Identity of preeclampsia and metabolic syndrome clinical manifestations: searching for substantiation
Aim. To investigate clinical and laboratory parameters in pregnant women with preeclampsia (PE) who have and do not have metabolic syndrome (MS) to identify common clinical and pathogenetic patterns.Lipatov I.S., Tezikov Yu.V., Azamatov A.R., Shmakov R.G.
Materials and methods. The study included 89 women with PE and high-risk factors (group I), 50 women with PE and MS (group II), 32 women with MS without PE (group III), and 30 women with a healthy pregnancy (group IV, control group). The study participants underwent comprehensive clinical and laboratory examinations.
Results. Compared to controls, patients with PE without somatic comorbidities and PE with MS were more likely to have abnormal 24‐h BP patterns (non-dipper, night-picker), episodes of gestational sleep apnea, insomnia, increased visceral adipose tissue, which are typical for MS outside of pregnancy. The laboratory parallels between MS and PE were confirmed by similar changes in metabolic and hormonal parameters, markers of proinflammatory and prothrombotic states, angiogenesis, and endothelial dysfunction.
Conclusion. The identity of clinical and pathogenetic patterns of MS and PE is based on pathological insulin resistance, hyperinsulinemia, and associated atherogenic dyslipidemias, hyperleptinemia, proinflammatory and immune-metabolic disorders, oxidative stress, hyperuricemia, prothrombotic and hyper-sympathetic states which account for similar clinical features.
Keywords
Preeclampsia (PE) remains a major global health problem and is one of the leading causes of maternal and perinatal morbidity and mortality worldwide [1, 2]. Changes in the current diagnostic criteria for PE, the search for new prognostic indicators suggest the absence of clear etiopathogenetic ideas about the mechanisms underlying this pregnancy complication. Therefore, PE is an equivocal clinical category. Considering the general clinical approach to arterial hypertension (AH), it should be noted that in the current guidelines, PE is regarded as a complicated hypertensive crisis [3]. At the same time, increased blood pressure has a significant relationship with metabolic disorders, which are believed to be the leading mechanism underlying essential hypertension [4, 5]. This assumption is confirmed by the fact that AH is one of the top components of metabolic syndrome (MS), which remains an area of extensive research due to its high prevalence [4, 6]. Existing criteria for MS (IDF, 2005) include waist circumference> 94 cm in men and> 80 cm in women, blood pressure >130 and 85 mm Hg. or antihypertensive therapy, blood triglyceride (TG) ≥1.7 mmol/L or specific treatment for dyslipidemia, high-density lipoprotein (HDL) <1.03 mmol/L in men and <1.29 mmol/L in women, fasting blood glucose ≥5.6 mmol/L or diagnosed type 2 diabetes mellitus [7]. Advancing knowledge and searching for a "platinum" standard for the diagnosis of MS has led to the emerging of additional factors to be considered. They included a high level of free fatty acids, hyperleptinemia, leptin resistance, microalbuminuria/proteinuria, obstructive sleep apnea, hyperuricemia, pro-inflammatory and prothrombotic states, oxidative stress, endothelial dysfunction, polycystic ovary syndrome, and hyperandrogenism in women [7, 8]. Diabetes-related conditions (insulin resistance (IR), chronic hyperinsulinemia (HI)), and atherogenic disorders) have been recognized by most authors as the leading mechanisms of MS [8, 9]. It is essential that during pregnancy, there are similar metabolic changes in response to placental anti-insulin factors aimed at the energy and nutrient supply of the developing fetus [10, 11]. At the same time, the disruption of the mechanisms of gestational adaptation, which occurs against the background of risk factors (somatic, infectious, autoimmune pathology, hormonal, metabolic, epigenetic disorders, gene deviations, stress, unhealthy behaviors, environmental factors, etc.), leads to an aggravation of the changes inherent in pregnancy and the development of its complications.
The present study aimed to investigate clinical and laboratory parameters in pregnant women with PE who have and do not have MS to identify common clinical and pathogenetic patterns.
Materials and methods
The study included 201 pregnant women who were managed at the Samara Regional Perinatal Center in 2016–2019 and underwent a comprehensive clinical and laboratory examination. Group I (comparison group) comprised 89 women with PE, who had high-risk factors including a history of PE [51.7% (46/89)], family history of PE [40.4% (36/89)]; 7.9% (7/89) were primigravida of late reproductive age (>35 years). Group II included 50 women with PE and MS. Group III included 32 pregnant women with MS without PE. Group IV (control group) consisted of 30 healthy pregnant women with a healthy pregnancy. The criteria for inclusion in group I were the presence of PE, body mass index 18.5–24.9 kg/m2, blood pressure <130 and 85 mm Hg, and absence of metabolic disorders according to pre-pregnancy examination registered in medical records.
Inclusion criteria in groups II and III were MS diagnosed before pregnancy (based on clinical measures including waist circumference> 80 cm, triglycerides>1.7 mmol/l, HDL cholesterol<1.29 mmol/l, BP≥130 and 85 mm Hg), early reproductive age; preserved menstrual and ovulatory ovarian cycles, and spontaneous pregnancy. It is important to note that the pre-gestational venous blood glucose level in women with MS was <6.1 mmol/L, which made it possible to limit the effect of existing hyperglycemia on the processes of gestational rearrangement. The exclusion criteria for all groups were carbohydrate metabolism abnormalities before pregnancy, gestational diabetes mellitus, severe somatic (except for MS in groups II and III), infectious and autoimmune comorbidities, genital anomalies, polycystic ovary syndrome, pregnancy after assisted reproductive technologies; congenital fetal pathology. Women in groups II and III had no statistically significant differences in MS criteria.
Clinical and laboratory evaluation was conducted at 30–36 weeks’ gestation. Women in groups I and II were examined at that time-points if PE occurred. Considering the similarity of the PE criterion dyad with the MS criteria (AH, proteinuria/microalbuminuria), in the study groups, we analyzed additional clinical signs (24-hour blood pressure profile, episodes of sleep apnea, subjective assessment of sleep characteristics, adipose tissue distribution type) that are typical for MS outside pregnancy [4, 7, 12]. 24-hour BP variability is an important chronobiological parameter and is divided into types, including dipper (normal) – decrease in night-time blood pressure by 10–20%, over-dipper – decrease> 20%, non-dipper – decrease in blood pressure by 0–10%, nigh-picker – an increase in blood pressure at night. MS is characterized by the latter two types [13]. Sleep apnea episodes are a sign of obstructive sleep apnea, a separate clinical and pathogenetic variant of MS. They play an important role in developing hypertension (hypoxia, the release of stress hormones, and oxidative stress at night) [14]. The presence of insomnia (daytime sleepiness, poor sleep quality, frequent awakenings, and negative dreams) was assessed using Ya.I. Levin’s questionnaire. (1995). Scores were classified as normal (score> 22), borderline (19–21), and insomnia (18 or less) [15]. Considering the importance of visceral fat in the development of MS, adipose tissue distribution was assessed using ultrasound (K. Tayama et al., 1999) and measuring the thickness of subcutaneous (SCFT) and pre-peritoneal (PPFT) fat with the calculation of the abdominal wall fat index (AWFI = PPFT/SCFT). AWFI > 1.0 indicates the visceral fat deposition [16].
The laboratory investigations were aimed to cover the main pathogenetic changes in MS and PE. Metabolic parameters included venous plasma glucose, IR index (HOMA-IR) [17], uric acid, total cholesterol (TC), TG, HDL cholesterol, TG/HDL ratio. Hormonal profile comprised insulin, leptin, placental lactogen (PL), and cortisol. Also, laboratory examination included markers of proinflammatory (tumor necrosis factor (TNF) -α, C-reactive protein, leukocyte count, leukocyte activation index (LAI) [18]) and prothrombotic (platelet count, mean platelet volume, platelet aggregation with collagen) states, endothelial dysfunction (nitric oxide metabolites, circulating endothelial cells (CEC), fibronectin (FN)), placental alpha-1-microglobulin (PAMG-1) and placental growth factor (PGF). Laboratory and instrumental investigations were carried out using an Architect c4000 biochemical analyzer (Abbotte, USA), a Sysmex XN-1000 hematology analyzer (Sysmex Corporation, Japan), an ALAT-2 laser platelet aggregation analyzer ALAT-2 LLC NPF Biola, and the Voluson E6 "GE Healthcare (Austria) ultrasound system. The severity of placental insufficiency was assessed according to the classification of A.N. Strizhakova et al. (2014) [19]. The diagnosis of PE was established following the WHO criteria approved by the Ministry of Health of the Russian Federation (2011).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25 HC IMAGO 5.0 software, license No. 5725-A54. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test with the Lilliefors and Shapiro–Wilk corrections. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD). Between-group differences in continuous variables were assessed by analysis of variance (ANOVA). Numerical variables that were not normally distributed were summarized as medians (Me) and inter-quartile range [Q1 (25%); Q3 (75%)]. Medians for continuous variables were compared using the Mann–Whitney U-test with Bonferroni correction. Categorical variables were reported as counts and proportions (%) and compared using contingency tables and the Pearson χ² test with Yates' correction. Spearman's correlation analysis was used to identify relationships. The critical level of significance when testing statistical hypotheses was considered at p<0.05. When using the Bonferroni correction for pairwise comparison of 4 groups, the number of compared pairs was six, and the new critical level corresponded to p<0.008.
Results and Discussion
There were no statistically significant differences between the study groups regarding participant medical and social characteristics (age, parity, marital status, living conditions and place of residence, professional affiliation) (p>0.05). Gestational complications are presented in Table 1. Women with MS were more likely to have early pregnancy complications than women in the group I. The differences were statistically significant when patients had MS and PE (early moderate gestational toxemia - χ2 = 5.53, р = 0.01; threatened miscarriage – χ2 = 6.80, p=0.009), which indicates an essential role of periconceptional changes in MS in the development of these complications. In groups I, II and III, grade II–III chronic placental insufficiency was found, in 34.8%, 36.0% and 28.1% of patients, respectively (χ2I-II = 0.02, pI-II =0.89; χ2I-III =0.48, pI-III =0.49; χ2II-III =0.55, pII-III=0.46). In groups, I and II, grade IIB, IIB, and III placental insufficiency rates were 4.7 and 5.2 times higher, respectively, than in group III (χ2I-III=5.75, pI-III=0.02; χ2II-III =6.12, pII-III=0.01), which suggests more severe chronic placental insufficiency in PE.
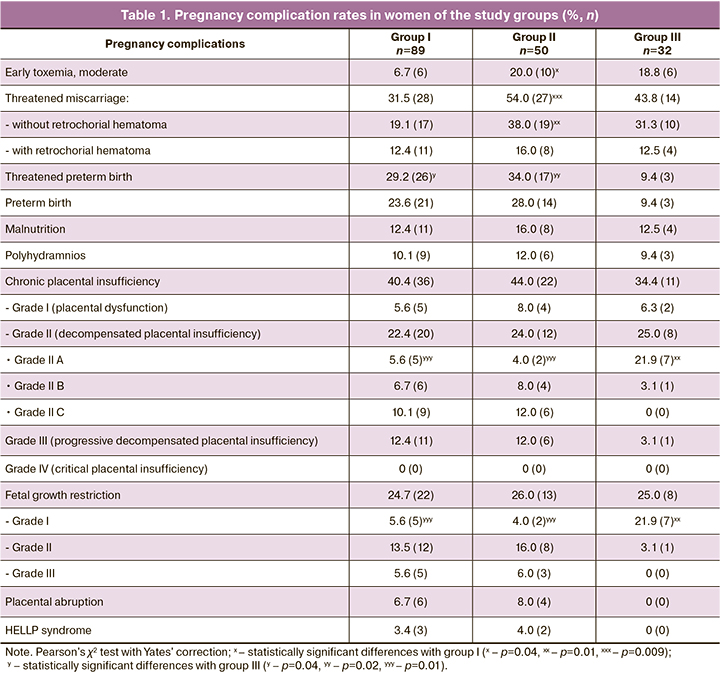
In groups I and II, early PE was diagnosed at 30–34 weeks of gestation in 34.8% (31/89) and 44.0% (22/50) of women, late PE (after 34 weeks) in 65.2% (58/89) and 56.0% (28/50), respectively (χ2=1.14, p=0.29). PE rates depending on the severity in groups I and II were as follows: moderate PE – 62.9% (56/89) and 60.0% (30/50), severe PE – 37.1% (33/89) and 40.0% (20/50) observations, respectively (χ2=0.12, p=0.73). Therefore, in terms of the time and severity of PE manifestation, pregnant women in the group I with PE without somatic pathology and group II with PE and MS were similar. At the same time, the levels of blood pressure and proteinuria, both with moderate and severe PE, were statistically significantly higher in women with PE and MS than in group I. In groups I and II groups: with moderate PE, systolic blood pressure corresponded to 148 [143; 152] and 156 [151; 158] mm Hg, diastolic blood pressure – 97 [94; 101] and 105 [100; 108] mm Hg, mean blood pressure was 109 [105; 114] and 120 [115; 124] mm Hg. Proteinuria in a single urine specimen was 1.58 [0.94; 2.05] and 2.48 [2.07; 2.89] g/l, proteinuria in 24-hour urine collection was 0.99 0.58; 1, 42] and 2.05 [1.74; 2.41] g/day. In severe PE, systolic blood pressure was 172 [165; 180] and 189 [181; 200] mm Hg, diastolic blood pressure – 120 [115; 125] and 128 [124; 133] mm Hg, mean blood pressure – 125 [122; 130] and 138 [132; 144] mm Hg. Proteinuria in a single urine specimen was 3.74 [3.25; 4.20] and 4.77 [4.35; 5.53] g/l, proteinuria in 24-hour urine collection was 3.86 [2, 56; 4.50] and 4.83 [3.86; 5.57] g/day (for all indicators p=0.01). In all pregnant women with PE, the level of systolic and diastolic blood pressure met the criteria of the developed pathology severity.
The identity of MS and PE's clinical features was found to include 24-hour variability of blood pressure, sleep characteristics, and adipose tissue distribution (Table 2). Pathological types of 24-hour blood pressure variability with an insufficient decrease (non-dipper) or increase (night-picker) of BP at night were observed in 53.9%, 60.0%, and 40.6% of women in groups I, II, and III, respectively (χ2=2.98, p=0.23). Hypertension and pathological 24-hour blood pressure profile are associated with obstructive sleep apnea episodes [5, 14, 20]. A conditional analogue of this syndrome during pregnancy is gestational sleep apnea which was found in 53.9%, 66.0%, 37.5% participants in groups I, II and III, respectively (χ2I-II =1.92, pI-II =0.17; χ2I-III=2.54, pI-III =0.11; χ2II-III=6.40, pII-III=0.01). The relationship between the pathological diurnal BP profile and gestational sleep apnea was confirmed moderate strength and a strong positive correlation (for non-dipper r=0.75, at p<0.05; for night-picker r=0.84, at p<0.001), which reflects the similarity of underlying mechanisms of clinical manifestations in PE and MS. Analysis of the subjective assessment of sleep characteristics showed that 60.7%, 66%, and 40.6% of pregnant women in groups I, II, and III had scores<22(χ2I-II=0,39, рI-II=0.53; χ2I-III=3,83, pI-III=0.05; χ2II-III=5,10, pII-III=0.02), which reflects the similarity of sleep disorders in PE and MS.
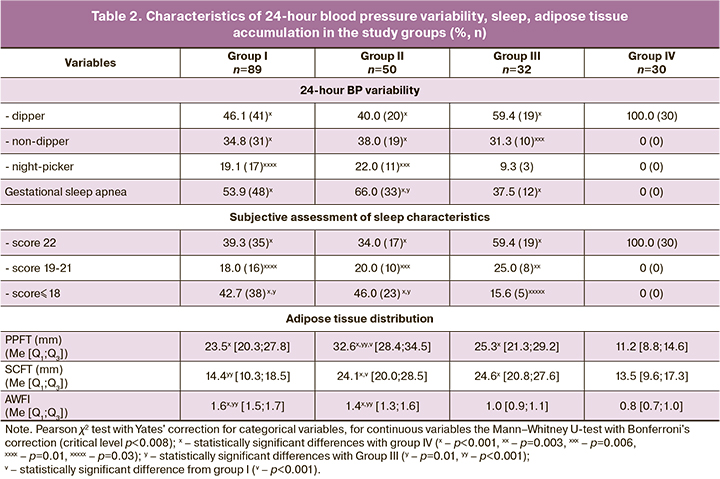
Visceral obesity is a primary diagnostic criterion for MS and the main trigger for pathophysiological changes [6, 21, 22]. In pregnant women with PE without somatic comorbidities, who did not have excess body weight before pregnancy, the pre-peritoneal (visceral) fat thickness was more twice higher as in controls (p <0.001) and approached that in group III (p=0.78). Besides, AWFI, the integrative indicator of the visceral type of fat deposition in groups I, II and III was 1.6 [1.5; 1.7], 1.4 [1.3; 1.6], 1.0 [0.9; 1.1], respectively (рI-II=0.04, pI-III<0.001, pII-III<0.001), which confirms the general similarity in adipose tissue distribution (abdominal visceral fat predominance) in MS and PE without somatic comorbidities.
The laboratory parameters reflecting the pathogenetic features of MS and PE are presented in Table 3. It is shown that pathological IR and HI characteristics of MS also occur in PE without somatic pathology. The insulin and HOMA-IR levels in group I were statistically significantly higher than in pregnant women with MS without PE and controls (p<0.001). This indicates that the physiological IR and compensatory HI existing within "pregnancy norm" acquire pathological character during PE formation [10]. The glucose level in pregnant women did not go beyond the normal range, determined by the inclusion and exclusion criteria in the study.
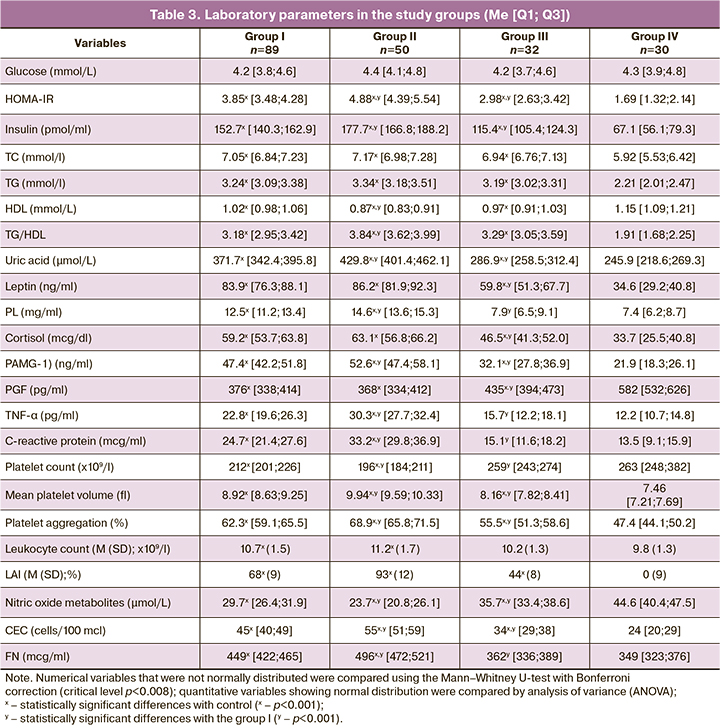
IR during pregnancy is aimed to increase energy nutrient availability, in particular glucose to the fetus [10, 11]. During physiological gestation, pregnant women in group IV had increased levels of lipid profile atherogenic fractions, which act as alternative sources of energy for the maternal organism under conditions of limited glucose intake against the background of IR. With the development of PE, the levels of TC, TG, the TG/HDL ratio were statistically significantly higher (p<0.001), and the HDL level is statistically significantly lower than in the control group (p<0.001), which additionally potentiates the formation of pathological IR and HI. Consequently, in pregnant women with PE and pathological IR and HI, there are pronounced atherogenic disorders, which are also criteria for MS, which indicates the similarity of pathophysiological changes in these pathologies.
Considering the diabetogenic and atherogenic disorders' trigger mechanism, an analogy can be drawn between MS and PE. Most researchers agree that visceral adipose tissue plays a leading role in MS [6, 16, 21]. At the same time, during pregnancy, a similar organ with a colossal secretory potential is the placenta, the anti-insulin activity of which is aimed at supplying the fetus with energy. In women with PE without somatic comorbidities and PE against the background of MS, the concentration of PL, the primary placental anti-insulin hormone, had a similar orientation and statistically significantly exceeded the levels in groups III and IV (p<0.001), which emphasizes the involvement of this indicator in the pathogenesis of PE through the formation of pathological IR and HI.
PAMG-1 also had the highest levels in groups I and II (pI-III, pI-IV, pII-III, pII-IV <0.001). This peptide, which reflects the state of the maternal part of the placenta, is synthesized by decidual cells and regulates the insulin-like growth factor's activity. Increased concentrations of PAMG-1 in pregnant women with PE blocks insulin-like growth factors, contributing to the formation of pathological IR and HI. The involvement of hyperleptinemia and leptin resistance in the mechanisms underlying hypersympathicotonia and hypertension, proinflammatory state, pathological IR, and HI in MS is widely known [23]. It is important to note that pregnancy is accompanied by additional leptin synthesis activation in the placenta and mammary glands [6,23]. In the groups with PE without somatic pathology and PE against MS's background, the level of this hormone did not differ statistically (p=0.65). Still, it significantly exceeded the values in women with MS without PE and in control subjects (pI-IV, pII-IV, pIII-IV <0.001), reflecting hyperleptinemia involvement in the pathogenesis of PE. Cortisol, a nonspecific stress hormone with a pronounced anti-insulin effect, also participates in the development of pathological IR and HI [8, 9]. The cortisol level in groups I, II, III was statistically significantly higher than in the control group (pI-IV, pII-IV, pIII-IV<0.001). An increase in these anti-insulin hormones synthesized by the placenta in pregnant women with PE confirms this organ's role as a trigger for pathological IR.
Hyperuricemia is one of the additional signs of MS [7]. We noted a statistically significant increase in uric acid level in the groups with PE and MS (pI-IV, pII-IV, pIII-IV<0.001), which once again reflects the similarity of laboratory changes in MS and PE.
Chronic low-intensity systemic meta-inflammation is an integral part of MS. In MS, adipose tissue acts as a source of TNF-α, a leading inflammatory cytokine, and IR mediator [24]. Pregnant women with PE and MS had higher levels of TNF-α than the controls (pI-IV, pII-IV <0.001, pIII-IV =0.03). In patients with PE without comorbidities and PE with MS, the TNF-α level had a unidirectional change and was higher than in the group with MS without PE (pI-III, pII-III <0.001). Therefore, the described pathogenetic relationship of TNF-α with diabetogenic and atherogenic disorders, leukocyte activation, endothelial dysfunction is similarly realized in MS and PE. Changes in C-reactive protein, the most common marker of inflammation, were similar to that in TNF-α. Increased leukocyte indices in groups I, II, III also indicate the formation of immune-metabolic disorders in PE and MS. The prothrombotic status in pregnant women with PE and MS is confirmed by lower platelet count due to an increase in their consumption (pI-IV, pII-IV <0.001, pIII-IV =0.56), an increase in the average cell volume due to young and activated forms (pI-IV, pII-IV, pIII-IV <0.001), an increase in platelet aggregation activity (pI-IV, pII-IV, pIII-IV <0.001), which also occurs in MS outside pregnancy [8.22]. The obtained data on the decrease in PGF, a placental protein from the family of vascular endothelial growth factor in pregnant women with PE and with MS, supplemented by the literature data on the deficiency of vascular endothelial growth factor in MS outside pregnancy [25], reflect the similarity of antiangiogenic changes in MS and PE.
Through the mechanisms associated with pathological IR and HI, the above parameters cause structural and functional destabilization and death of vascular endotheliocytes, which leads to hypertension, proteinuria, and multiple organ failure [8, 26]. This is confirmed by a pronounced decrease in nitric oxide metabolites (the most essential vasodilator synthesized by the vascular endothelium), an increase in CEC and FN released during endothelial cell desquamation in pregnant women of groups I, II, and III compared to controls. These changes are also reflected in MS outside of pregnancy [9, 26].
The occurrence of these pathologies in the presence of predisposing risk factors and changes in homeostasis is an individual process. In our opinion, the absence of PE in group III (pregnant women with MS) can be explained by the existing clinical variability of MS manifestations, the criteria for which include hypertension, type 2 diabetes mellitus, hyperuricemia with gout manifestations, clinical manifestations of hyperandrogenism and thrombogenic state. The limited occurrence of PE in pregnant women with MS may be associated with the long-term preservation of reserve capacities of adaptation and autoregulation mechanisms (“norm of compensated pathology”) without a breakdown in the pathological process in the absence of expression of characteristic gene deviations and epigenetic disorders [2, 6, 8, 11]. Our findings suggest that in pregnant women in group III with MS without PE, the anti-insulin activity of the placenta corresponds to the level of placental anti-insulin activity in a healthy pregnancy (PL 7.9 [6.5; 9.1] mg/ml versus 7.4 [6.2; 8.7] mg/ml, p=0.27). This observation indicates the absence of additional stimulation, along with fatty tissue, by pathological IR placental factors.
 There were no statistically significant differences between pregnant women in the III and control groups in platelet counts and the levels of FN, as integrative clinically significant indicators of endothelial dysfunction and prothrombotic state, which indicates the absence of excessive platelet consumption, severe damage to the vascular endothelium and the level of prothrombotic state characteristic of pregnant women with PE. Therefore, PE in MS is associated with individual features in the presence or absence of high-risk factors and the severity of metabolic, inflammatory, oxidative, prothrombotic, antiangiogenic changes.
There were no statistically significant differences between pregnant women in the III and control groups in platelet counts and the levels of FN, as integrative clinically significant indicators of endothelial dysfunction and prothrombotic state, which indicates the absence of excessive platelet consumption, severe damage to the vascular endothelium and the level of prothrombotic state characteristic of pregnant women with PE. Therefore, PE in MS is associated with individual features in the presence or absence of high-risk factors and the severity of metabolic, inflammatory, oxidative, prothrombotic, antiangiogenic changes.
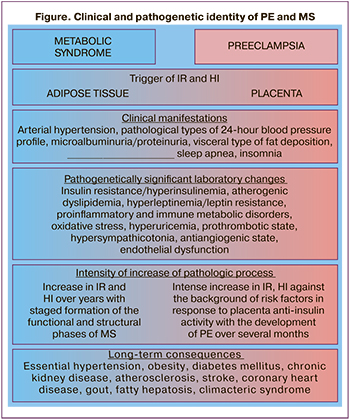
The identified clinical and laboratory parallels between MS and PE are schematically shown in the figure.
Conclusion
The clinical similarity between PE and MS, including AH and proteinuria/microalbuminuria as criteria, convincingly proves and complements the study results. Its findings showed that clinical manifestations characteristic of MS outside pregnancy were found in pregnant women with PE without somatic pathology: pathological types of diurnal blood pressure variability (non-dipper, night-picker), visceral type of fat deposition, episodes of sleep apnea, and insomnia.
Clinical manifestations of MS and PE are only the tip of the iceberg of the most complex pathophysiological disorders, the study of which made it possible to prove that the revealed identity of PE and MS is due to similar underlying mechanisms. They include pathological IR, HI and associated with them atherogenic dyslipidemia, hyperleptinemia and leptin resistance, pro-inflammatory and immune metabolic disorders, oxidative stress, prothrombotic state, hypersympathicotonia, antiangiogenic state, and endothelial dysfunction.
The proven identity of clinical and pathogenetic patterns of PE and MS opens up fundamentally new approaches to the study of pathogenesis, the development of methods for predicting and preventing, early diagnosis and treatment of PE. They may help improve gestational and perinatal outcomes in this pregnancy complication and optimize the consequences of long-term complications associated with a high risk of cardiovascular disease.
References
- Сидорова И.С., Никитина Н.А., Гусева Е.В. Результаты конфиденциального аудита материнской смертности от преэклампсии и эклампсии в России в 2017–2018 гг. Акушерство и гинекология. 2020; 1: 119-27. [Sidorova I.S., Nikitina N.A., Guseva E.V. The results of a confidential audit of maternal mortality due to preeclampsia and eclampsia in Russia in 2017–2018. Obstetrics and gynecology. 2020; 1: 119-27. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.119-127.
- Phipps E.A., Thadhani R., Benzing T., Karumanchi S.A. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019; 15(5): 275‐89. https://dx.doi.org/10.1038/s41581-019-0119-6.
- Общероссийская общественная организация «Содействия профилактике и лечению артериальной гипертензии «Антигипертензивная Лига». Алгоритмы ведения пациента с артериальной гипертензией гипертоническим кризом. СПб.; 2019. 90 с. [All-Russian public organization "Assistance in the prevention and treatment of arterial hypertension" Antihypertensive League". Algorithms for managing a patient with arterial hypertension, hypertensive crisis. First edition. St. Petersburg, 2019. 90 p. (in Russian)].
- Saklayen M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018; 20(2): 12. https://dx.doi.org/ 10.1007/s11906-018-0812-z.
- Saxena T., Ali A.O., Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev. Cardiovasc. Ther. 2018; 16(12): 879-87. https://dx.doi.org/10.1080/14779072.2018.1540301.
- Rochlani Y., Pothineni N.V., Kovelamudi S., Mehta J.L. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017; 11(8): 215-25. https://dx.doi.org/10.1177/1753944717711379.
- Международная федерация диабета (IDF): консенсус по критериям метаболического синдрома. Ожирение и метаболизм. 2005; 3: 47-9. [The IDF consensus worldwide definition of the metabolic syndrome. Obesity and metabolism. 2005; 3: 47-9. (in Russian)].
- Zafar U., Khaliq S., Ahmad H.U., Manzoor S., Lone K.P. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens). 2018; 17(3): 299‐313. https://dx.doi.org/10.1007/s42000-018-0051-3.
- Nolan C.J., Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 2019; 16(2): 118-27. https://dx.doi.org/10.1177/1479164119827611.
- Гордюнина С.В. Инсулинорезистентность при беременности (обзор литературы). Проблемы эндокринологии. 2013; 59(5): 61-6. [Gordyunina S.V. Pregnancy insulin resistance (literature review). Problemy endokrinologii/ Problems of endocrinology. 2013; 59(5): 61-6. (in Russian)].
- Chen X., Stein T.P., Steer R.A., Scholl T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res. Care. 2019; 7(1): e000632. https://dx.doi.org/10.1136/bmjdrc-2018-000632.
- Мирошниченко А.И., Иванов К.М. Влияние ночного повышения артериального давления на ремоделирование сердца у пациентов с артериальной гипертонией. Аспирантский вестник Поволжья. 2019; 1-2: 65-9. [Miroshnichenko A.I., Ivanov K.M. The effect of nocturnal increase in blood pressure on remodeling of the heart in patients with arterial hypertension. Aspirantskii vestnik Povolzh'ya/ Postgraduate Bulletin of the Volga Region. 2019; 1-2: 65-9. (in Russian)]. https://dx.doi.org/10.17816/2072-2354.2019.19.1.65-69.
- Altikardes Z.A., Kayikli A., Korkmaz H., Erdal H., Baba A.F., Fak A.S. A novel method for dipper/non-dipper pattern classification in hypertensive and non-diabetic patients. Technol. Health Care. 2019; 27(S1): 47-57. https://dx.doi.org/10.3233/THC-199006.
- Osman A.M., Carter S.G., Carberry J.C., Eckert D.J. Obstructive sleep apnea: current perspectives. Nat. Sci. Sleep. 2018; 10: 21‐34. https://dx.doi.org/10.2147/NSS.S124657.
- Ляшенко Е.А., Левин О.С. Расстройства сна в клинической практике. Современная терапия в психиатрии и неврологии. 2017; 1: 22-8. [Lyashenko E.A., Levin O.S. Sleep disorders in clinical practice. Sovremennaya terapiya v psikhiatrii i nevrologii/ Modern therapy in psychiatry and neurology. 2017; 1: 22-8. (in Russian)].
- Чабанова Н.Б., Василькова Т.Н. Особенности жирового обмена у беременных в зависимости от срока гестации, массы тела и характера жироотложения. Современные проблемы науки и образования. 2018; 5: 27. [Chabanova N.B., Vasil'kova T.N. Features of fat metabolism in pregnant women depending on gestational age, body weight and the nature of fat deposition. Sovremennye problemy nauki i obrazovaniya/ Modern problems of science and education. 2018; 5: 27. (in Russian)].
- Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27(6): 1487-95. https://dx.doi.org/10.2337/diacare.27.6.1487.
- Герасимов А.М., Малышкина А.И., Сотникова Н.Ю., Богатова И.К., Мартынченко Д.А. Роль индуцированной воспалительно-подобной реакции в репродуктивном тракте женщины в процессе размножения (обзор литературы). Проблемы репродукции. 2018; 24(5): 20-6. [Gerasimov A.M., Malyshkina A.I., Sotnikova N.Yu. et al. The role of the induced inflammatory-like reaction in a reproductive tract of woman during process of reproduction (a review). Problemy reproduktsii/Problems of reproduction. 2018; 24(5): 20-6. (in Russian)]. https://dx.doi.org/10.17116/repro20182405120.
- Стрижаков А.Н., Тезиков Ю.В., Липатов И.С., Шарыпова М.А., Анпилогова И.В., Азизов К.У., Костянова Е.В. Стандартизация диагностики и клиническая классификация хронической плацентарной недостаточности. Вопросы гинекологии, акушерства и перинатологии. 2014; 13(3): 5-12. [Strizhakov A.N., Tezikov Yu.V., Lipatov I.S. et al. Diagnosis standardization and clinical classification of chronic placental insufficiency. Voprosy ginekologii, akusherstva i perinatologii/Questions of gynecology, obstetrics and perinatology. 2014; 13(3): 5-12. (in Russian)].
- Калачин К.А., Пырегов А.В., Шмаков Р.Г. Гестационное сонное апноэ. Связь беременности и преэклампсии с синдромом обструктивного апноэ сна. Альманах клинической медицины. 2019; 47(3): 266-75. [Kalachin K.A., Pyregov A.V., Shmakov R.G. Gestational sleep apnea. The relationship of pregnancy and preeclampsia with obstructive sleep apnea syndrome. Al'manakh klinicheskoi meditsiny/ Almanac of Clinical Medicine. 2019; 47(3): 266-75. (in Russian)]. https://dx.doi.org/10.18786/2072-0505-2019-47-031.
- Серов В.Н. Метаболический синдром (нейрообменно-эндокринный синдром). Medica mente. Лечим с умом. 2015; 1: 16-9. [Serov V.N. Metabolic syndrome (neuro-endocrine syndrome). Medica mente. 2015; 1: 16-9. (in Russian)].
- Хромылев А.В., Макацария А.Д. Ожирение, метаболический синдром и тромбофилия. Акушерство и гинекология. 2017; 10: 27-33. https://dx.doi.org/10.18565/aig.2017.10.27-33. [Khromylev A.V., Makatsariya A.D. Obesity, metabolic syndrome and thrombophilia. Obstetrics and gynecology. 2017; 10: 27-33. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.27-33.
- Ökdemir D., Hatipoğlu N., Kurtoğlu S., Siraz Ü.G., Akar H.H., Muhtaroğlu S., Kütük M.S. The role of irisin, insulin and leptin in maternal and fetal interaction. J. Clin. Res. Pediatr. Endocrinol. 2018; 10(4): 307-15. https://dx.doi.org/10.4274/jcrpe.0096.
- Романцова Т.И., Сыч Ю.П. Иммунометаболизм и метавоспаление при ожирении. Ожирение и метаболизм. 2019; 16(4): 3-17. [Romantsova T.I., Sych Yu.P. Immunometabolism and metainflammation in obesity. Obesity and metabolism. 2019; 16(4): 3-17. (in Russian)].
- Шепель Р.Н., Драпкина О.М. Новые векторы в диагностике метаболического синдрома: оценка уровня сосудистого эндотелиального фактора роста, пентраксина-3 и трансформирующего фактора роста бета. Кардиоваскулярная терапия и профилактика. 2019; 18(6): 57-61. [Shepel R.N., Drapkina O.M. New directions in metabolic syndrome diagnosis: assessment of vascular endothelial growth factor, pentraxin-3 and transforming growth factor beta levels. Kardiovaskulyarnaya terapiya i profilaktika/ Cardiovascular therapy and prevention. 2019; 18(6): 57-61. (in Russian)]. doi: 10.15829/1728-8800-2019-6-57-61.
- Echeverria C., Eltit F., Santibanez J.F., Gatica S., Cabello-Verrugio C., Simon F. Endothelial dysfunction in pregnancy metabolic disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2020; 1866(2): 165414. https://dx.doi.org/10.1016/j.bbadis.2019.02.009.
Received 08.10.2020
Accepted 31.01.2021
About the Authors
Igor S. Lipatov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.E-mail: i.lipatoff2012@yandex.ru. ORCID: 0000-0001-7277-7431. 443099, Russia, Samara, Chapaevskaya str., 89.
Yurii V. Tezikov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.
E-mail: yra.75@inbox.ru. ORCID: 0000-0002-8946-501X. 443099, Russia, Samara, Chapaevskaya str., 89.
Amir R. Azamatov, Resident at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.
E-mail: azamatov.amir@yandex.ru. ORCID: 0000-0003-0372-6889. 443099, Russia, Samara, Chapaevskaya str., 89.
Roman G. Shmakov, Dr. Med. Sci., Professor of the RAS, Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: mdshmakov@mail.ru. ORCID: 0000-0002-2206-1002. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Lipatov I.S., Tezikov Yu.V., Azamatov A.R., Shmakov R.G. Identity of preeclampsia and metabolic syndrome clinical manifestations: searching for substantiation.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 81-89 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.81-89



