
The cases when the time of achieving maximal endometrial receptivity differed by 12 or more hours out of 120 hours of progesterone action (or 156 hours after the administration of the ovulation trigger in the modified menstrual cycle) were taken as displaced implantation window. Such displacement is significant in clinical practice, since it can lead to a decrease in the effectiveness of treatment in patients who missed the time of beginning of progesterone therapy or in case of changes in the timetable of the clinic and the operating room.
The changes in the time of the formation of the endometrial receptivity in the group of patients with extragenital endometriosis in comparison with the normal time (120 hours of progesterone effect) ranged from 12 to 19 hours. Biopsy did not reveal the time of formation of the receptivity in 2/20 (10.0%) patients with a post-receptive expression profile; repeated biopsy was performed, which confirmed the displaced implantation window for more than a day. The obtained results are consistent with the available data and show that the endometrial receptivity in patients with endometriosis is not fundamentally impaired [26], however, a more frequent displacement in time of maximal endometrial receptivity in these patients can certainly be of clinical interest.
Selective embryo transfer was performed in 19/20 (95.0%) patients with extragenital endometriosis and 36/42 (85.7%) patients from the control group within 2–6 months after receiving the results of the ERA-test; the transfer was performed at the blastocyst stage with embryo quality of 2BB and higher. In our study we took into account the result of the first transfer, but not the cumulative pregnancy rate. Clinical pregnancy occurred in 12/19 (63.2%) patients with extragenital endometriosis and in 25/36 (69.4%) patients of the control group, this difference was not statistically significant (p=0.65). Another patient from the study group had a biochemical pregnancy. The transfer of euploid blastocysts after the results of preimplantation genetic testing was carried out in 4/19 (21.1%) patients with extragenital endometriosis and 9/36 (25.0%) patients of the control group. All patients had a clinical pregnancy after testing. Thus, despite the available literature data on a decrease in the frequency of pregnancy in ART programs in patients with extragenital endometriosis [27], personalized embryo transfer based on the results of the ERA test allows such patients to achieve an increase in pregnancy rate which is comparable to that in the control group and does not differ statistically significantly from it.
Conclusion
The multifactorial negative impact of endometriosis on female fertility requires a comprehensive approach to patients with the disease at all stages of management: starting from the development of tactics, determining the extent and time of surgical intervention, time of administration of ART treatment, ending with the selection of the optimal scheme of stimulation and preparation for embryo transfer.
Due to the high rate of displaced implantation windows in patients with extragenital endometriosis, the ERA-test may be recommended as a standard practice for preparing for embryo transfer in a cryopreservation protocol. According to the results of the ERA-test, personalized embryo transfer contributes to an increase in the pregnancy rate in this group of patients.
References
- Адамян Л.В., ред. Клинические рекомендации Министерства здравоохранения Российской Федерации «Эндометриоз». М.; 2020. 32с. [Adamyan L.V., ed. Clinical recommendations of the Ministry of Health of the Russian Federation «Endometriosis». Moscow, 2020. 32 p. (in Russian)].
- National Guideline Alliance (UK). Endometriosis: diagnosis and management. London: National Institute for Health and Care Excellence (UK); 2017 Sep. 25p.
- Радзинский В.Е., ред. Бесплодный брак: версии и контраверсии. М.: ГЭОТАР-Медиа; 2018. 404с. [Radzinskiy V.E., ed. Infertile marriage: versions and contraversions. M.: GEOTAR-Media; 2018. 404 p. (in Russian)].
- Шмидт А.А., Замятнин С.А., Гончар И.С., Коровин А.Е., Городнюк И.О., Коцур А.В. Эпидемиология бесплодия в России и за рубежом. Клиническая патофизиология. 2019; 1: 9-12. [Shmidt A.A., Zamyatnin S.A., Gonchar I.S., Korovin A.E., Gorodnyuk I.O., Kocur A.V. Epidemiology of infertility in Russia and abroad. Clinical pathophysiology. 2019; 1: 9-12. (in Russian)].
- Evans M.B., Decherney A.H. Fertility and endometriosis. Clin. Obstet. Gynecol. 2017; 60: 497-502. https://dx.doi.org/10.1097/GRF.0000000000000295.
- Хабаров С.В. Гинекологическая заболеваемость сельских жительниц по данным медицинских осмотров. Вестник новых медицинских технологий. 1997; 4(1): 63-5. [Khabarov S.V. Gynecological morbidity of rural women according to medical examinations. Vestnik novyh medicinskih tekhnologij/Journal of New Medical Technologies. 1997; 4(1): 63-5. (in Russian)].
- Российская Ассоциация Репродукции Человека. Регистр ВРТ. Отчет за 2019 год. 2021. 55с. [Russian Association of Human Reproduction. ART register. Report for 2019. 2021. 55 p. (in Russian)].
- Sebastian-Leon P., Garrido N., Remohi J., Pellicer A., Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum. Reprod. 2018; 33(4): 626-35. https://dx.doi.org/10.1093/humrep/dey023.
- Куликова Г.В., Абдурахманова Н.Ф., Файзуллина Н.М., Асатурова А.В., Щеголев А.И., Зиганшина М.М., Долгушина Н.В. Рецептивность «тонкого» эндометрия у пациенток в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2019; 10: 100-7. https://dx.doi.org/10.18565/aig.2019.10.100-107. [Kulikova G.V., Abdurakhmanova N.F., Fayzullina N.M., Asaturova A.V., Shchegolev A.I., Ziganshina M.M., Dolgushina N.V. Receptivity of the thin endometrium in patients in assisted reproductive technology programs. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 10: 100-7. https://dx.doi.org/10.18565/aig.2019.10.100-107. (in Russian)].
- Хабаров С.В., Горская О.С., Русанова Г.П. Опыт применения ультразвуковой кавитации у пациенток с хроническим эндометритом перед проведением программы ЭКО. Акушерство и гинекология. 2020; 11: 197-204. https://dx.doi.org/10.18565/aig.2020.11.197-204. [Khabarov S.V., Gorskaya O.S., Rusanova G.P. Experience with ultrasonic cavitation in patients with chronic endometritis before IVF. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 11: 157-64. https://dx.doi.org/10.18565/aig.2020.11.197-204. (in Russian)].
- Rosario G.X., Stewart C.L. The Multifaceted Actions of Leukaemia Inhibitory Factor in Mediating Uterine Receptivity and Embryo Implantation. Am. J. Reprod. Immunol. 2016; 75: 246-55. https://dx.doi.org/10.1111/aji.12474.
- Парамонова Н.Б., Коган Е.А., Колотовкина А.В., Бурменская О.В. Морфологические и молекулярно-биологические признаки нарушения рецептивности эндометрия при бесплодии женщин, страдающих наружным генитальным эндометриозом. Архив патологии. 2018; 3: 11-8. https://dx.doi.org/10.17116/patol201880311-18 [Paramonova N.B., Kogan E.A., Kolotovkina A.V., Burmenskaya O.V. Morphological and molecular biological signs of endometrial receptivity disorders in infertility of women suffering from external genital endometriosis. Arkhiv Patologii/Archive of Pathology. 2018; 3: 11-8. https://dx.doi.org/10.17116/patol201880311-18 (in Russian)].
- Довгань А.А., Зиганшина М.М., Долгушина Н.В. Современные тренды в поиске маркеров рецептивности эндометрия – от отдельных параметров к комплексному подходу. Акушерство и гинекология. 2020; 11: 26-32. https://dx.doi.org/10.18565/aig.2020.11.26-32. [Dovgan A.A., Ziganshina M.M., Dolgushina N.V. Current trends in the search for endometrial receptivity markers: from individual parameters to a comprehensive approach. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 11: 26-32. https://dx.doi.org/10.18565/aig.2020.11.26-32. (in Russian)].
- Гохберг Я.А., Тимофеева А.В., Калинина Е.А. Молекулярные маркеры рецептивности эндометрия в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2021; 11: 56-62. https://dx.doi.org/10.18565/aig.2021.11.56-62. [Gokhberg Ya.A., Timofeeva A.V., Kalinina E.A. Molecular markers of endometrial receptivity in assisted reproductive technology programs. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 11: 56-62. https://dx.doi.org/10.18565/aig.2021.11.56-62. (in Russian)].
- Marquardt R.M., Kim T.H., Shin J.H., Jeong J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019; 20(15): 3822. https://dx.doi.org/10.3390/ijms20153822.
- Wölfler M.M., Küppers M., Rath W., Buck V.U., Meinhold-Heerlein I., Classen-Linke I. Altered expression of progesterone receptor isoforms A and B in human eutopic endometrium in endometriosis patients. Ann. Anat. 2016; 206: 1-6. https://dx.doi.org/10.1016/j.aanat.2016.03.004.
- Moberg C., Bourlev V., Ilyasova N., Olovsson M. Levels of oestrogen receptor, progesterone receptor and alphaB-crystallin in eutopic endometrium in relation to pregnancy in women with endometriosis. Hum. Fertil. (Camb.) 2015; 18: 30-7. https://dx.doi.org/10.3109/14647273.2014.922705.
- Vasquez Y.M., Wang X., Wetendorf M., Franco H.L., Mo Q., Wang T. et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018; 14(11): e1007787. https://dx.doi.org/10.1371/journal.pgen.1007787.
- Ruiz-Alonso M., Valbuena D., Gomez C., Cuzzi J., Simon C. Endometrial Receptivity Analysis (ERA): data versus opinions. Hum. Reprod. Open. 2021 Apr 14; 2021(2): hoab011. https://dx.doi.org/10.1093/hropen/ hoab011.
- Tan J., Kan A., Hitkari J., Taylor B., Tallon N., Warraich G. et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J. Assist. Reprod. Genet. 2018; 35(4): 683-92. https://dx.doi.org/10.1007/s10815-017-1112-2.
- Mahajan N., Kaur S., Alonso M.R. Window of implantation is significantly displaced in patients with adenomyosis with previous implantation failure as determined by endometrial receptivity assay. J. Hum. Reprod. Sci. 2018; 11(4): 353-8. https://dx.doi.org/10.4103/jhrs.JHRS_52_18.
- Schmitz C.R., Oehninger S., Genro V.K., Chandra N., Lattanzio F., Yu L., Cunha-Filho J.S. Alterations in expression of endometrial milk fat globule-EGF factor 8 (MFG-E8) and leukemia inhibitory factor (LIF) in patients with infertility and endometriosis. JBRA Assist. Reprod. 2017; 21(4): 313-20. https://dx.doi.org/10.5935/1518-0557.20170056.
- Da Broi M.G., Rocha C.V. Jr, Carvalho F.M., Martins W.P., Ferriani R.A., Navarro P.A. Ultrastructural evaluation of eutopic endometrium of infertile women with and without endometriosis during the window of implantation: A pilot study. Reprod. Sci. 2017; 24(10): 1469-75 https://dx.doi.org/10.1177/1933719117691142.
- Garcia-Velasco J.A., Fassbender A., Ruiz-Alonso M., Blesa D., D'Hooghe T., Simon C. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reprod. Biomed. Online. 2015; 31(5): 647-54. https://dx.doi.org/10.1016/j.rbmo.2015.07.014.
- Senapati S., Sammel M.D., Morse C., Barnhart K.T. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 2016; 106(1): 164-71.e1. https://dx.doi.org/10.1016/j.fertnstert.2016.03.037.
- Miravet-Valenciano J., Ruiz-Alonso M., Gómez E., Garcia-Velasco J.A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: it is not affected, and let me show you why. Fertil. Steril. 2017; 108(1): 28-31. https://dx.doi.org/10.1016/j.fertnstert.2017.06.002.
- Zhong C., Gao L., Shu L., Hou Z., Cai L., Huang J. et al. Analysis of IVF/ICSI outcomes in endometriosis patients with recurrent implantation failure: influence on cumulative live birth rate. Front. Endocrinol. (Lausanne). 2021 Jul 30; 12: 640288. https://dx.doi.org/10.3389/fendo.2021.640288.
Received 26.11.2021
Accepted 06.12.2021
About the Authors
Nora Kh. Maeva, obstetrician-gynecologist, VITROMED Co., +7(910)086-36-90, dr.maeva@yandex.ru, https://orcid.org/0000-0001-9517-0181,125424, Russia, Moscow, Volokolamskiy proezd str., 1A.
Sergey V. Khabarov, Dr. Med. Sci., Merited Doctor of the Russian Federation, Professor at the Department of Obstetrics and Gynecology, Medical Institute of Tula State University; Professor of the Department of Clinical Laboratory Diagnostics and Pathological Anatomy, Academy of Postgraduate Education of the FMBA of Russia, +7(916)726-51-26, s.v.habarov@mail.ru, https://orcid.org/0000-0002-1736-9408, 300028, Russia, Tula, Boldin str., 128.
Authors’ contributions: Maeva N.Kh., Khabarov S.V. – development of the concept and design of the study, statistical data processing, writing the text of the manuscript; Maeva N.Kh. – collecting and processing of the material, review of publications on the subject of the article, analysis of the obtained data; Khabarov S.V. – editing the text of the manuscript.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without any financial support.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Maeva N.Kh., Khabarov S.V.: Experience in using ERA-test for diagnosis of endometrial receptivity in frozen embryo transfers in patients with endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2021; 12: 128-134 (in Russian)
https://dx.doi.org/10.18565/aig.2021.12.128-134



