The composition and stability of the vaginal microbiota in pregnant women during dynamic observation
Aim. To investigate the characteristics and stability of the vaginal microbiota in pregnant women during dynamic observation.Khodzhaeva Z.S., Priputnevich T.V., Murav'eva V.V., Guseinova G.E., Gorina K.A., Mishina N.D.
Materials and methods. The study comprised 26 pregnant women aged 18 to 40 years with all trimesters of pregnancy. Inclusion criteria were primigravida status, age 18 to 40 years, and singleton pregnancy. Exclusion criteria were congenital fetal malformations and somatic comorbidities. All patients were investigated for vaginal microbiota composition.
Results. The vaginal microbial communities were categorized into five Community State Types (CST) including CSTI (L.crispatus), CSTII (L.gasseri), CSTIII (L.iners), CSTIV (L. jensenii), and CSTV, which was dominated by conditional pathogens. The study findings showed that CST II constituted the largest proportion (23.1–42.3%), followed by CSTI (26.9–38.5%) and CSTIV (23.1%), while CSTIII type comprised 7.7%. In the overwhelming majority (96.2%) of pregnant women, the composition of vaginal microbiota remained relatively constant, and only in one (3.8%) pregnant woman significant changes were observed. An analysis of pregnancy outcomes showed that 20 (76.9%) women achieved a full term delivery and gave birth to healthy infants, and 6 (23.1%) women had premature rupture of membranes at 27–35 weeks’ gestation. Women with premature rupture of membranes had markedly dysbiotic vaginal microflora.
Conclusion. A comprehensive microbiological evaluation provided new information on the composition of the vaginal microbiota in pregnant women and changes in the microbial community that may affect the pregnancy outcome. Timely correction of microecological disorders of the vaginal biotope will enable prevention of pregnancy and postpartum infectious complications. Evaluation of vaginal microbiota during pregnancy may have prognostic, diagnostic, and therapeutic value.
Keywords
According to the literature, there is a relationship between the state of the vaginal microbiota and pregnancy complications [1-3]. Vaginal microflora is represented by a variety of species that are classified into obligate and transient. Colonization resistance of the vaginal biotope is maintained by vaginal lactobacilli, which are resident components of the vaginal microflora. The transient component of microflora is represented by conditional pathogenic microorganisms (CPM), which, due to competitive restraint, colonize the vagina in low titers. Under the influence of endogenous and exogenous effects on the woman’s body, the harmonious equilibrium between lactobacilli and CPM may be disturbed, which leads to an imbalance in the microbial community and the development of infections [4, 5]. The current literature reported some evidence suggesting the role of dysbiotic disorders of the vaginal microbiocenosis in the development of infectious complications of pregnancy, childbirth and the postpartum period [6], their possible association with some types of lactobacilli and the instability of the vaginal microbiota. The previous studies suggested that the composition of the vaginal microflora varies depending on the gestational age, and is mostly dominated by the following species: L. crispatus, L. jensenii, L. gasseri and L. iners [7].
This study aimed to investigate the characteristic features and stability of the vaginal microbiota in pregnant women during dynamic observation.
Material and methods
The study examined 26 women aged 18-40 during the three trimesters of pregnancy. Inclusion criteria were primigravida status, age 18 to 40 years, and singleton pregnancy. Exclusion criteria were congenital fetal malformations and somatic comorbidities.
The comprehensive microbiological analysis included an assessment of vaginal microbiocenosis by microscopy of a Gram-stained vaginal smear and microbiological culture according to the medical technology «Integral assessment of the state of the vaginal microbiota. Diagnosis of opportunistic vaginitis». Vaginal discharge was cultured in a standard culture medium. Facultative anaerobic microorganisms were isolated using Colombian agar, mannitol-salt agar (Сonda, Spain), Endo medium, and Saburo agar (FGUN “GITPM & B,” Obolensk, Russia). Lactobacilli were cultured in a Laktobakagar medium (FGUN “GITSPM and B,” Obolensk, Russia); cultivation of strong anaerobes was performed in pre-reduced Schaedler agar (Сonda, Spain) with the necessary additives. The culture was incubated in a CO2 incubator (Jouan, France). Strong anaerobes and lactobacilli were cultured in an anaerobic chamber (Jouan, France) in an atmosphere of 10% H2, 10% CO2, and 80% N2. Identification of microorganisms was performed by the time-of-flight mass spectrometry (MALDI-TOF MS) using an AutoFlex III with MALDI BioTyper software (BrukerDaltoniks, Germany), which previously helped identify 101 strains of Lactobacillus to the species level, of which 76.3% and 23.7% had high (lgscore - 1.9 ≥ 2) and low (lgscore <1.9) titers, respectively.
Statistical analysis Significance of differences between trimesters in detection rates of microbiota states (normocenosis and microcenosis disorders) was analyzed with the χ2. Dynamics of microbiota species representation was analyzed, taking into account the frequency of changes in the composition by trimesters. The proportion of species that did not vary in representation from trimester to trimester was estimated for each patient. Fisher’s exact test for each patient was used to estimate the dependence of the number of species that differently change their degree of colonization during the transition between trimesters: the frequency of an increase and decrease in the concentration of species (increase or decrease: binary variable) were compared during the transition between trimesters I – II (group I) and the transition between trimesters II – III (group II). This calculation was carried out for 26 times for each pregnant woman participating in the study.
For all types of analysis, p <0.05 was considered statistically significant. Statistical analysis was performed using the Statistica 7.0 statistical software and Python 2.7 libraries and packages for statistical analysis Sklearn, Skipy, Pandas, and Matplotlib.
The data were reported in the following formats:
Categorical variables were reported as counts (n) and percentages (%) and presented as n (%):
- detection rates of various microbiota states in pregnant women during dynamic observation by trimesters;
- detection rates of vaginal microbial communities with varying degrees of colonization by trimesters;
- detection rates of pregnant women with a relatively stable or variable microbiota composition between trimesters.
Quantitative variables that were not normally distributed were reported as a median (Me) and quartiles Q1 and Q3 and presented as Me (Q1; Q3):
- the number of species with a constant degree of colonization by trimesters; distribution by the patient.
Results
The findings of the comprehensive microbiological evaluation of the state of vaginal microbiocenosis in the study participants during dynamic observation are presented in Table 1; statistical significance of detection rates of the main microbiota states by trimesters was analyzed with the χ2 (Table 2).
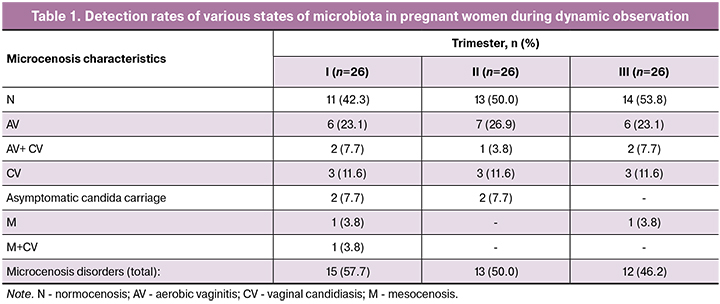
Comparison of detection rates of normocenosis (H) and microcenosis disorders (χ2 test) showed no statistically significant differences between the three trimesters (I and II trimesters, p = 0.31, II and III trimesters, p = 0.07, I and III trimesters, p = 0.69). However, the most frequent states and trends were identified. As shown in table 1, the most common states of the microbiota were normocenosis (N) with detection rates of 42.3%, 50.0%, and 53.8% in I – III trimesters of pregnancy, respectively. Vaginal microecology disorders were observed in 57.7%; 50.0%, and 46.2% of cases, respectively. It was established that an increase in gestational age was accompanied by a reduction in detection rates of abnormalities from 57.7% to 46.2%, which seems to be a favorable prognostic factor for successful pregnancy prolongation. The second most common state of microcenosis was aerobic (non-specific) vaginitis (AV). In dynamics, its rates remained almost unchanged (30.8% of cases in each trimester) and was observed as a single agent infection (23.1%; 26.9%, and 23.1% of pregnant women, respectively) or in combination with vulvovaginal candidiasis (CV) (7%, 3.8%, and 7.7% respectively). Yeast fungi of the genus Candida were most frequently isolated from patients in the first trimester (30.8%) and somewhat less frequently in the second and third trimesters (23.1% and 23.1%, respectively). Candida fungi are known to be capable of vegetating in the vagina in association with many types of CPM under both acidic and alkaline pH. In our study, they were detected in patients the classic variant of CV, with the combination of CV with AB, mesocenosis (M), as well as in asymptomatic candida carriers. There was not a single case of bacterial vaginosis (BV). In three cases, M was found (intermediate between N and BV): in two patients in the first trimester (7.7%) and one in the third trimester (3.8%).

The figure summarizes the species composition of the vaginal microbiota by trimesters.
As shown in table 3, there were five predominant CSTs (lactobacilli concentration 105 and more CFU/ml). Four of these CSTs were dominated by a different Lactobacillus species: CSTI – L. crispatus; CSTII - L.gasseri; CSTIII - L.iners; CSTIV - L.jensenii, whereas CSTV was dominated by conditional pathogens.
The spectrum of Lactobacillus species was represented by 11 species. Leading were 4 types including L. crispatus (26.9-38.5%), L.gasseri (23.1-42.3%), L. jensenii (23.1%) and L.iners (7.7 %). The percentage of other species (L.fermentum, L..salivarius, L.Galinarum, L.oris, L.delbrueckii, L.paracasei, L.rhamnosus) ranged from 1.0 to 3.8%.
The change in total species representation between I–II and II–III trimesters was estimated by the number of species in which the degree of colonization increased, decreased, and remained unchanged. A total of 26 species of microorganisms were examined. Between I and II trimesters, the degree of colonization in 22 (22; 23) species did not change; between II and III trimesters no changes were found in 22 (21; 23.75) species. These data indicate the stability of the species composition of microbial communities during pregnancy. Analysis of detection rates of species that differently change their degree of colonization during the transition between trimesters I – II or II – III was performed using Fisher’s exact test, which showed a high level of significance (p = 0.02) only in one (3,8%) of 26 patients (for details of the Fisher’s exact test, see Supplementary materials, Table 4).
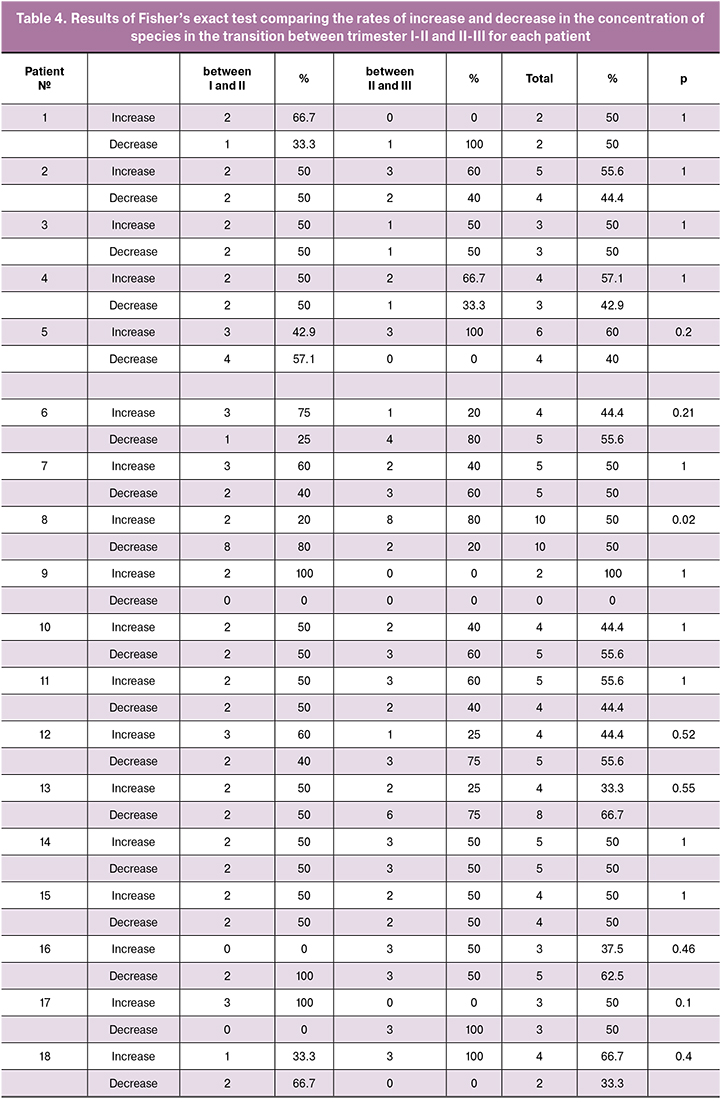

Therefore, the vaginal microbial community composition remained stable throughout pregnancy for the majority (25/26;96.2%) of the study participants, even taking into account local variations in the changes of species representation between trimesters, and only for one of 26 (3.8%) pregnant women it has undergone significant changes throughout the pregnancy.
In pregnant women with prelabor rupture of membranes (PRM), dysbiotic disorders were observed at 27–35 weeks’ gestation. Culture of cervical canal discharge taken from 5 patients after the rupture of membranes identified CPM. Of these, in one case, Enterococcus faecalis was isolated as a monoculture, while the others had associations of E. coli + E.faecalis, S.hominis + C. albicans, e. coli + S.lugdunensis + S.anginosus + G.vaginalis and S.epidermidis + S.agalactiae + E.faecalis + S.lugdunensis, respectively. None of the patients was found to have CPM. Lactobacilli were also isolated in 4 of 6 women, including L. Gasseri in 3 and L. iners in 1 of them, respectively. In 2 women without lactobacillus growth in the previous cultures, L. gasseri species were also identified. It is also noteworthy that in 5 of 6 patients, at least one episode of AV was noted during the observation. These findings may serve as a base for future studies investigating the relationship between vaginal microbiota and adverse pregnancy outcomes.
Discussion
The study of vaginal microbiota showed the absence of statistically significant differences in the rates of N and microecological disorders (total) during all periods of observation. At the same time, there was a tendency for N cases to increase by the end of gestation from 42.3% in the first trimester to 53.8% in the third trimester. Among microbiota abnormalities in all trimesters, AV was most common, being noted in every third woman in each trimester. In patients with N, etiologically significant CPM Enterococcus faecalis (18.2–42.9%) and Escherichia coli (18.2-21.4%) were isolated, always in low titers. In patients with AV, the most commonly cultured were Enterococcus faecalis, with a pronounced tendency to increase from I to III trimester from 16.7 to 87.5%, Escherichia coli (25.0–37.5%) and 3 species of Streptococcus: S.anginosus, S.agalactiae and S.oralis (only S.agalactiae was found in trimester III - 25.0%). In patients with AV, CPMs were more often cultured in moderate or high titers. These CPMs were highly present in patients with both N and AV. Our findings on the detection rates of facultative anaerobic CPMs are consistent with other studies [8]. Yeast fungi of the genus Candida were most frequently isolated from patients in the first trimester (30.8%) and somewhat less frequently in the second and third trimesters (23.1% and 23.1%, respectively) with different variants of microbiocenosis. As for BV and M, associated with strong anaerobes and Gardnerella vaginalis, in our study, these variants of microbiocenosis were rare. R. Romero et al. [9] reported that the abundance of species associated with BV is rarely seen in women with term delivery. In patients with a pathological course of pregnancy, these disorders are more common [10].
An analysis of pregnancy outcomes showed that 20 (76.9%) women delivered at term and gave birth to healthy infants, and 6 (23.1%) women had PRM at 27–35 weeks’ gestation and low birth weight deliveries. Pregnant women with PRM had markedly dysbiotic vaginal microbiota composition. The spectrum of CPM species in patients with PRM was associated with the most common types of CPM colonizing the vaginal biotope during pregnancy, both in N and the pathological state of the microbiota, especially in AV (E.faecalis, E.coli). PRM is membrane rupture before labor and before 37 weeks of gestation. One of the causes of PRM is bacterial enzymes that induce the degradation of collagen, the main component of the extracellular matrix of the fetal membranes. B. Hammond et al. [11] showed that vaginal CPMs have a role in PRM. The abnormal vaginal microbiota is a major risk factor for PRM. For a deeper understanding of the role of bacteria in the pathogenesis of PRM, the authors investigated the ability of G. vaginalis and other BV-associated microorganisms to directly or indirectly affect the integrity of fetal membranes by co-cultivating G. vaginalis under anaerobic conditions with mammalian ED27 cells (cell line-derived trophoblast). The results showed that G. vaginalis does not produce bacterial collagenases that directly destroy fetal membranes, but can disrupt the tissue integrity by releasing substances affecting the metalloproteinase expression of in the fetal membrane tissues. Contact of the pathogen with fetal membranes is most often associated with ascending infection. One of the mechanisms known to contribute to the infiltration of CPMs, including those associated with BV, into the upper genitalia is their ability to break down protective barriers. The female reproductive tract is rich in glycoproteins, proteins containing sialic acids, which provide high viscosity for secretions of the genital tract mucous membranes, protecting them from mechanical damage and bacterial exposure. It has been established that many BV-associated microorganisms and other CPMs, including E. coli, are capable of producing enzymes (sialidase, mucinase) that destroy glycoproteins of the protective mucous layer. Mucin destruction is a necessary step in the colonization of the cervical epithelium and the infiltration of the vaginal CPMs into the uterus [12]. Also, there is evidence that the destruction of vaginal mucins leads to a decrease in the production of specific immunoglobulin A [13], thereby increasing the risk of ascending infection in pregnant women. Cauci S. et al. [14] found that elevated sialidase levels in vaginal discharge at 12-week’ gestation correlated with early spontaneous preterm labor and late miscarriage. Our observations on CPM species composition in patients with PRM are consistent with the studies of other authors. Li-nan Zeng et al. [15] investigated the spectrum of pathogens isolated from Chinese women experiencing PRM over a 32-year period and reported that the species spectrum of 1706 CPM isolates included Staphylococcus spp., Enterococcus spp., Streptococcus spp., Escherichia coli, Enterobacter spp., Gardnerella vaginalis, strong anaerobes, Candida fungi, Staphylococcus spp., and Escherichia coli.
The role of Lactobacillus species composition in healthy women and those with vaginal infections has been subject to considerable discussion in the literature. Lactobacilli are known to constitute the resident part of the microflora of the female urogenital tract in the reproductive years. Colonizing the parietal zone of the vaginal mucosa, they form microbial-tissue complexes, including lactobacilli microcolonies and their metabolites, epithelial cells, mucus (mucin), stroma cells (fibroblasts), leukocytes, lymphocytes, neuro-endocrine cells [16]. Our data showed that the Lactobacillus species spectrum was represented by 11 species. Leading were 4 types: L.crispatus (26.9–38.5%), L.gasseri (23.1–42.3%), L.jensenii (23.1%), and L.iners (7.7 %) both in terms of isolation rates and the degree of vaginal discharge colonization (105 or more CFU/ml). The proportions of other species (L.fermentum, L.salivarius, L.Galinarum, L.oris, L.delbrueckii, L.paracasei, L.rhamnosus) ranged from 1.0% to 3.8%. In women with N, during the whole pregnancy (I – III trimesters) three types dominated: L.crispatus (54.5%, 46.2%, and 28.6%, respectively); L.jensenii (45.4%, 30.8%, and 35, 7%, respectively) and L.gasseri (18.2%, 46.2%, and 42.9%, respectively).
By the end of pregnancy, there was a slight decrease in isolation rates of L.crispatus to 28.6% and an increase in L.gasseri from 18.2% to 42.9%. The species dominating among lactobacilli in patients with AV, unlike N, were L.gasseri with isolation rate of 50% in the first trimester and 37.5% in II and III. The isolation rate of L. crispatus and L. jensenii that dominated in patients with N, were significantly lower in patients with AV, accounting for 25.0–12.5% in I – III trimesters for L. crispatus and 25.0% (only in the II trimester) for L.jensenii. In the overwhelming majority (25/26; 96.2%) of pregnant women, the composition of vaginal microbiota remained relatively stable, and only in one (3.8%) pregnant woman significant changes were observed. Similar findings were reported by R.Romero et al. [10]. The most productive species in maintaining the stability of normal flora were L. crispatus and L. jenseniii; on the contrary, dysbiotic disorders (AV, M) occurred during colonization of the vagina by L.gasseri. It should be noted that all cases of PRM were associated with the presence of lactobacillus species L.gasseri along with CPM. Melkumyan A.R. et al. [17], Medzhidova N. [18] showed the dominant role of L. crispatus in healthy women. H.Verstraelen et al. [19] noted that the maximal stability of the vaginal ecosystem was determined by the species L.crispatus, and the minimal by L.gasseri and L. iners. They showed that vaginal colonization by the lactobacillus species L.gasseri / L. iners results in a 10-fold higher risk of dysbiotic disorders. The unique data obtained by Ghartey J.P. et al. [20] suggest that the highest inhibition of Escherichia coli proliferation in pregnant women occurs in patients with vaginal colonization by L. crispatus. Besides, the authors demonstrated the inhibitory effect of the L. Crispatus supernatant on the growth of Escherichia coli in vitro.
Two cases of PRM in our study were caused by CPM associations that included high titer of Escherichia coli. In both cases, L.gasseri species were present in the associations. Moreover, all six cases of PRM were associated with L.gasseri /L iners. L. Petricevic et al. [21] analyzed the isolation rate of L.iners in patients with preterm labor and women with term delivery and found that this type of lactobacilli was found in 85% of cases of preterm birth and only in 16% of women with a favorable pregnancy outcome.
Conclusion
This study reveals new insights into the composition of the vaginal microbiota in pregnant women and changes in the microbial community that may affect the pregnancy outcome. Timely correction of microecological disorders of the vaginal biotope will enable prevention of pregnancy and postpartum infectious complications. Evaluation of vaginal microbiota during pregnancy may have prognostic, diagnostic, and therapeutic value.
References
- Ryckman K.K., Simhan H.N., Krohn M.A., Williams S.M. Predicting risk of bacterial vaginosis: the role of race, smoking and corticotropin-releasing hormone-related genes. Mol. Hum. Reprod. 2009; 15(2): 131-7.
- Sobel J.D. Bacterial vaginosis. Annu. Rev. Med. 2000; 51: 349-56.
- Brotman R.M. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J. Clin. Invest. 2011; 121(12): 4610-7.
- Gajer P., Brotman R., Bai G., Sakamoto J., Schuette U., Zhong X. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012; 4(132): 132-52.
- Yildirim S., Yeoman C., Janga S., Thomas S., Ho M., Leigh S. et al. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J. 2014; 8(12): 2431-44.
- Macintyre D., Chandiramani M., Lee Y., Kindinger L., Smith A., Angelopoulos N. et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015; 5: 8988.
- Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Bieda J. et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014; 2: 18.
- Савченко Т.Н., Макаров О.В., Алешкин В.А., Афанасьев С.С., Воропаева Е.А., Мельников А.Н., Стрыгина В.А. Нарушение микробиоценоза влагалища как фактор невынашивания беременности. Лечение и профилактика. 2012; 1: 30-43. [Savchenko Т.N., Makarov O.V., Aleshkin V.A., Afanasiev S.S., Voropaeva E.A., Melnikov A.N., Strigina V.A. Violation of microbiocenosis of the vagina as a factor in pregnancy loss. J. Treatment and Prevention 2012; 1 (2): 30-43. (in Russin)]
- Romero R., Hassan S.S., Gajer P., Tarca A.L., Fadrosh D.W., Nikita L. et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014; 2(1): 4.
- Карапетян Т.Э. Акушерская и перинатальная патология при вагинальной инфекции и дисбиозе: дисс. … д-ра мед. наук. М.; 2014.[Karapetyan T.E. Obstetric and perinatal pathology in vaginal infection and dysbiosis Thesis for the degree of doctor of medical Sciences. M.; 2014.(in Russian)]
- Hammond B., Enriquez F., Wilstermann A. Role of bacteria in the premature rupture of fetal membranes. Science Division and McGregor Summer Research Poster Fair, October 19, 2012.
- Roberton A.M., Wiggins R., Horner P.J., Greenwood R., Crowley T., Fernandes A.et al. A novel bacterial mucinase glycosulfatase is associated with bacterial vaginosis. J. Clin. Microbiol. 2005; 43(11): 5504-8.
- Cauci S., Driussi S., Monte R., Lanzafame P., Pitzus E., Quadrifoglio F. Immunoglobin A response against Gardnerella vaginalis hemolysin and sialidase activity in bacterial vaginosis. Am. J. Obstet. Gynecol. 1998; 178(3): 511-5. Given this, along with the importance of glycoproteins to the integrity, growth and function of vaginal epithelial cells, disruption of the mucin-layer may predispose hosts to further complications, such as those currently associated with BV, including an increased risk of infection with HIV and other sexually transmitted diseases [8] .
- Cauci S., Culhane J.F. High sialidase levels increase preterm birth risk among women who are bacterial vaginosis-positive in early gestation. Am. J. Obstet. Gynecol. 2011; 204(2): 142. e1-9.
- Zeng L.N., Zhang L.L., Shi J., Gu L.L., Grogan W., Gargano M.M., Chen C. The primary microbial pathogens associated with premature rupture of the membranes in China: A systematic review. Taiwan. J. Obstet. Gynecol. 2014; 53(4): 443-51.
- Ljungh A., Wadstrom T. Lactobacillus. Molecular biology from genomics to probiotics. Causter Academic Press, UK; 2009. 205 с.
- Мелкумян А.Р., Припутневич Т.В., Анкирская А.С., Трофимов Д.Ю., Муравьева В.В., Муллабаева С.М., Завьялова М.Г. Видовой состав лактобактерий при различном состоянии микробиоты влагалища у беременных. Клиническая микробиология и антимикробная химиотерапия. 2013; 15(1): 73-9. [Melkumyan A.R., Priputnevich T.V., Ankirskaya A.S., Trofimov D.Yu., Muravyeva V.V., Mullabaeva S.M., Zavyalova M.G. Species composition of lactobacilli in different States of vaginal microbiota in pregnant women. Wedge Microbiology antimicrob khimioter 2013.15; 1: 73-79. (in Russian)]
- Меджидова М.К. Течение послеродового периода в зависимости от особенностей микробиоценоза и локального иммунитета влагалища у беременных перед родами: дисс. … канд. мед. наук. М.; 2012. [Medzhidova M.K. The course of the postpartum period according to characteristics of the microbiocenosis and local immunity of the vagina in pregnant women before childbirth Dissertation on competition of a scientific degree of the candidate of medical Sciences, Moscow 2012. (In Russian)]
- Verstraelen H., Vilchez-Vargas R., Desimpel F., Jauregui R., Vankeirsbilck N., Weyers S. et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ. 2016; 4: e1602.
- Ghartey J.P., Smith B.C., Chen Z., Buckley N., Lo Y., Ratner A.J. et al. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One. 2014; 9(5): e96659.
- Petricevic L., Domig K.J., Nierscher F.J., Sandhofer M.J., Fidesser M., Krondorfer I. et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014; 4: 5136.
Received 07.12.2018
Accepted 22.02.2019
About the Authors
Khodzhaeva Zulfiya S., Dr.Med.Sci., Professor, Head of #1 Obstetric Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Address: 117997, Russia, Moscow, Oparina str. 4, Phone: +7 (916) 407-75-67. E-mail: zkhodjaeva@mail.ru
Priputnevich Tatiana V., Dr.Bio.Sci., Head of Laboratory of Microbiology, Department of Microbiology, Clinical Pharmacology and Epidemiology, V.I. Kulakov NMRC
for OG&P of Minzdrav of Russia. Address: 117997, Russia, Moscow, Oparina str. 4. Phone: +7 (495) 438-25-77. E-mail:t_priputnevich@oparina4.ru
Murav'eva Vera V., Ph.D. (Bio.Sci), Senior Researcher at the Laboratory of Microbiology, Department of Microbiology, Clinical Pharmacology and Epidemiology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Address: 117997, Russia, Moscow, Oparina str. 4. Phone:+7 (495) 438-25-77. E-mail: v_muravieva@oparina4.ru
Guseinova Gulnara E., Ph.D. Student at the #1 Obstetric Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Oparina str. 4, Phone: +7 (967) 153-18-81E-mail: g_guseynova@oparina4.ru
Gorina Ksenia A., Junior Researcher at the #1 Obstetric Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Oparina str. 4, Phone:+7(495)438-06-74 E-mail:kseniagorina@gmail.com
Mishina Nataliia D., Junior Researcher at the Laboratory of Genomics Data Analysis, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 119997, Russian Federation, Moscow, Oparina street, 4. Phone: +7(985)217-29-89. E-mail: mis7ha@gmail.com
For citations: Khodzhaeva Z.S., Priputnevich T.V., Murav'eva V.V., Guseinova G.E., Gorina K.A., Mishina N.D. The composition and stability of the vaginal microbiota in pregnant women during dynamic observation. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (7):30-8 (in Russian).
http://dx.doi.org/10.18565/aig.2019.7/30-38



