Informative value of oxidative stress markers in predicting outcomes of infertility treatment using ART methods
Objective: To assess the level of reactive oxygen species (ROS) and total antioxidant capacity (TAC) in the peripheral blood and follicular fluid in women with various types of infertility and to identify the informative value of these markers of oxidative stress in predicting the outcomes of assisted reproductive technologies (ART) up to 12 weeks gestation.Agadzhanyan D.S., Smolnikova V.Yu., Krasnyi A.M., Lobanova N.N., Shchipitsyna V.S., Sadekova A.A., Makarova N.P., Kalinina E.A.
Materials and methods: A total of 75 patients received ART treatment for infertility. The level of ROS was measured in the peripheral blood and follicular fluid of all patients by means of the FORM 3000 device using the FORT kit. The level of TAC was determined in the peripheral blood and follicular fluid of 45 patients by means of the FORM 3000 device using the FORD kit.
Results: The likelihood of implantation was higher in women with higher levels of ROS in the follicular fluid (p=0.024). A positive correlation between the level of ROS in the follicular fluid and body mass index (BMI) was identified. There was a correlation between the level of ROS in the peripheral blood and the follicular fluid of patients, therefore, it was possible to determine the level of oxidative stress only in one biological fluid. The level of ROS in the follicular fluid of patients who had implantation and ongoing pregnancy up to 12 weeks was higher compared to the patients who had implantation failure and pregnancy loss up to 12 weeks, respectively. The level of TAC in these fluids was higher in the groups of patients who had pregnancy loss up to 12 weeks gestation and implantation failure in comparison with the pregnant patients. The measurement of ROS and TAC is not interchangeable.
Conclusion: According to the data on the parameters of oxidative stress, the levels of ROS and TAC have an influence on the outcomes of ART treatment. It is necessary to continue studying oxidative stress in patients with various types of infertility in order to develop a mathematical model for predicting the results of ART treatment and improving the preconception care of couples prior to infertility treatment.
Keywords
Assisted reproductive technologies (ART) hold a prominent place in the treatment of both male and female infertility as more than 6.5 million children in the world have been born after in vitro fertilization and embryo transfer into the uterine cavity [1–3]. Such an increase in the use of ART is caused by socio-economic and environmental impact which is often associated with the development of infertility in women of late reproductive age. However, the effectiveness of treatment in all countries has not changed dramatically in recent decades; pregnancy rate is 30–35% after embryo transfer into the uterine cavity. It is possible to solve the problem of infertile couples by increasing the rate of pregnancy and implantation only with the help of fundamental scientific projects aimed at studying the characteristics of human reproduction and the impact of various endogenous and exogenous factors on fertility. It has been found that one of these factors is oxidative stress which negatively affects the results of ART treatment.Recent studies have shown that increased production of reactive oxygen species (ROS) is a factor that affects female reproductive function [4]. Oxidative stress can be caused by an excess of ROS in the body or antioxidant deficiency. ROS are known to be side products of normal metabolism; moreover, it has been proven that in case of physiological concentrations they have a positive effect on various processes in the body including the normal development and functioning of germ cells [5]. The increased number of ROS in the body has a damaging effect and leads to a deterioration in the quality of cellular material. Therefore, there is a risk for a decrease in the frequency of fertilization if there is a disbalance between ROS and total antioxidant capacity (TAC) [6].The role of oxidative stress can be seen in the pathogenesis of various diseases, though its influence on the processes of folliculogenesis and oogenesis has not been studied enough to date [7]. There is a number of studies with contradictory results on the effect of oxidative stress on the female reproductive function. These studies are mainly focused on the microenvironment of developing oocytes, namely on the levels of ROS and antioxidants in the follicular fluid [8–12].Follicular fluid is the liquid surrounding the oocyte in the ovarian follicle.The composition of the follicular fluid includes steroid hormones, cytokines, enzymes, electrolytes, various amino acids, lipids, growth factors, reactive oxygen species and antioxidants (superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase, as well as fat- and water-soluble antioxidants such as vitamins A, C, E and glutathione). Follicular fluid is an available substrate that is why it is possible to evaluate various biochemical indicators of oocyte quality.The aim of this study is to assess the level of ROS and TAC in the peripheral blood and follicular fluid in women with various types of infertility.
Materials and methods
The prospective study included 65 patients aged 21–38 years with preserved ovarian reserve estimated on the basis of hormonal profile (AMH>1.2) and ultrasound examination. The patients received treatment for infertility at B.V. Leonov Department of Assisted Reproductive Technologies in the Treatment of Infertility, National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow in the period from 2020 to 2021. All patients were not contraindicated to undergo ovarian stimulation and ART treatment. The written informed consent to participate in the study was obtained from each couple. All the patients included in the study were examined in accordance with the Order of the Russian Ministry of Health dated July 31, 2020 No. 803n «On the procedure for the use of assisted reproductive technologies, contraindications and restrictions to their use». Ovarian stimulation was performed with gonadotropin-releasing hormone (GnRH) antagonists, the dose of gonadotropins depended on the ovarian reserve of the patients. The trigger of the final oocyte maturation was administered once 35-36 hours before transvaginal puncture in the presence of follicles in the ovaries with a diameter of ≥ 17 mm. Human chorionic gonadotropin (hCG) at a standard dose of 10,000 IU or a gonadotropin-releasing hormone (GnRH) agonist at a dose of 0.2 mg was prescribed as a trigger. On the day of transvaginal follicle puncture, peripheral blood was taken from the patients before giving intravenous anesthesia. Moreover, follicular fluid was taken to measure the parameters of oxidative stress. All the obtained follicular fluid was pooled from one patient from all punctured follicles. Individual oocytes and follicular fluid were not isolated.
The level of oxidative stress was assessed immediately after collecting biological material in the cytology laboratory. In order to assess oxidative stress, the FORM 3000 device (Callegari, Italy) was used in accordance with the manufacturer’s recommendations. Blood was collected in test tubes with sodium heparin. The level of TAC in peripheral blood was measured in units equivalent to the activity of vitamin E (mmol/L trolox eq.) using the FORD kit, while the level of free radicals was determined in units equivalent to the molar concentration of H2O2 (mmol/L) using the FORT kit.
All patients underwent intracytoplasmic sperm injection into the oocyte (ICSI) to fertilize the obtained mature oocytes. One good quality embryo was transferred in the uterine cavity on the 5th day of cultivation. The embryos were evaluated using morphological characteristics in accordance with the criteria presented in Table 1. The luteal phase and posttransfer period were supported with progesterone preparations in all patients. On the 12th–14th day after the transfer all patients had blood tested for β-hCG to confirm pregnancy. The results of pregnancy course were found out by a phone call to the patient at the estimated date of 12 weeks gestation. The end point in this study was a developing pregnancy at 12 weeks gestation.
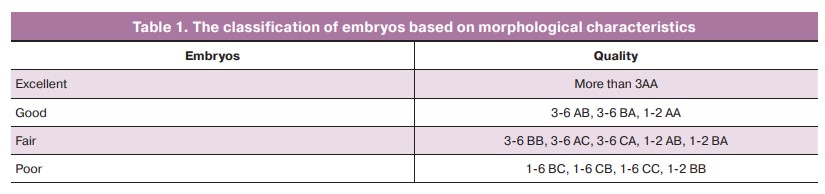
In order to identify differences in the mean values of the main clinical and embryological parameters, all patients were divided into groups depending on the presence of tuboperitoneal factor (TPF), presence or absence of pregnancy, as well the course of pregnancy up to 12 weeks.
Statistical analysis
Sampling was tested for normality using the Shapiro–Wilk test; mean and standard deviation (M (SD)) were used to characterize parameters with a normal distribution (the level of ROS in the blood, height, the level of TAC in the blood), and percentiles (25%, 75%) were used for the rest parameters.
The mean values and standard deviations were calculated to evaluate the parametric data. To assess nonparametric data (data with a distribution other than normal), a median (Me) with interquartile ranges (25%, 75%) was calculated. Student’s t-test was used to compare the mean values of parameters with a normal distribution in two independent groups, and the Mann–Whitney U test was used for parameters with a distribution other than normal one. In order to assess the correlation between the main clinical and embryological parameters of ART treatment, the Pearson correlation coefficient (Rs) was calculated for the parameters with a normal distribution, and the Spearman correlation coefficient (Rp) was calculated for the parameters with a distribution other than the normal one. When interpreting the results of the statistical analysis, the significance level of p-value=0.05 was accepted as critical.
Results
Descriptive statistics were used to characterize the total sample of the study (Table 2).
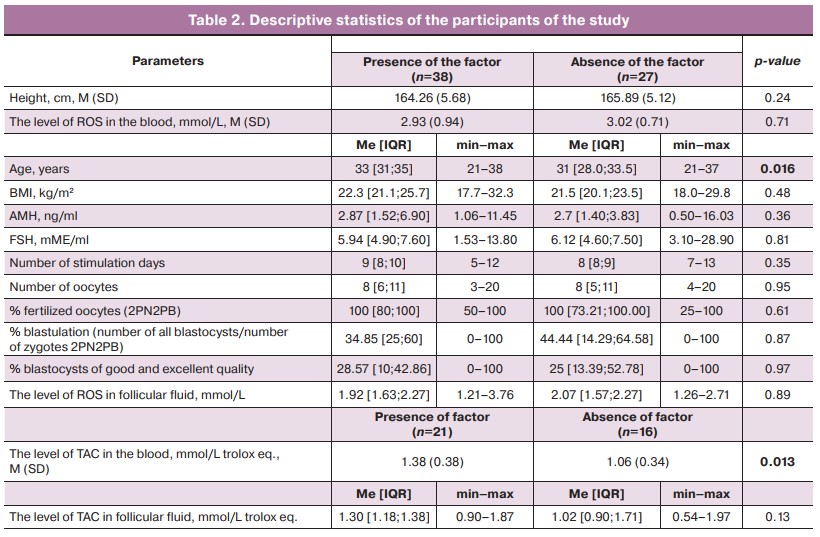
The correlation analysis revealed a positive correlation between the level of ROS in peripheral blood and the number of stimulation days (Rs=0.294; p=0.010), as well as between the level of ROS in follicular fluid and BMI (Rs=0.238; p=0.039). It is worth noting that the compared variables were interdependent. There were no significant correlations between the parameters of TAC in blood and follicular fluid and the main clinical and embryological parameters of ART treatment.
There was a significant positive correlation between the parameters of ROS in peripheral blood and ROS in follicular fluid (Rs=0.503; p<0.001), as well as between the parameters of TAC in peripheral blood and TAC in follicular fluid (Rs=0.620; p<0.001). The compared variables were interdependent.
The male factor of infertility can influence the embryological stage; therefore, we analyzed the influence of the male factor on the embryological and clinical parameters of ART treatment. There were no significant differences, so it can be concluded that the male factor and its effect on embryological and clinical parameters in this case can be neglected.
Significant differences and a tendency to differences between the mean values of the level of ROS in the blood and follicular fluid were found in the groups of pregnant patients, patients with implantation failure, and patients with ongoing pregnancy up to 12 weeks (Table 3).
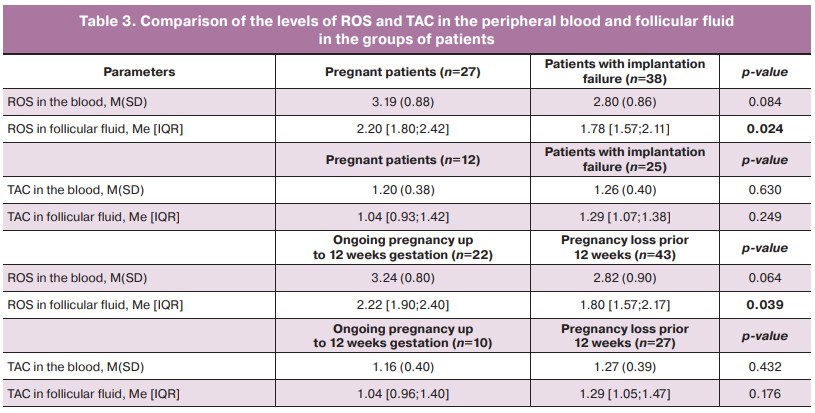
The comparison of the level of ROS in follicular fluid in the groups of pregnant women and patients with implantation failure revealed significant differences (p=0.024). The level of ROS in follicular fluid in the group of patients with ongoing pregnancy to 12 weeks gestation was also significantly higher (p=0.039) (Table 3). There were statistical trends (0.1>p-value>0.05) to the difference in the mean values of ROS in the peripheral blood in patients with ongoing pregnancy and pregnancy loss (p=0.064).
The mean values of TAC in the peripheral blood in patients with TPF were significantly (p=0.013) higher than in patients without TPF (Table 2).
Discussion
Follicular fluid provides a microenvironment for the developing oocyte and has a direct impact on the quality of oocytes, implantation and early development of the embryo. The impaired production of ROS in the follicular fluid of the ovaries may have an adverse effect on the above processes. It was previously shown that increased ROS activity in the follicular fluid may be toxic for embryo development, whereas the physiological ROS level may be indicative of healthy developing oocytes [13, 14]. The results of our study showed the absence of the influence of TAC on the embryological stage in the participants of the study. There were no significant differences in the fertilization rate and embryo development before the 5th and 6th days of cultivation in women with different levels of TAC and ROS. Perhaps this was due to the pooling of the total follicular fluid and the assessment of the overall TAC and ROS from all collected follicles during transvaginal puncture of the ovaries. Therefore, a team of researchers headed by Nishihara T. presented other results where the total antioxidant capacity was higher in the follicles, where the oocytes were successfully fertilized, and significantly lower in follicular fluid, where the obtained embryo was of good quality [14].
Currently, contradictory data have been published on the effect of oxidative stress in follicular fluid on the quality of oocytes and embryos, as well as the rate of fertilization and pregnancy. The results of our study showed that a decrease in the level of ROS in blood and follicular fluid may be associated with negative IVF outcomes. That means it is necessary to maintain ROS at a certain physiological level.
A new method for evaluating ROS and TAC in follicular fluid was tested in this study. The revealed correlation can contribute to predicting the outcomes of ART treatment if oxidative stress is detected in the blood and follicular fluid prior to the treatment; however, a larger set of materials is required and it is necessary to develop a mathematical model on the basis of the revealed patterns.
It should be noted that the present study has a number of limitations: a small number of patients, the lack of accurate physiological concentrations of ROS and TAC in healthy women in natural menstrual cycles as a normalizing value. The level of ROS in follicular fluid and peripheral blood was shown to be higher in women who had positive outcomes of ART treatment. At the same time, these indicators relate to the number of stimulation days and BMI. These facts suggest that a greater number of obtained oocytes may lead to higher probability of cultivating mature oocytes at stage MII, higher chances of developing embryo of excellent quality on the 5th day, and higher chances of achieving pregnancy. Many parameters are often related to each other in ART treatment and this relationship cannot always be differentiated from the influence of external factors. The future studies will be aimed at finding the threshold values of ROS and TAC in fertile women. The correlation between the levels of ROS and TAC with the outcomes of ART treatment demonstrated that it is possible to study these parameters as new biomarkers for predicting the onset of pregnancy, including natural conception.
Conclusion
The study showed that the level of ROS in follicular fluid is higher in groups of pregnant women and in patients with ongoing pregnancy up to 12 weeks. The level of ROS increases in pregnant women and in patients with ongoing pregnancy up to 12 weeks gestation. There was a positive correlation of the level of ROS with BMI and the number of stimulation days. The evaluation of TAC and the level of ROS in the blood and follicular fluid is not identical; it makes sense to measure them separately and subsequently evaluate their possible impact on the outcome of ART treatment in total.
The correlation between the outcomes of ART treatment and indicators of oxidative stress suggests that in the future it is possible to develop a prognostic model of the relationship between oxidative stress and pregnancy rate in ART treatment, as well as the likelihood of its development at 12 weeks gestation in couples suffering from infertility.
References
- Tamrakar S.R., Bastakoti R. Determinants of infertility in couples. J. Nepal Health Res. Counc. 2019; 17(1): 85-9. https://dx.doi.org/10.33314/jnhrc.1827.
- Isupova O.G. Assisted Reproductive Technologies: New Opportunities. Demographicheskoe obozrenie/Demographic review. 2017; 1: 33-63 (in Russian).
- Thurston L., Abbara A., Dhillo W.S. Investigation and management of subfertility. J. Clin. Pathol. 2019; 72(9): 579-87. https://dx.doi.org/10.1136/jclinpath-2018-205579.
- Wojsiat J., Korczyński J., Borowiecka M., Żbikowska H.M. The role of oxidative stress in female infertility and in vitro fertilization. Postepy Hig. Med. Dosw. (Online). 2017; 71(1): 359-66. https://dx.doi.org/10.5604/3001.0010.3820.
- Agarwal A., Maldonado Rosas I., Anagnostopoulou C., Cannarella R., Boitrelle F., Munoz L.V. et al. Oxidative stress and assisted reproduction: a comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment. Antioxidants (Basel). 2022; 11(3): 477. https://dx.doi.org/10.3390/antiox11030477.
- Di Renzo L., De Lorenzo A., Fontanari M., Gualtieri P., Monsignore D., Schifano G.; SIERR. Immunonutrients involved in the regulation of the inflammatory and oxidative processes: implication for gamete competence. J. Assist. Reprod. Genet. 2022; 39(4): 817-46. https://dx.doi.org/10.1007/
s10815-022-02472-6.
- Camaioni A., Ucci M.A., Campagnolo L., De Felici M., Klinger F.G.; Italian Society of Embryology, Reproduction and Research (SIERR). The process of ovarian aging: it is not just about oocytes and granulosa cells. Assist. Reprod. Genet. 2022; 39(4): 783-92. https://dx.doi.org/10.1007/s10815-022-02478-0.
- Ivancha K.A., Syrkasheva A.G., Volodina M.A., Pyataeva V., Sukhanova Yu.A., Vysokikh M.Yu., Kalinina E.A., Ponizovkina A.I., Dolgushina N.V. Role of oxidative stress markers in predicting the outcomes of assisted reproductive technologies. Obstetrics and Gynecology. 2017; 5: 98-103. (in Russian). https://dx.doi.org/10.18565/aig.2017.5.98-103.
- Nie Z., Zhang N., Guo L., Lv C., Zhang Y., Wang C., Wu H. Growth hormone improved oxidative stress in follicle fluid by influencing Nrf2/Keap1 expression in women of advanced age undergoing IVF. Gynecol. Endocrinol. 2022; 38(3): 222-6. https://dx.doi.org/10.1080/09513590.2021.2003325.
- Ramgir S.S., Renu K., Vellingiri B., George A., Tirupapuliyur D., Thiagarajan P., Valsala Gopalakrishnanash A. Phytomedicinal therapeutics for male infertility: critical insights and scientific updates. J. Nat. Med. 2022; 76(3): 546-73. https://dx.doi.org/10.1007/s11418-022-01619-0.
- Mendes S., Sá R., Magalhães M., Marques F., Sousa M., Silva E. The role of ROS as a double-edged sword in (In)fertility: the impact of cancer treatment. Cancers (Basel). 2022; 14(6): 1585. https://dx.doi.org/10.3390/cancers14061585.
- Bedaiwy M.A., Elnashar S.A., Goldberg J.M., Sharma R., Mascha E.J., Arrigain S. et al. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Endocrinol. 2012; 28(1): 51-5. https://dx.doi.org/10.3109/09513590.2011.579652.
- Singh A.K., Chattopadhyay R., Chakravarty B., Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013; 42: 116-24. https://dx.doi.org/10.1016/j.reprotox.2013.08.005.
- Nishihara T., Matsumoto K., Hosoi Y., Morimoto Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Med. Biol. 2018; 17(4): 481-6. https://dx.doi.org/10.1002/rmb2.12229.
Received 11.04.2022
Accepted 10.08.2022
About the Authors
Diana S. Agadzhanyan, MD, postgraduate student, Prof. B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, agadzhanyand@inbox.ru, 117997, Russia, Moscow, Academician Oparin str., 4.Veronika Yu. Smolnikova, PhD, Associate Professor, Leading researcher, Prof. B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, v_smolnikova@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Aleksey M. Krasnyi, PhD in Biology, Head of the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, alexred@list.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Natalia N. Lobanova, Junior Researcher, Prof. B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, n_lobanova@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Alsu A. Sadekova, PhD in Biology, Researcher at the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, sialsad@gmail.com, 117997, Russia, Moscow, Academician Oparin str., 4.
Valeriya S. Shchipitsyna, Junior Researcher at the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, v_shchipitsyna@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher at the Prof. B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, np_makarova@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Prof. B.V. Leonov Department of IVF, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, e_kalinina@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Authors’ contributions: Agadzhanyan D.S. – collecting and analyzing literature data, collecting biological material, processing results, writing the article; Smolnikova V.Yu., Makarova N.P., Lobanova N.N. – developing the design of a clinical trial, selecting patients, editing the text of the article; Krasnyi A.M. – measuring the parameters of oxidative stress, analyzing the results; Shchipitsyna V.S. – measuring the parameters of oxidative stress; Sadekova A.A. – measuring the parameters of oxidative stress, editing the text of the article; Kalinina E.A. – editing the text of the article.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was carried out without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Agadzhanyan D.S., Smolnikova V.Yu., Krasnyi A.M.,
Lobanova N.N., Shchipitsyna V.S., Sadekova A.A., Makarova N.P.,
Kalinina E.A. Informative value of oxidative stress markers
in predicting outcomes of infertility treatment using ART methods.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 9: 64-70 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.64-70



