Склеротический лихен (СЛ) – это хронический рецидивирующий воспалительный дерматоз неизвестной этиологии, который поражает как кожу, так и слизистую и локализуется преимущественно в генитальной области, хотя может формировать и экстрагенитальные очаги поражения. Данное заболевание неминуемо приводит к нарушению четырех основных функций слизистой вульвы: гидратации, эластичности, способности противостоять механическим воздействиям, проприоцептивной чувствительности. Большинство симптомов заболевания связаны с данными нарушениями, которые неуклонно прогрессируют и приводят к разрушению анатомических структур и формированию функциональных нарушений [1, 2].
Генитальный СЛ у женщин преимущественно поражает большие и малые половые губы, межгубную борозду, клитор и клиторальный капюшон, промежность и перианальную область, а участки поражения могут образовывать характерную форму «цифра 8» [1].
Ранние стадии заболевания проявляются в виде изменений местной чувствительности, чаще всего описываемых как зуд или жжение, чрезмерная чувствительность к прикосновениям – аллодиния. При осмотре могут наблюдаться побледнения на ограниченных участках слизистой оболочки. На данном этапе трудно установить клинический диагноз. На более поздних стадиях СЛ появляются стабильные трещины, происходит формирование атрофии и синехий малых половых губ, атрофии больших половых губ. На этом этапе субъективные симптомы связаны с такими нейросенсорными расстройствами, как зуд, жжение и аллодиния, независимо от половой активности. Формируется стеноз входа во влагалище, возможен уретральный стеноз, что приводит к нарушению мочеиспускания и апареунии.
Многие исследователи считают, что диагноз СЛ является клиническим [3]. Однако клиническая и даже гистопатологическая диагностика часто бывают затруднены, особенно на ранних стадиях заболевания, которое часто диагностируется как «неспецифический вульвит» [4]. Также свой вклад вносят различные подтипы заболевания [5], изменения слизистой из-за постоянного повреждения (цикл зуд–царапина), ассоциация с другими дерматозами вульвы (наиболее часто это хронический простой лихен), морфологические изменения, возникающие в результате применения лекарственных средств (в основном топических кортикостероидов) [6].
Клиническая диагностика СЛ и других вульварных дерматозов затруднена, в том числе из-за отсутствия методов прижизненной визуализации с объективной оценкой специфичных критериев. Эту проблему может решить новый метод мультимодальной оптической когерентной томографии (ОКТ), который обеспечивает неинвазивную прижизненную визуализацию тканей на глубину до 1,5 мм с пространственным разрешением 10–15 мкм [7, 8]. Данный метод позволяет оценить общее состояние слизистой вульвы, состояние эпителия, соединительной ткани и микроциркуляцию (крови и лимфы) без применения контрастирующих веществ [9, 10]. Метод ОКТ нашел применение в гинекологической практике для оценки структуры слизистой шейки матки [11, 12]. Поляризационно-чувствительный вариант технологии был запатентован как способ диагностики патологии шейки матки [13].
Мультимодальный вариант ОКТ, включающий оценку состояния слизистой оболочки в ко- и кросс-поляризации, а также микроциркуляторного русла кровеносных и лимфатических сосудов вульвы, демонстрируется впервые. Важно, что сосуды микроциркуляторного русла отвечают на изменения, происходящие в соединительной ткани при СЛ. Уже на начальных стадиях капиллярные петли расширены и перемещаются из вертикальной в горизонтальную ориентацию параллельно дермально-эпидермальному переходу, что, вероятно, связано с формированием зоны гиалиноза непосредственно под эпителием и потерей сосочков подслизистой основы. При длительно текущем СЛ папиллярное сплетение опускается вниз до уровня средней дермы [14]. Данный факт может лечь в основу ОКТ-ангиографических признаков СЛ.
Цель исследования – изучение возможности прижизненного метода мультимодальной ОКТ для задач диагностики СЛ вульвы.
Материалы и методы
Характеристика пациентов
Исследование проведено на базе I отделения гинекологии ГБУЗ НО «НОКБ им. Н.А. Семашко» (Нижний Новгород). Было обследовано 6 пациенток, которые разделены на 2 группы на основании гистологических критериев СЛ [4] после биопсии слизистой оболочки вульвы. Первая группа была контрольной, наблюдались 3 пациентки без видимых клинических и симптоматических критериев вульварных дерматозов, которые проходили лечение по поводу пролапса стенки влагалища. Во 2-й группе наблюдались 3 пациентки со СЛ на поздней стадии течения заболевания. Для проведения ОКТ-исследований были отобраны больные без выраженного гиперкератоза слизистой, который препятствует проникновению инфракрасного ОКТ-излучения.
Исследование одобрено этическим комитетом ФГБОУ ВО «ПИМУ» Минздрава России (протокол №2 от 29 января 2018 г.). ОКТ-исследования проведены с информированного согласия пациентов.
Мультимодальная ОКТ
Исследования слизистой вульвы проведено с помощью спектрального ОКТ-устройства [15], разработанного в Институте прикладной физики РАН (г. Нижний Новгород) [15, 16]. ОКТ-прибор использует инфракрасное излучение с длиной волны 1310±100 нм. ОКТ характеризуется высоким пространственным разрешением до 10–15 мкм на глубину 1–2 мм. 3D-массив данных получается в реальном времени в течение 26 секунд и имеет размер 3,4×3,4×1,25 мм3. ОКТ-прибор оснащен контактным оптическим зондом (длина 15 см, диаметр 1 см) (рис. 1). Методом мультимодальной ОКТ получали информацию о структуре слизистой вульвы (кросс-поляризационная ОКТ) (срез вглубь), а также о состоянии ее кровеносных (ОКТ-ангиография) и лимфатических (ОКТ-лимфангиография) сосудов (вид сверху). Визуализация кровеносных и лимфатических сосудов основана на анализе спектровой структуры и не требует применения контрастирующих веществ [17, 18]. Количественная оценка сосудистого компонента (кровеносных и лимфатических сосудов) проводилась путем подсчета плотности сосудов, которая рассчитывалась как отношение общей длины сосудов на ОКТ-изображении к площади всего изображения. Значение выражено в процентном отношении [19].
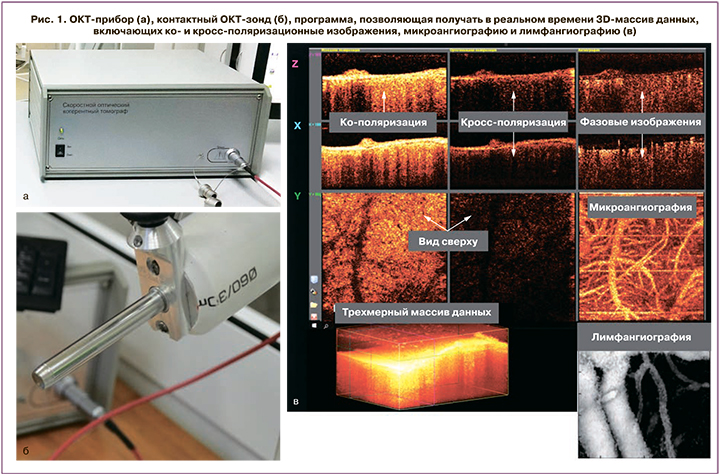
3D-ОКТ-данные были получены до взятия биопсии в том же самом месте, расположенном в центральной зоне на границе «внутреннего» и «среднего вульвоскопического кольца» [20] (симметрично справа и слева). Участок слизистой в месте проведения ОКТ исследования отмечался метиленовым синим для прицельного взятия биопсии.
Гистологический анализ
Гистологический анализ биоптатов слизистой вульвы проводился с целью подтверждения диагноза, оценки состояния эпителия, подслизистой основы и сосудов. Окраска препаратов производилась гематоксилином и эозином. Структура коллагеновых волокон изучалась с помощью окраски пикрофуксином по Ван–Гизону. Идентификация и изучение лимфатических сосудов слизистой вульвы осуществлялись иммуногистохимически с помощью антител на маркеры эндотелиоцитов лимфатических сосудов Podoplanin. Подсчет сосудов осуществлялся непосредственно под эпителием на глубину до 500 мкм в 10 полях зрения на каждый препарат. Диаметр поля зрения составлял 500 мкм (объектив 40×, окуляр 10×/20 мм).
Гистопатологическое исследование проводилось с использованием микроскопа Leica DM 2500 (Leica Microsystems, Германия) c CCD-камерой DFC 245 C.
Биопсийный материал забирался из участков слизистой, расположенной до линии Харта, который соответствует высокогликогенированному многослойному плоскому эпителию, непосредственно после ОКТ-исследования. Гистологический анализ биопсийного материала проводился тремя патологоанатомами независимо с последующим анализом описания и постановкой диагноза.
Статистический анализ
Статистическую обработку данных проводили с помощью программы SPSS Statistics с использованием непараметрического критерия Манна–Уитни. Результаты представляли как среднее значение ± стандартное отклонение.
Результаты и обсуждение
Гистологическое описание слизистой вульвы в норме (рис. 2, верхний ряд)
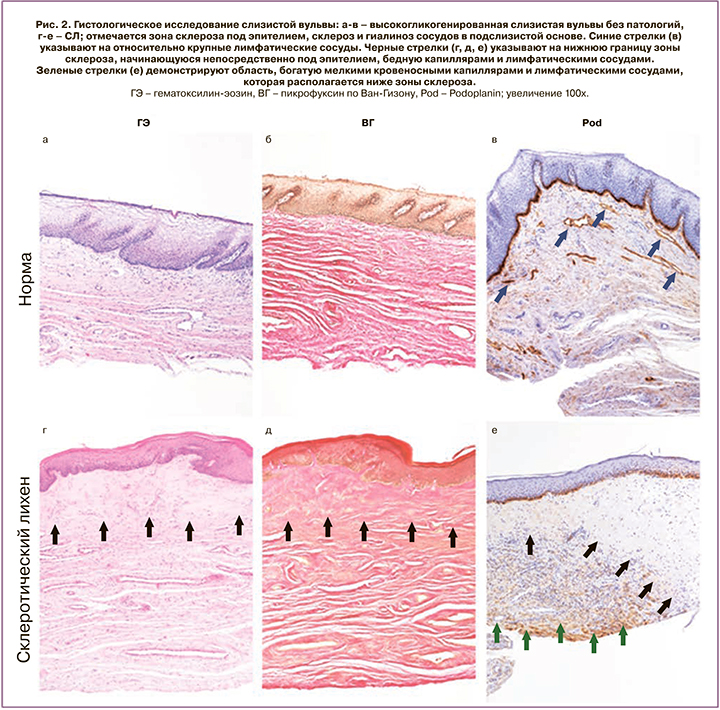
Слизистая вульвы в норме представляет собой неороговевающий многослойный плоский эпителий, богатый гликогеном (рис. 2а). По данным гистологического исследования было продемонстрировано, что толщина эпителия варьировала от 100 до 300 мкм за счет хорошо развитых эпителиальных сосочков, эпителий высокогликогенированный, не ороговевает. В подслизистой основе наблюдалось много капилляров и лимфатических сосудов (окраска Podoplanin), непосредственно под эпителием. Так, количество кровеносных сосудов в неизмененной слизистой вульвы составило 11±2,94, а лимфатических – 3,6±1,26 в поле зрения.
При окрашивании по Ван–Гизону наблюдались хорошо развитые, тонкие коллагеновые волокна.
ОКТ-визуализация слизистой вульвы в норме (рис. 3)
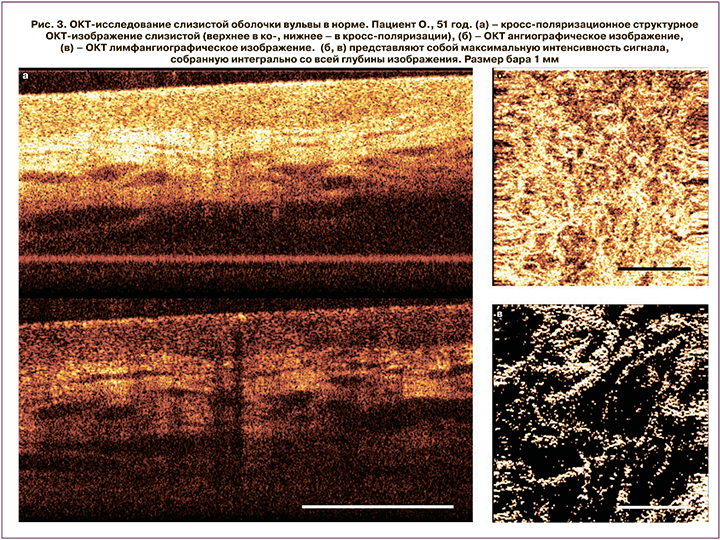
В норме слизистая оболочка вульвы на структурных ОКТ-изображениях демонстрирует слоистую структуру (рис. 3а). Эпителий на ОКТ-изображениях имеет низкую интенсивность сигнала и контрастную четкую границу с собственной пластинкой слизистой. Толщина эпителия равномерная на всем протяжении. Собственная пластинка слизистой характеризуется высокоинтенсивным ОКТ-сигналом. Подслизистый слой имеет высокий, однако менее интенсивный, чем в собственной пластинке слизистой ОКТ, сигнал, быстро затухающий в глубину. В подслизистом слое визуализируются крупные включения с низким ОКТ-сигналом неправильной формы, которые в соответствии с гистологическими данными являются лимфатическими сосудами. Наличие большого количества лимфатических сосудов в подслизистом слое подтверждено иммуногистохимическим окрашиванием (рис. 2в). ОКТ ангиографические изображения демонстрируют плотную сеть кровеносных сосудов (рис. 3б), представленную как сосудами капиллярного типа, так и более крупными артериолами и венулами. Плотность кровеносных сосудов по данным ОКТ составила 3,9±0,23%, плотность лимфатических сосудов – 3,7±0,54% площади ОКТ- изображения.
На ОКТ/лимфангиографических изображениях в норме визуализируется сеть лимфатических сосудов, представленная преимущественно относительно крупными сосудами (рис. 3в), что также демонстрирует иммуногистохимия (рис. 2в, синие стрелки).
Гистологическое описание слизистой вульвы при СЛ (рис. 2, нижний ряд)
Эпителий, как правило, атрофичный, отсутствует гликоген в кератиноцитах, толщина его равномерна (около 65 мкм), эпителиальные сосочки уплощены или отсутствуют в результате склероза, гиалиноза и отека стромы. Наблюдается резко выраженный ортокератоз. Клетки базального слоя вакуолизированы, что свидетельствует о гидропической дистрофии. В субэпителиальной зоне стромы имеется широкая полоса склерозированной ткани. На ее границе со средней третью подслизистой основы имеются массивные очагово-диффузные лимфогистиоцитарные инфильтраты, которые иногда содержат эозинофилы. Склерозированная и гиалинизированная область содержит малое количество сосудов; так, количество кровеносных капилляров составило 3,5±1,43 в поле зрения. Лимфатические сосуды отмечаются в единичных экземплярах, спавшиеся, среднее значение – 0,7±0,82 в поле зрения (рис. 2д). В средней трети подслизистой, ниже зоны гиалиноза, располагаются экстатические капилляры и лимфатические сосуды в большом количестве (рис. 2е, зеленые стрелки).
Однако наравне с классической картиной СЛ, которая описана выше, присутствуют образцы, которые имеют эпителий неравномерной толщины (132–275 мкм) с формированием акантоза, нарушением стратификации клеточных слоев. При этом сохраняются участки гликогенированного эпителия, а гидропическая дистрофия отсутствует. Изменения в подслизистой основе аналогичны классической форме. Данный вариант описан в литературе как гипертрофический СЛ и не является редкой находкой [7].
ОКТ-визуализация слизистой вульвы при СЛ (рис. 4)
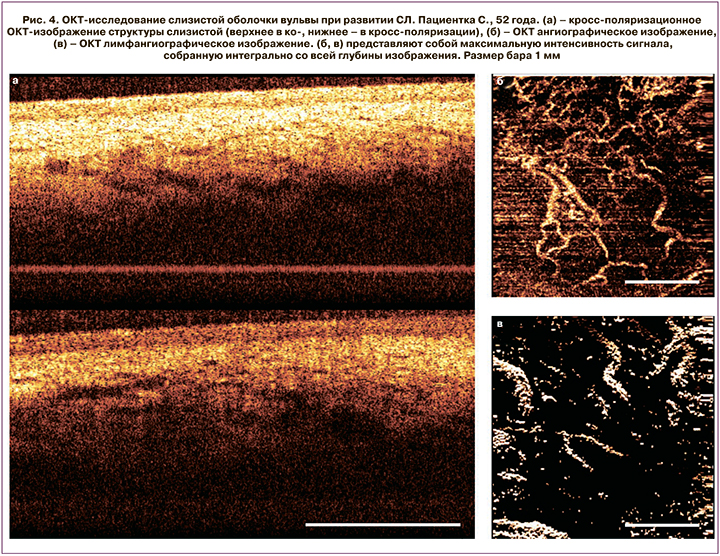
Слоистая структура на кросс-поляризационных ОКТ-изображениях сохраняется даже при поздней стадии СЛ, за исключением случаев с сильно выраженным ороговением. Такие случаи были исключены из исследования ввиду низкой информативности метода ОКТ. Эпителий в случае СЛ имеет уровень ОКТ сигнала выше, чем в норме, из-за чего граница с собственной пластинкой слизистой становится менее контрастной, однако остается по-прежнему четкой и ровной. Толщина эпителия существенно меньше по сравнению с нормальным эпителием. В подслизистом слое визуализируются преимущественно мелкие включения с низким ОКТ-сигналом, однако сигнал включений выше, чем в случае нормы, а потому они выглядят менее контрастно. ОКТ ангиографические изображения демонстрируют заметное снижение количества кровеносных сосудов по сравнению с нормальным состоянием слизистой. Плотность кровеносных сосудов в области гиалиноза достоверно снизилась и составила 2,5±0,79% против 3,9±0,23% в норме (р=0,0003). Плотность сосудистой сетки неравномерна по всей площади изображения. Встречаются аваскулярные участки (рис. 4б). Одной из возможных причин снижения плотности сосудистой сетки является периваскулярный склероз стенок сосудов, приводящий к сужению просвета. ОКТ/лимфангиографические изображения также демонстрируют достоверное снижение количества лимфатических сосудов, их плотность составила 1,7±0,75% против 3,7±0,54% в норме (р=0,02). Гистологически подтверждено достоверно сниженное количество как кровеносных (3,5±1,43 против 11±2,94 (р=0,003) в поле зрения в норме), так и лимфатических сосудов в зоне склероза и гиалиноза коллагеновых волокон (0,7±0,82 против 3,6±1,26 (р=0,0003) в поле зрения в норме), которое имеет место в собственной пластинке слизистой и подслизистой основе.
Заключение
Впервые была продемонстрирована взаимосвязь между гистологическими особенностями и ОКТ-картиной слизистой вульвы в норме и при СЛ. Метод мультимодальной ОКТ с функциями ко- и кросс-визуализации, ангиографии и лимфангиографии обеспечивает объективную визуализацию гистологических изменений эпителия, подслизистой основы, а также сосудов микроциркуляторного русла (как лимфатических, так и кровеносных) в очагах локализации СЛ. Данный прижизненный метод визуализации не травматичен, не инвазивен, в отличие от биопсии, следовательно, снижает шанс спровоцировать формирование новых очагов поражения в результате феномена Кебнера. Метод одновременно обеспечивает оценку слизистой оболочки в реальном времени, что может быть использовано для выявления ранних форм СЛ, дифференциальной диагностики вульварных дерматозов, оценки тяжести и глубины поражения, контроля состояния во время лечения.



