Novel coronavirus infection (covid-19): guiding principles for obstetric care under pandemic conditions
The literature review analyzes the latest data on the principles of diagnosis and treatment of the novel coronavirus infection, management of pregnancy, childbirth, and the postpartum period during a pandemic, as well as the benefits and risks of breastfeeding with confirmed COVID-19 infection on the basis of the clinical guidelines of the Russian and international professional associations and the results of several basic studies and clinical trials.Ignatko I.V., Strizhakov A.N., Timokhina E.V.,Denisova Yu.V.
In late 2019, the global medical community faced the new infection transmitted through airborne droplets. Being initially identified as one of the acute respiratory viral infections, the novel coronavirus infection was included in the list of diseases that pose a risk to others on January 31, 2020. A little later, on March 11, 2020, the World Health Organization (WHO) stated that the infection reached the level of a pandemic. The high-risk group includes the elderly, patients with severe concomitant diseases and immunodeficiency, as well as pregnant women with altered immune responsiveness. At the same time, the pregnancy status restricts the use of some methods for instrumental diagnosis and anti-coronavirus therapy. An obstetrician-gynecologist is responsible not only for the health of an infected woman, but also for that of her unborn child, therefore the specialist must know all the transmission routes of infection, follow epidemiological precautions, be able to identify early symptoms of the disease, perform thorough clinical assessment and provide timely adequate anti-coronavirus therapy. The management of women during pregnancy, childbirth, and postpartum should be carried out according to the potential risk of this infection. The paper provides useful algorithms for actions during the initial hospital admission of a pregnant woman, her further examination and treatment.
Keywords
Introduction
Coronavirus strains have been identified and studied for decades, but it is SARS-CoV-2 that has caused such serious complications and high mortality and resulted in the disease that reached the scale of a pandemic. A new coronavirus infection is a challenge for doctors of all specialties, and it is necessary to be totally prepared to respond to the challenge. The most relevant domestic and foreign data on etiology, epidemiology, variants of clinical course and possible preventive measures of the infection are presented below. The paper provides action plans for pregnant women and doctors from the moment of confirming pregnancy to breastfeeding with its advantages and disadvantages in patients diagnosed with COVID-19. Special attention is paid to the issues of diagnosis, therapy and management of childbirth in such patients.
Etiology
The novel coronavirus causing COVID-19, subsequently named SARS-CoV-2 originates from the betacoronavirus genus containing RNA. It was originally registered in the Wuhan city of Hubei province in the People’s Republic of China. To date, there are seven known strains of coronavirus that affect people; four of them (229E, NL63, OC43 and HKU1) cause only mild and moderate upper respiratory tract diseases, the remaining three strains (MERS-CoV, SARS-CoV and SARS-CoV-2) belong to pathogenicity group II and can lead to the development of middle East (MERS-CoV) and severe acute respiratory syndromes (SARS-CoV).
Epidemiology
The first strain of the coronavirus family, namely infectious bronchitis virus (IBV), which is now known as avian coronavirus, was discovered in 1931. Before 2002 scientists revealed human coronaviruses 229E, NL63, OC43 and HKU1 that are constantly present in the morbidity pattern of acute respiratory viral infection (ARVI) and rarely cause death. At the end of 2002, a new coronavirus strain SARS-CoV, a causative agent of atypical pneumonia, was identified. Bats are the most likely to be the reservoir for this strain, while camels and Himalayan civets are intermediate hosts. This strain caused an epidemic in 37 countries with the registration of more than 8,000 cases of the disease including 774 deaths.
In 2012, a new strain of coronavirus MERS-CoV, a causative agent of middle East respiratory syndrome, was revealed. Camels are implicated as the reservoir of this virus and its endemic territory is the Arabian Peninsula. New cases of the disease are still registered, some of them causing death.
The first cases of the new coronavirus SARS-CoV-2 were registered in December 2019. To date, the number of infected worldwide has almost reached 3.5 million people, the number of deaths has risen above 230 thousand.
The routes of transmission include direct transmission, such as cough, sneeze, droplet inhalation transmission, and contact transmission, such as the contact with oral, nasal, and eye mucous membranes. Infection may also be transmitted via fecal–oral transmission route. Vertical transmission of the virus is not excluded: in the neonatal period IgM antibodies to SARS-CoV-2 were detected in the blood plasma of infants born to infected mothers [1, 2]. Analysis of nasopharyngeal aspirate obtained from newborns also showed a positive result for SARS-CoV-2. Since IgM antibodies do not pass through the placenta, their detection may indicate the development of an immune response as a result of intrauterine infection, however, postnatal infection cannot be excluded. All studies of amniotic fluid, placenta, cord blood, genital secretions, and breast milk were negative for SARSCoV-2 [3–6].
The population has been highly susceptible to this pathogen. The source of infection is virus carriers. The pathogen may be detected in blood plasma, substances from the respiratory tract, feces and fomites (inanimate objects contaminated with a viral strain, for example, patient’s belongings, etc.).
There is no evidence of an increased risk for infection during pregnancy, however, changes in the immunological reactivity in pregnant women can lead to more severe forms of the disease in such patients.
Clinical Picture
The clinical picture of the disease is dominated by the symptoms of a cold and mild or moderate flulike symptoms. The most typical of these are cough, fever, headache, shortness of breath, and anosmia. Among pregnant women, there were cases of atypical clinical picture of coronavirus infection which was not accompanied by fever or leukocytosis [7]. The severity of symptoms is of key importance in the third trimester of pregnancy, although the absolute risk of severe complications is low [8]. Elderly patients, people with immunodeficiency, chronic diseases, and oncological pathologies are at greater risk for complications such as pneumonia and severe respiratory failure [9]. The most common obstetric complications in pregnant women are preterm birth, premature rupture of membranes (RPOM) and preeclampsia [10]. In case of confirmed COVID-19, there is a higher percentage of cesarean section due to the development of fetal distress syndrome [11]. There is no data on an increased risk of miscarriage or early pregnancy loss in patients infected with COVID-19.
The National Institutes of Health in the USA have proposed the following classification of the COVID-19 infection according to its severity [12]:
- asymptomatic infection: individuals who test positive for SARS-CoV-2 but have no symptoms;
- mild illness: individuals who have any of various signs and symptoms (for example, fever, cough, sore throat, malaise, headache, muscle pain) without shortness of breath, or abnormal imaging;
- moderate illness: individuals who have evidence of lower respiratory disease by clinical assessment or imaging and a saturation of oxygen (SpO2) >93%;
- severe illness: individuals who have respiratory rate (RR) >30 breaths per minute, SpO2 ≤93%, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300, or lung infiltrates >50%;
- critical illness: individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction
P. Karami, M. Naghavi, A. Feyzi et al. (2020) published a clinical case of stillbirth in a 27-year-old patient with intrauterine COVID-19 infection [13]. The patient was admitted to the obstetric department at 33 weeks of gestation; a few hours after stillbirth, the patient died due to acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and septic shock, despite active therapy provided according to treatment protocols of ARDS.
Pregnant women may experience blood thickening due to physiology of pregnancy. Since coagulopathy is observed in COVID-19 patients, the risk of developing venous thromboembolism (VTE) increases during pregnancy. The increased risk of VTE is also caused by lack of movement during self-isolation [14].
Prevention
The main preventive measures against the coronavirus infection in pregnant women are maintaining social distance and self-isolation. All vulnerable groups of the population including pregnant women are recommended to avoid contact with people infected with coronavirus or those having its characteristic symptoms [15], individuals and families with symptoms of prolonged cough and fever [16]. These recommendations are also relevant for people who are at high risk of infection, namely pregnant women with congenital or acquired diseases of the cardiovascular system, cancer patients, people with severe respiratory diseases (cystic fibrosis, severe persistent asthma), rare diseases and congenital metabolic disorders (sickle cell anemia, etc.) [17]. Strict adherence to the above measures is especially important after the 28th week of pregnancy.
Prenatal care
Prenatal visits to health facilities should be minimized. Antenatal care registration after establishing pregnancy should be done by phone or video, faceto-face consultations are recommended at 12–13 and 20–22 weeks of gestation [20]. If a pregnant woman or members of her family show the symptoms of the coronavirus infection, it should be reported to the women’s consultation or maternity department. In case of symptoms progression and if the symptoms are not persistent (except prolonged cough), the planned consultation should be postponed for 7 days from the moment of their appearance. If members of the family have characteristic symptoms, the planned consultation of a pregnant woman who is in self-isolation should be postponed for 14 days. In case of the delay of the planned consultation for 3 weeks, an emergency consultation should be scheduled. In order to minimize the risk of infection of a pregnant woman during a doctor’s consultation, it is essential to strictly observe the precautions against airborne transmission of infection using personal protective equipment (PPE). Pregnant women with concomitant pathologies should be provided with masks during their visits to health facilities. Algorithm for actions on admission of a pregnant woman to the inpatient department is presented in Fig. 1.
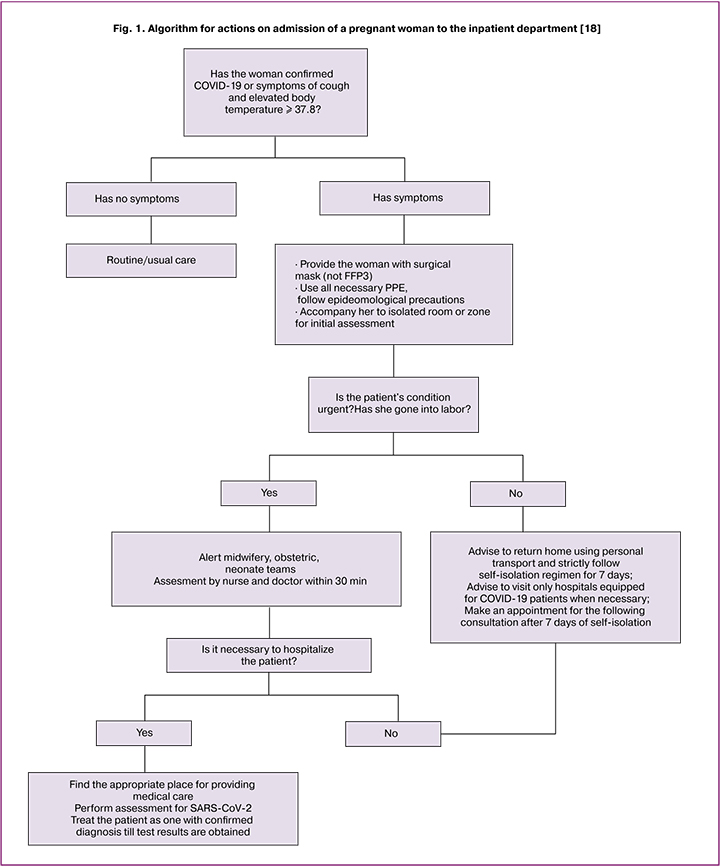
When pregnancy is established, it is necessary for doctors to find out the information about the woman’s smoking habit and recommend her to give up smoking for the whole period of pregnancy. According to the studies of W.J. Guan, Z.Y. Ni, Y. Hu et al. (2020), smokers are 1.4 times more likely to develop severe COVID-19 symptoms than non–smokers; transfer to the intensive care unit, connection to a ventilator and fatal outcome are 2.4 times higher in smokers [9]. The increased incidence may be due to the negative effect of smoking on the human immune system, other mechanisms remain understudied [21]. If family members of a pregnant woman are smokers they should be offered nicotine replacement therapy to eliminate secondhand smoking. During consultations it is important to inquire about a woman’s mental state; since isolation, financial difficulties, feelings of insecurity, and inability to obtain support are risk factors for developing mental disorders. Self-isolation appears to result in more frequent episodes of domestic violence. One should remember that the main symptom of COVID-19 is fever; maternal fever during early pregnancy may be associated with skull or face defects, heart defects, and neurological dysfunctions in the infant.
P. Dashraath, J. L. J. Wong, M. X. K. Lim, et al. (2020) performed the assessment of the data on 84 women who had the coronavirus infections during pregnancy (COVID-19 in 55 women, SARS in 17 women, MERS in 12 women). They analyzed the outcomes of these infections for mother and child. Maternal mortality was noted in 0%, 18%, 25% of cases, respectively; ventilation was necessary in 2%, 35%, 41% of cases; abortion/stillbirth was in 2%, 25%, 18% of cases; intrauterine growth retardation was revealed in 9%, 13%, 9% of cases; pre-term delivery was noted in 43%, 25%, 27% of cases, neonatal death was identified in 2%, 0%, 9% of cases, respectively.
Pregnant women are not recommended to refuse antenatal screening during the pandemic, since the risk of increased maternal mortality, stillbirth and other adverse perinatal outcomes significantly outweighs the risk of infection [22, 23]. The patients should be provided distance planned consultations. If a pregnant woman with the confirmed novel coronavirus infection shows preeclampsia, magnesium sulfate should be used carefully, since respiratory depression is one of the side effects of this drug. In severe preeclampsia, the intravenous infusion of 4 g of magnesium sulfate is given for 15–20 minutes and followed by an intravenous administration of a maintenance dose of 1 g every hour [24]. Maternal and Fetal Medical Society suggested that patients with mild degree of respiratory failure (RF) should be administered a single bolus dose of 4 g of magnesium sulfate instead of the standard dosage. In cases of moderate to severe RF, as well as mild preeclampsia, magnesium therapy should be abandoned [25].
Diagnostics
Public Health England (PHE) of the Department of Health and Social Care in the United Kingdom defines the following criteria for taking a nasopharyngeal swab with subsequent analysis for SARS-CoV-2 in pregnant women who are hospitalized or newly admitted to hospital:
- clinical/radiological signs of pneumonia;
- acute respiratory distress syndrome (ARDS)
- fever ≥ 37.8º C and at least one of the following symptoms: prolonged severe cough, hoarseness of voice, rhinorrhea/nasal congestion, shortness of breath, sore throat, wheezing, sneezing.
According to the recommendations of Royal College of Obstetricians and Gynecologists (Great Britain) for the management of pregnant women with the coronavirus infection, the patients who have fever ≥ 37.8º C without any other symptoms should also be examined [18]. SARS-CoV-2 screening may be recommended to all pregnant women with lower respiratory tract diseases who have travelled by public transport or had a close contact with a person with the confirmed or suspected COVID-19 diagnosis. The examination includes complete blood count (CBC) and a nasopharyngeal swab followed by RT-PCR assays for the detection of SARSCoV-2 RNA. In the presence of symptoms, a negative RT-PCR result does not rule out COVID-19 diagnosis due to a large percentage of false negative results. In this case, WHO recommends to perform RT-PCR for samples obtained from the lower respiratory tract (for example, induced sputum, bronchoalveolar lavage) when possible [26]. Detection of leukopenia in any patient is also an indication for SARS-CoV-2 screening. If a patient experiences leukocytosis accompanied by normal or reduced number of lymphocytes, the bacterial infection should be considered as the cause of pyrexia and antibacterial therapy should be prescribed. Indications and algorithm for assessment of a pregnant woman for SARS-CoV-2 are presented in Fig. 2.
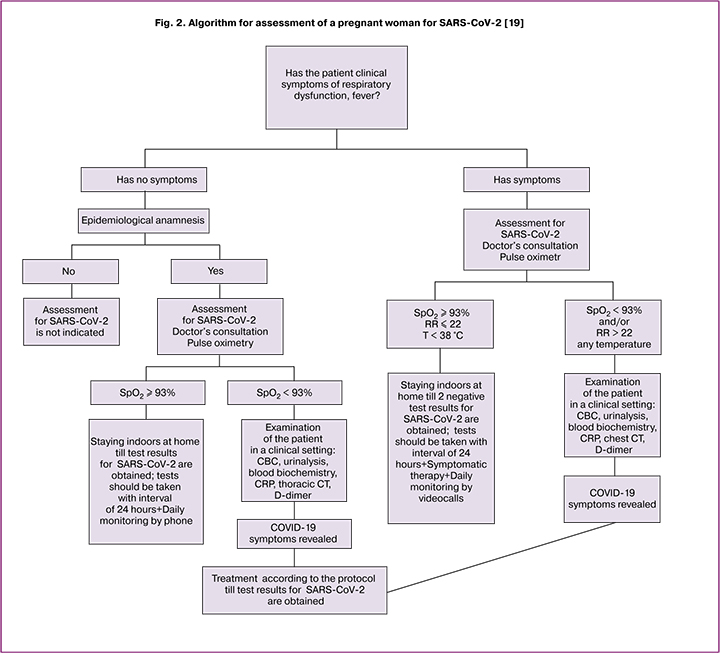
Before obtaining the results of the SARS-CoV-2 test, the patient should receive the treatment administered for the patient with a confirmed coronavirus infection. Chest CT has the highest sensitivity for detecting COVID-19, moreover, the radiation dose to the fetus is low. Thus, this method for diagnosing can be considered as the main one for pregnant women during the pandemic [7, 27]. Patients with RF signs are recommended to perform echo CT due to the possible risk of periportal cardiomyopathy [28].
If the coronavirus infection is moderate to severe and there is a high risk of perinatal complications before 12 weeks of gestation, it is recommended to terminate pregnancy after curing the infection. In case of refusal to terminate pregnancy at 12-14 weeks, a chorionic villus or placenta biopsy should be performed to exclude fetal pathology, and amniocentesis should be performed at 16 weeks to detect chromosomal abnormalities in the fetus [19].
Treatment
Women with suspected COVID-19 and mild to moderate symptoms, as well as patients with a confirmed mild form of the disease, should be treated as outpatients strictly following the regimen of self-isolation under the supervision of a district therapist and an obstetriciangynecologist from the women’s consultation. Routine instrumental evaluation and laboratory studies performed in the normal course of pregnancy should be postponed for the period of self-isolation. In high-risk pregnancies, the need for examination and testing should be considered by specialists individually in each case (Table).
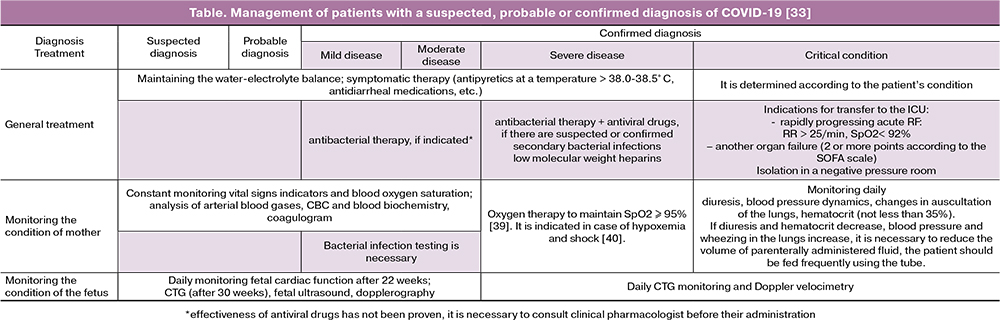
Etiotropic therapy for COVID-19 has not been developed for pregnant women, women in labor, and women who have recently had a baby. Ribavirin, recombinant interferon beta-1b and antimalarial drugs are strictly contraindicated during pregnancy. It is possible to use antiviral drugs taking into account their effectiveness against SARS-CoV-2. When antiviral therapy is prescribed, the decision about breastfeeding depends on the severity of the symptoms and the presence of complications. Timely administration of antiviral drugs is recommended. Patients can be prescribed a combination of lopinavir and ritonavir orally when the potential benefit to the mother outweighs any potential risk to the fetus. The preparations are administered according to the following scheme: 400 mg of lopinavir and 100 mg of ritonavir every 12 hours for 14 days. If the preparation cannot be taken orally, the medicines should be prescribed in the form of suspension at a dose of 5 ml and taken through a nasogastric tube.
The use of remdesivir turns out to be promising since it is a nucleotide analog that has shown its effectiveness against SARS-CoV-2 under laboratory conditions [29] and against other human coronavirus strains, including MERS-CoV and SARS-CoV, both in vitro and in animal models [30]. When it was used in pregnant women in the treatment of Ebola and Marburg hemorrhagic fevers, no embryotoxicity was detected [31].
Pathogenetic therapy involves administration of antipyretic drugs. Paracetamol can be prescribed at doses of 500–1000 mg up to 4 times per day (not more than 4 g/day). In case of oral rehydration, infusion therapy should be administered; the fluid is taken gradually, 250–500 ml at a time, since the transfusion of fluid in large volume can worsen blood oxygen saturation and cause ARDS exacerbation [32].
If the patients experience such severe symptoms as intoxication, abdominal discomfort, nausea, vomiting, they are prescribed intestinal sorbents.
During the epidemic in China, IL-6 receptor inhibitors appeared to be effective in severe RDS; however, tocilizumab, the drug that is best known among them, is contraindicated during pregnancy, so their use should be considered only after delivery. The drugs of this group are the most effective when they are taken within a period from 8 to 14 days after onset of the disease [33].
Patients with severe disease are recommended to take low-molecular-weight heparins to reduce the risk of thromboembolic complications, if they do not have contraindications [33]. In conditions of self-isolation, pregnant women should lead an active lifestyle to exclude hypodynamia.
As symptomatic therapy, it is possible to use isotonic saline solutions and nasal decongestants for rhinitis or rhinopharyngitis (hypertonic saline solutions are used for nasal congestion), mucolytics (ambroxol) and bronchodilators are applied in the case of bronchitis (combination of phenoterol and ipratropium bromide, in the first trimester, salbutamol may be used with a mesh nebulizer). Adequate respiratory support is important: SpO2 should not decrease below 95%.
Contraindications to aspirin in low doses were not revealed in pregnant women with confirmed coronavirus infection. Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in patients diagnosed with COVID-19 in severe and critical condition [34]. Among tocolytics, nifedipine should be preferred. In cases with pregnant women in critical condition, when preterm delivery is necessary, the use of corticosteroids for the prevention of RDS development in the fetus may lead to further deterioration of the patient’s condition [35, 36].
Regional analgesic techniques (TAP block, QL block) in infected patients are preferable in the prenatal and postpartum period. The use of narcotic analgesics should be abandoned due to the risk of hypoventilation [37].
Antibacterial therapy. In complicated forms of the disease, intravenous antibacterial therapy should be initiated within the first 2–3 hours after hospitalization [19]. The choice of an antibacterial drug depends on the form of pneumonia: in secondary viral and bacterial pneumonia, the first-line therapy is the third-generation cephalosporins or protected aminopenicillins with or without macrolides; in tertiary bacterial pneumonia, the patients are prescribed the fourth-generation cephalosporins with or without macrolides, carbapenems, vancomycin, linezolid. Tetracyclines, fluoroquinolones and sulfonamides are contraindicated during pregnancy. Due to the fact that during previous outbreaks of the coronavirus infection there was a high incidence of Staphylococcus aureus infection, some patients (who have permanent intravenous catheter, recent short-term surgical interventions, dialysis) should be prescribed antistaphylococcal antibiotics (ceftaroline fosamil, linezolid, etc.) in combination with azitromycin (intravenously) or respiratory fluoroquinolone (intravenously) [33].
If there is an increased risk of pseudomonas infection (after prolonged administration of glucocorticosteroids, recent administration of systemic antibiotics, cystic fibrosis, secondary bronchiectasis), a combination of a β-lactam antibiotic with antipseudomonal activity (piperacillin/tazobactam, imipenem/cilastatin, meropenem , etc.) with ciprofloxacin or levofloxacin should be prescribed. Alternatives to ciprofloxacin and levofloxacin are second- and third-generation aminoglycosides and macrolides or respiratory fluoroquinolones. In severe cases, antibiotics are administered intravenously.
A promising method of therapy is the use of convalescent plasma, pathogen-reduced plasma, or plasma from recovered patients, which is based on the principles of passive immunization according to the recommendations of WHO [38]. Protocols for the use of convalescent plasma are currently under development.
The criteria for discharging the patient with a laboratory-confirmed diagnosis of COVID-19 from the hospital are the absence of clinical manifestations of the disease (the patient must have normal body temperature for 3 days), normal laboratory finding, and negative result of laboratory tests for SARS-CoV-2 RNA which is done twice with interval of at least one day [19]. After hospital discharge, the recovered patients should continue taking low-molecular-weight heparins for 10 days. It is recommended to proceed routine examinations 14 days after termination of the acute phase of the disease in the absence of urgent cases. The patient should not refuse an ultrasound examination. There is no direct link between coronavirus strains infection and the development of fetal pathology. However, according to a number of researchers, two thirds of pregnant women carrying the SARS-CoV virus strain were diagnosed with fetal growth retardation; there was a description of a clinical case of placental abruption in a pregnant woman infected with MERS-CoV. Thus, some specialists recommend ultrasound examination to assess the dynamics of fetal growth and amniotic fluid volume, as well as umbilical artery Doppler screening [41–43].
Intrapartum care
In order to rationalize the work of obstetric hospitals during pandemic, it is necessary to reduce the incidence of labor induction [44] or perform it in an outpatient setting when emergency transportation to the hospital is possible; fetometry should be minimized as well if there are no certain indications to perform it according to the current recommendations. At the onset of the disease, the planned induction of labor should be postponed until clinical indicators improve. For labor induction in a hospital setting, the simultaneous use of two methods should be considered (for example, mechanical method + misoprostol or mechanical method + oxytocin) to speed up this procedure.
In the latent phase of labor, women with mild symptoms of COVID-19 should be at home. All women with a suspected, probable and confirmed diagnosis of coronavirus infection should be admitted to tertiary obstetric hospitals equipped with facilities for effective isolation and PPE. Patients with severe and critical forms of COVID-19 should be isolated in negative pressure rooms, if hospitals are furnished with such rooms.
The woman should be warned about the potentially high risk of infection of the fetus during the intranatal period [3]. To prevent transmission of coronavirus, the number of visitors to the prenatal and postpartum wards should be limited. The number of staff present at birth should be kept to a minimum. Personnel must be equipped with PPE and observe all epidemiological precautions.
Epidural anesthesia is recommended to women in labor with severe comorbidities, suspected or confirmed COVID-19 diagnosis. In case of administering endotracheal anesthesia, medical personnel must be equipped with PPE due to the increased risk of aerosolization. Intravenous anesthesia is not recommended due to the risk of hypoventilation. Some authors believe that the presence of a patient’s healthy partner at birth should be encouraged due to the proven positive effect of his support on the condition of the woman in labor [45]. The partner can be present provided that he has no symptoms of COVID-19, he has not contacted with COVID-19 infected people or diagnosed coronavirus infection for 14 days. If the partner has had such symptoms as fever, prolonged severe cough, rhinorrhea, nasal congestion, shortness of breath, sore throat, wheezing and sneezing in the last 7 days, he is not allowed to visit the maternity department [46]. If a pregnant woman has a suspected and confirmed infection, partner’s presence at delivery is excluded. Partner’s support of a woman in labor by videocall could be considered.
In cases of suspected or confirmed COVID19, the use of baths in delivery rooms is not recommended due to the increased risk of fecaloral transmission of the virus.
The mode of delivery does not depend on the presence or absence of infection. Cesarean section is preferable, if it is impossible to eliminate hypoxia associated with ventilation and/or RF, development of pulmonary alveolar edema and in case of refractory septic shock [19]. It is recommended to perform cesarean section in non-intubated patients with pneumonia after 32–34 weeks gestation, since tracheal intubation accompanied by RF progression may cause obstetric complications. Pregnant women with suspected and confirmed diagnosis of COVID-19 are recommended to postpone planned cesarean section until a negative test result or recovery (at least 7 days after the onset of the disease) to minimize the risk of postnatal infection. At 20-23 weeks gestation, emergency cesarean section is performed in order to save the life of the mother, and after 24 weeks it is done for saving the lives of both mother and fetus.
According to the data of R. Chen, Y. Zhang, and L. Huang (2020), hypotension developed in more than 85% of infected women in labor after epidural anesthesia for cesarean section [47]. All patients with a confirmed diagnosis of COVID-19 are recommended intravenous injections of norepinephrine or phenylephrine for the prevention of hypotension during cesarean section [48].
In case of spontaneous onset of labor, vaginal delivery is preferable during the peak of the disease [33].
During childbirth women infected with COVID-19 should undergo pulse oximetry and have respiratory rate measured every hour. Blood oxygen saturation should not be lower than 94%, otherwise oxygen therapy should be used. It is worth noting that young women leading an active lifestyle are able to maintain oxygen saturation at a high level which can be followed by a sudden development of decompensation. Decompensation is characterized by the following signs: increased oxygen demand or oxygen concentration in the inhaled mixture > 40%, respiratory rate > 30/min, oliguria, sleepiness (SpO2 values are normal). Continuous electronic fetal monitoring (CTG) is necessary.
If these patients show the progression of RF which is accompanied by severe preeclampsia, magnesia therapy should be discontinued [48].
Delayed ligation of the umbilical cord is not recommended [19]. Cord blood collection for banking can be performed normally, since the risk of COVID-19 transmission with cord blood has not been documented. Abortion material and placenta should be disposed of as potentially infected tissues.
Postpartum care
If a child is born to mother diagnosed with coronavirus, a medical and nursing team should be ready in advance for the newborn; smears from the pharynx, oropharynx, as well as (according to indications) aspirate from the tracheobronchial tree, blood and stool should be taken immediately after transportation from the delivery room and on day 3 [33]. A child is considered potentially infected if coronavirus is confirmed in the mother within the period of 14 days before birth and 28 days after birth, or if the mother was in self-isolation after the contact with an infected person. All items and care products for each infant should be individual. Personnel should wear PPE and observe epidemiological precautions. The American Academy of Pediatrics suggests bathing the baby as soon as possible after birth to remove viral agents which are potentially present on the skin [49]. The mother and infant should be isolated in separate wards for a period of 14 days. Then the condition of the mother and fetus is assessed:
- the severity of COVID-19 symptoms in the mother and their presence in the infant are evaluated;
- the mother’s temperature and respiratory rate are measured, pulse oximetry is performed (in mild form of the disease - every 4 hours within 24 hours after vaginal birth and 48 hours after cesarean section, in moderate and severe forms of the disease - every hour for 24-48 hours after birth); the risk of developing thromboembolic complications is assessed (in case of increased risk, the first dose of low-molecularweight heparins should be administered as soon as possible after birth; in case of regional anesthesia, it is administered not earlier than 4 hours after the last spinal injection or removal of the epidural catheter); • women with a suspected or confirmed diagnosis of COVID-19 take low-molecular-weight heparins as a thromboprophylaxis for at least 10 days;
- fetal cardiotocography is performed;
- further patient’s management is discussed by a multidisciplinary team with the participation of an infectious disease specialist;
- all women should be screened for postpartum depression 4-8 weeks after delivery, using, for example, the Edinburgh Postnatal Depression Scale.
An infected mother and a healthy child should be separated until:
- the patient is apyrexial within 72 hours without the use of antipyretics;
- at least 7 days have passed since the appearance of clinical manifestations of the disease in the mother;
- at least two nasopharyngeal smear tests for SARSCoV-2 performed at interval of ≥ 24 hours are negative.
In case of positive test result for SARS-CoV-2 in the infant, separation is no longer relevant.
The mothers with a confirmed diagnosis of COVID19 are required to follow a self-isolation regime for 7 days after the appearance of the first symptoms. After self-isolation regime is over, they must keep a distance of ≥ 2 m from other people and wear a medical mask covering their nose and mouth in public places.
All women who have given birth are recommended early discharge from hospital, namely one day after natural childbirth and maximum two days after cesarean section, if there are no postpartum complications [50]. Postpartum examinations (wound examination, blood pressure measurement) should be performed via video link. Face-to-face visits to the doctor may be recommended to patients with comorbidities.
Breastfeeding
H. Chen, J. Guo, C. Wang et al. (2020) conducted a study of breast milk for coronavirus. All 6 tests were negative [3]. However, due to the close contact of an infected mother and a healthy infant during breastfeeding, one cannot say that breastfeeding is safe. In order to prevent virus transmission, it is necessary:
- to consider breastfeeding a child by a healthy woman who has given birth;
- to wash one’s hands thoroughly before feeding;
- to avoid coughing or sneezing during breastfeeding;
- to wear a face mask to prevent airborne infection;
- to use a breast pump for extracting breast milk when possible and to follow all the principles of sterilization before and after its use;
- to sterilize carefully all items that come into contact with the baby during formula feeding.
Since breast milk is the only source of antibodies and other various factors of specific and non-specific protection, it is impossible to give a definite answer whether breastfeeding has more risks or benefits if the woman is infected with COVID-19.
Conclusion
The following conclusions can be drawn from this study: when establishing the fact of pregnancy antenatal care registration should be done by phone or video, the need for additional face-to-face appointments (in addition to prenatal screening) is determined by the doctor individually; the main preventive measures are keeping the social distance and following self-isolation regime; if the first symptoms appear, it is necessary to have an analysis for SARS-CoV-2, the negative results of which should be complemented by a much more sensitive chest CT scan in case of persistent symptoms; treatment should be prescribed as early as possible; the volume of therapy and the form of medical care (outpatient, inpatient) are determined according to the severity of the disease; thromboprophylaxis should be carried out throughout pregnancy; hospital staff should wear PPE and strictly observe epidemiological precautions; infected patients should be isolated in separate rooms and contact other people only in face masks; the final decision on the method of feeding a baby is made by the woman who has recently given birth. Only strict adherence to national and international recommendations will improve the quality of medical care and reduce the rate of complications and mortality in obstetric hospitals.
References
- Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020; Mar 26. https://dx.doi.org/10.1001/jama.2020.4621.
- Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020; Mar 26. https://dx.doi.org/10.1001/jama.2020.4861.
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395(10226): 809-15. https://doi.org/10.1016/S0140-6736(20)30360-3.
- Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., Liu Y. Infants born to mothers with a new coronavirus (COVID-19). Front. Pediatr. 2020; 8: 104. https://dx.doi.org/10.3389/fped.2020.00104.
- Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin. Infect. Dis. 2020. Mar 30. pii: ciaa352. https://dx.doi.org/10.1093/cid/ciaa352.
- Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020; 9(1): 51-60. https://dx.doi.org/10.21037/tp.2020.02.06.
- Liu H., Liu F., Li J., Zhang T., Wang D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J. Infect. 2020; 80(5): e7-e13. https://dx.doi.org/10.1016/j.jinf.2020.03.007.
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). World Health Organisation; 2020.
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382(18): 1708-20. https://dx.doi.org/10.1056/NEJMoa2002032.
- Ashokka B., Loh M.H., Tan C.H., Su L.L., Young B.E., Lye D.C. et al. Care of the pregnant woman with COVID-19 in labor and delivery: anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel. Am. J. Obstet. Gynecol. 2020; Apr 10. pii: S0002-9378(20)30430-0. https://dx.doi.org/10.1016/j.ajog.2020.04.005.
- Liu D., Li L., Wu X., Zheng D., Wang J., Yang L., Zheng C. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID19) pneumonia: a preliminary analysis. AJR Am. J. Roentgenol. 2020; Mar 18: 1-6. https://dx.doi.org/10.2214/AJR.20.23072.
- NIH COVID-19 Treatment Guidelines. Available at: https://covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ Accessed April 22 2020.
- Karami P., Naghavi M., Feyzi A., Aghamohammadi M., Novin M.S., Mobaien A.et al. Mortality of a pregnant patient diagnosed with COVID-19: A case report with clinical, radiological, and histopathological findings. Travel Med. Infect. Dis. 2020; Apr 11: 101665. https://dx.doi.org/10.1016/j.tmaid.2020.101665.
- Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. 2020. Available at: https://thrombosisuk. org/covid-19-thrombosis.php Accessed 07 April 2020.
- COVID-19: guidance on social distancing and for vulnerable people 2020 Available at: https://www.gov. uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people Accessed 17 March 2020.
- Stay at home: guidance for households with possible coronavirus (COVID-19) infection. 2020. Available at: https://www.gov.uk/government/publications/covid-19-stay-at-home-guidance/stay-at-home-guidancefor-households-with-possible-coronavirus-covid-19-infection Accessed 17 March 2020.
- Major new measures to protect people at highest risk from coronavirus. 2020. Available at: https://www. gov.uk/government/news/major-new-measures-to-protect-people-at-highest-risk-from-coronavirus Accessed 26 March 2020.
- The Royal College of Obstetricians and Gynaecologists (RCOG). Coronavirus (COVID-19) Infection in Pregnancy. Version 8. London,2020.
- Министерство здравоохранения РФ. Временные методические рекомендации «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19)» Минздрава России, версия 7. М.; 2020. [Ministry of Health of the Russian Federation. The temporary guidelines «Prevention, diagnosis and treatment of novel coronavirus infection (COVID-19)» of the Ministry of Health of Russia, Version 7. Moscow; 2020.(in Russian)].
- IFMSS. Guidance fetal diagnosis and therapy during COVID-19 pandemic. Leuven; 2020.
- Zhou Z., Chen P., Peng H. Are healthy smokers really healthy? Tob. Induc. Dis. 2016; 14: 35. https://dx.doi.org/10.1186/s12971-016-0101-z.
- Dowswell T., Carroli G., Duley L., Gates S., Gülmezoglu A.M., Khan-Neelofur D., Piaggio G. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst. Rev. 2015; (7): CD000934. https://dx.doi.org/10.1002/14651858.CD000934.pub3.
- Knight M., Bunch K., Tuffnell D., Jayakody H., Shakespeare J., Kotnis R. et al. Saving Lives, Improving Mothers’ Care. Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2014-16. MBRRACE-UK; 2018.
- Altman D., Carroli G., Duley L., Farrell B., Moodley J., Neilson J., Smith D.; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002; 359(9321): 1877-90. https://dx.doi.org/10.1016/s0140-6736(02)08778-0.
- Society for Maternal Fetal Medicine and Society for Obstetric and Anesthesia and Perinatology. Labor and delivery COVID-19 considerations. Available at: https://s3.amazonaws.com/cdn.smfm.- org/media/2277/SMFM-SOAP_COVID_LD_Considerations_3-27- 20_(final)_PDF.pdf AccessedApril 1 2020.
- World Health Organization. Coronavirus disease (COVID-19) technical guidance: Surveillance and case definitions. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/surveillance-and-case-definitions AccessedFebruary 28 2020.
- СанПиН 2.6.1.1192-03. Гигиенические требования к устройству и эксплуатации рентгеновских кабинетов, аппаратов и проведению рентгенологических исследований. Утвержденные Главным государственным санитарным врачом Российской Федерации 14 февраля 2003 года, с 1 мая 2003 года. [SanPiN 2.6.1.1192-03. Hygienic requirements for the arrangement and use of X-ray rooms and apparatuses and for X-ray studies, approved by the Principal State Sanitary Inspector of the Russian Federation on February 14, 2003, from May 1, 2003. (in Russian)].
- Juusela A., Nazir M., Gimovsky M. Two cases of COVID-19 related cardiomyopathy in pregnancy. Am. J. Obstet. Gynecol. 2020; 3 April. https://dx.doi.org/10.1016/j.ajogmf.2020.100113.
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30(3): 269-71. https://dx.doi.org/10.1038/s41422-020-0282-0.
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017; 9(396). pii: eaal3653. https://dx.doi.org/10.1126/scitranslmed.aal3653.
- Mulangu S., Dodd L.E., Davey R.T. Jr., Tshiani Mbaya O., Proschan M., Mukadi D. et al. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. 2019; 381(24): 2293-303. https://dx.doi.org/10.1056/NEJMoa1910993.
- Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 2020. Available at: https://www.who.int/publications-detail/clinical-management-of-severeacute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed 05 March 2020.
- Министерство здравоохранения РФ. Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19. Методические рекомендации. Версия 1. М.; 24.04.2020. [Ministry of Health of the Russian Federation. Organization of medical care for pregnant, parturient, and puerperant women and newborns in relation to the novel coronary viral infection COVID-19. Guidelines. Version 1. Moscow; April 24, 2020. (in Russian)].
- Updated SOGC Committee Opinion – COVID-19 in Pregnancy (March 13th).
- Poon L.C., Yang H., Lee J.C.S., Copel J.A., Leung T.Y., Zhang Y. et al. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet. Gynecol. 2020; 55(5): 700-8. https://dx.doi.org/10.1002/uog.22013.
- Ди Ренцо Д.К., Макацария А.Д., Цибизова В.И., Капанна Ф., Разеро Б., Комличенко Э.В., Первунина Т.М., Хизроева Д.Х., Бицадзе В.О., Шкода А.С. О принципах работы перинатального стационара в условиях пандемии коронавируса. Вестник РАМН. 2020; 75(1): 83-92. https://dx.doi.org/10.15690/vramn1324. [Di Renzo G.C., Makatsaria A.D., Tsibizova V.I., Capanna F., Rasero B., Komlichenko E.V., Pervunina T.M., Khizroeva D.Kh., Bitsadze V.O., Shkoda A.S. On the principles of activities of a perinatal hospital during the coronavirus pandemic. Vestnik RAMN (Bulletin of RAMS). 2020; 75 (1): 83-92. (in Russian)] https://dx.doi.org/10.15690/vramn1324.
- Анестезиолого-реанимационное обеспечение пациентов с новой коронавирусной инфекцией COVID-19. Методические рекомендации. Версия №2 от 18 апреля 2020 года. Общероссийская общественная организация «Федерация анестезиологов и реаниматологов»; 2020. 92с. [Anesthetic and intensive care support for patients with the novel coronavirus infection COVID-19. Guidelines. Version No. 2 dated April 18, 2020. All-Russian Public Organization «The Federation of Anesthesiologists and Resuscitators»; 2020. 92 p.(in Russian)].
- Al-Tawfiq J.A., Memish Z.A. Update on therapeutic options for Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Expert Rev. Anti Infect. Ther. 2017; 15(3): 269-75. https://dx.doi.org/10.1080/14787210.2017.1271712.
- Røsjø H., Varpula M., Hagve T.A., Karlsson S., Ruokonen E., Pettilä V., Omland T.; FINNSEPSIS Study Group. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011; 37(1): 77-85. https://dx.doi.org/10.1007/s00134-010-2051-x.
- World Health Organization. Clinical management of severe acute respiratory infection when COVID-19 is suspected. 13 March 2020. Available at: https://www.who.int/publications-detail/clinicalmanagement-of-severe-acute-respira tory-infection-when-novel-coronavirus-(ncov)-infection-issuspected Accessed March 15 2020.
- Swartz D., Graham A. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020; 12(2). pii: E194. https://dx.doi.org/10.3390/v12020194.
- Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East Respiratory Syndrome Coronavirus (MERS - CoV) on pregnancy and perinatal outcome. BMC Infect. Dis. 2016; 16: 105. https://dx.doi.org/10.1186/s12879- 016-1437-y.
- Dashraath P., Jing Lin Jeslyn W., Mei Xian Karen L., Li Min L., Sarah L., Biswas A. et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020; Mar 23. pii: S0002-9378(20)30343-4. https://dx.doi.org/10.1016/j.ajog.2020.03.021.
- National Institute for Health and Care Excellence. Inducing Labour. 2008.
- Bohren M.A., Berger B.O., Munthe-Kaas H., Tunçalp Ö. Perceptions and experiences of labour companionship: a qualitative evidence synthesis. Cochrane Database Syst. Rev. 2019; (3): CD012449. https://dx.doi.org/10.1002/14651858.CD012449.pub2.
- COVID-19: investigation and initial clinical management of possible cases 2020. Available at: https://www. gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigationand-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection Accessed 05 March 2020.
- Chen R., Zhang Y., Huang L., Cheng B.H., Xia Z.Y., Meng Q.T. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can. J. Anaesth. 2020; 67(6): 655-63. https://dx.doi.org/10.1007/s12630-020-01630-7.
- Bauer M., Bernstein K., Dinges E., Delgado C., El-Sharawi N., Sultan P. et al. Obstetric Anesthesia during the COVID-19 pandemic. Anesth. Analg. 2020; Apr 6. https://dx.doi.org/10.1213/ANE.0000000000004856.
- Puopolo K.M., Hudak M.L., Kimberline D.W., Cummings J. INITIAL GUIDANCE: Management of infants born to mothers with COVID-19. Available at: https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf AccessedApril 03 2020.
- Boelig R.C., Manuck T., Oliver E.A., Di Mascio D., Saccone G., Bellussi F., Berghella V. Labor and delivery guidance for COVID-19. Am. J. Obstet. Gynecol. MFM. 2020. https://dx.doi.org/10.1016/j.ajogmf.2020.100110. Available at: https://www.sciencedirect.com/science/article/pii/S2589933320300409.
Received 07.05.2020
Accepted 12.05.2020
About the Authors
Irina V. Ignatko, MD, PhD, professor at the Department of Obstetrics, Gynaecology and Perinatology, I. M. Sechenov First Moscow State Medical University of the Ministryof Health of the Russian Federation (Sechenov University). Tel.: +7(499)782-33-07. E-mail: iradocent@mail.ru. 8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Alexander N. Strizhakov, Academician of the Russian Academy of Sciences, MD, professor, Head of the Department of Obstetrics, Gynecology and Perinatology, Faculty
of Medicine, I. M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University). Tel.:
+7(499)782-33-07. E-mail: kafedra-agp@mail.ru. 8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Elena V. Timokhina, Professor, Doctor of Medical Sciences, Professor of the Department of Obstetrics, Gynecology and Perinatology, Faculty of Medicine, I. M. Sechenov
First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University). Tel.: +7(499) 782-33-07. E-mail: kafedra-agp@mail.ru.
8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
Yulia V. Denisova, student of the I. M. Sechenov First Moscow State Medical University (Sechenov University). Tel.: +7(495)609-14-00. E-mail: yuliya.sheveleva.97@mail.ru.
8/2 Trubetskaya str., Moscow, 119991, Russian Federation.
For reference: Ignatko I.V., Strizhakov A.N., Timokhina E.V., Denisova Yu.V. Novel Coronavirus Infection (COVID-19): Guiding Principles for Obstetric Care under Pandemic Conditions.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 5: 22-33 (In Russian).
https://dx.doi.org/10.18565/aig.2020.5.22-33



