Genetic predictors of necrotizing enterocolitis in neonates
Aim. To reveal molecular genetic predictors of necrotizing enterocolitis (NEC) in preterm neonates.Nikitina I.V., Donnikov A.E., Krogh-Jensen O.A., Krasheninnikova R.V., Nepsha O.S., Lenyushkina A.A., Degtyarev D.N.
Materials and methods. The study included 590 neonates (gestational age (GA) 24–41 weeks) admitted to the Neonatal Intensive Care Unit during the first hours of life from January 2015 to December 2017. All neonates underwent sampling of biological material (venous blood and buccal swabs) before the start of medical treatment and enteral feeding. The reverse transcription method was used to measure gene expression levels: IL1b, IL6, IL8, IL10, IL12а, IL15, IL18, TNFa, TGFb1, TBX21, GATA3, RORC2, CD45, CD68, CD69, TLR2, TLR4, TLR9 and MMP8. We compared gene expression in venous blood and buccal swabs of neonates who developed NEC and those without NEC.
Results. The development of NEC was noted in 25 out of 590 newborns initially included in the study (4.2%). After the technical assessment of biomaterial and exclusion of 172 newborns, there were remaining 418 patients with 18 cases of NEC. After statistical adjustment according to GA there were 130 patients remaining: 16 neonates with NEC and 114 patients in control group (without NEC). In premature infants, a statistically significant increase in the level of expression of the TLR4 in blood was associated with the development of NEC. The study also revealed a downward trend in GATA3 in neonates with NEC compared with the control group. The decimal Lg (TLR4/GATA3) then was calculated and ROC analysis showed decent prognostic value of this criterion. Threshold value of 0.74, the sensitivity of the model – 88%, and the specificity – 77%; positive predictive value (PPV) – 39%, negative predictive value (NPV) – 97%. The same analysis in the buccal epithelium didn’t show significant differences between the groups. The expression level of other genes did not differ statistically significantly between groups.
Conclusion. The determination of the TLR4 gene expression in very premature infants born on 24–32 weeks of gestation can be used as a predictor of necrotizing enterocolitis.
Keywords
The genetic mechanisms of the development of various diseases have recently aroused scientific interest. The research in this area is expected to help develop and implement modern medicines or innovative diagnostic approaches that can speed up diagnostic search and start targeted therapy for patients as quickly as possible.
Among the severe diseases of the newborns that can result in serious complications is necrotizing enterocolitis (NEC). NEC is an inflammation of the intestinal wall which is followed by its necrosis [1]. This disease most frequently occurs in newborns who are in critical condition, and/or in premature infants. NEC is characterized by an excessive inflammation of the intestine, which causes damage to the mucous membrane of various severity and area. The main conditions that contribute to the development of NEC in newborns include prematurity, artificial feeding, birth asphyxia, and exchange blood transfusion.
Although the risk factors for NEC in newborns are currently well known, the exact cause of the disease remains unclear. It has previously been shown that a combination of various pathological mechanisms plays a role in the development of NEC. The complex interaction of the immune system, intestinal epithelium, commensal bacteria and signaling molecules has been under consideration.
Despite significant advances in neonatal intensive care and surgery, the mortality rate of newborns with NEC has not changed significantly in recent decades and ranges from 20 to 30% [2]. NEC also remains one of the main causes of disability among premature infants. Unfortunately, surgical treatment does not always improve the prognosis of the disease, since the inflammation in the intestine, as an organ with a large surface area, extensive vascularization and a high degree of concentration of immune cells, can contribute not only to the perforation of the intestinal wall, but also to the development of systemic effects of the disease that influence other tissues and organs [3]. According to recent studies, more than 50% of premature infants with NEC have a delayed development of the nervous system [3].
In experimental models, NEC is associated with serious morphological changes in the brain: proinflammatory reactions in the brain change the homeostasis and density of brain cell populations. G. Biouss et al. showed that the severity of inflammatory reactions in the brain correlated with the severity of NEC [4].
The initial clinical manifestations of NEC are non-specific, especially in infants with very and extremely low birth weight; therefore, it is difficult to diagnose and start treatment in a timely manner.
Nowadays, the main task for neonatologists is to develop a diagnostic complex that could allow the doctors to predict NEC, identify its early stages, differentiate it from other diseases of the neonatal period, and, accordingly, start etiopathogenetic therapy as quickly as possible. Such an approach should help to reduce the frequency and, in some cases, avoid the development of disabling complications in newborns, as well as in premature infants.
The analysis of the expression of genes involved in the immune response is now considered to be one of the most promising tools for identifying NEC biomarkers. The multicenter study conducted by Tremblay et al. showed that a significant number of differentially expressed genes are involved in the development of NEC [5]. The authors studied the expression of toll-like receptor (TLR) genes TLR4, TLR10 and CXCL8/IL8 in the samples of the resected intestines of premature infants. The analysis of the gene expression profile revealed a predominantly pro-inflammatory orientation of the immune response in the intestines of newborns with NEC [5]. The experimental study performed by Yin et al. demonstrated that the expression of TLR4 and NF-κB mRNA in the samples of the intestines of mice with NEC was significantly higher than in the control group without signs of the disease [6].
Currently, there is also evidence that measuring the expression of activated TLR4 is also representative in blood samples obtained from newborns with early-onset sepsis [7]. Taking into account the fact that the altered immune response of newborns is one of the main pathogenetic pathways of NEC development which has systemic effects, we suggested that mRNA detection can be performed in blood samples and buccal swabs (BS). Obtaining biomaterial samples from these loci during the first examination of newborns after birth can be considered as the least invasive method for determining NEC biomarkers.
In this study, we evaluated the relationship between the level of expression in buccal epithelial cells and in the blood of genes involved in the immune response, and the development of NEC in newborns in critical condition. The aim of the research was to identify molecular genetic predictors of NEC in premature infants of different gestational age.
Materials and Methods
The study included 590 neonates (gestational age (GA) 24–41 weeks) who were born at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia, from January 2015 to December 2017. Among them, there were 130 very preterm infants born at 24–32 weeks’ gestation. After the initial stabilization of the condition in the delivery room, all infants were transferred to the Neonatal Intensive Care Unit (NICU) for examination and treatment.
The exclusion criteria from the study were numerous birth defects which were fatal or required emergency surgical correction, as well as chromosomal abnormalities in infants.
 The parents of the newborns signed an informed consent to the children’s participation in the study. This study was approved by the Local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
The parents of the newborns signed an informed consent to the children’s participation in the study. This study was approved by the Local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
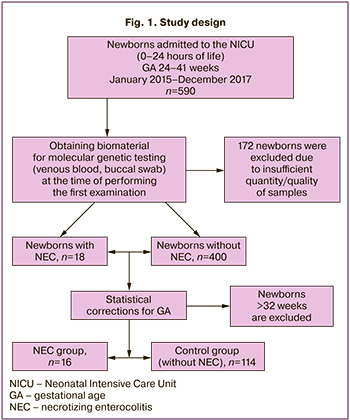
Anthropometric data of premature newborns were evaluated using the Fenton growth chart for preterm infants [8]. The evaluation of birth weight less than the 10th percentile indicated an intrauterine growth restriction. NEC was diagnosed using the classification based on clinical staging which was proposed by Bell et al. (1978) and modified by Walsh and Kliegman (1987) [1]. The study design is shown in Figure 1.
In order to reveal or exclude the congenital infection, all newborns underwent a standard clinical and laboratory examination, which included chest X-ray, hemoculture, complete blood count with estimation of the number of leukocytes, platelets, neutrophils and determining neutrophil index, control of the level of proteins in the acute phase of inflammation (C-reactive protein). According to the results of the clinical, laboratory and instrumental examination of the newborns 72 hours of age, a conclusion was made about the presence or absence of a congenital infection.
Early-onset neonatal sepsis was diagnosed after detecting a focus of infection in a newborn, or positive hemoculture, signs of systemic inflammatory response syndrome, if at least one of them was hematological, and symptoms of multiple organ failure [9, 10]. The differential diagnosis between congenital pneumonia, early-onset neonatal sepsis, respiratory distress syndrome, and transient tachypnea of the newborn was made in accordance with clinical guidelines [11, 12].
All the newborns included in the study underwent sampling of biological material (venous blood (VB) and buccal swabs (BS)) during the first day after birth.
The biological material was obtained from newborns during the first hours of life, immediately after admission to the NICU; it was carried out simultaneously with performing a clinical and laboratory examination, prior to the beginning of medication therapy and enteral feeding. BS cells were obtained by taking swabs from the cheek area into test tubes with a solution for mRNA stabilization (lyse solution of the Proba NK kit (DNA-Technology, Russia)). Blood for the study was collected in a test tube with an anticoagulant (EDTA). In order to avoid mRNA degradation, the blood tubes were immediately transported to the biobank department, where they were aliquoted (100 ml each) into the lyse solution of the Proba NK kit (DNA-Technology, Russia) for mRNA stabilization. Prior to the study, all samples were stored at -80°C. After completing the course of treatment of all infants included in the study, the biomaterial was analyzed. RNA isolation was performed using the Proba NK kit (DNA-Technology, Russia) with phenolic deproteinization. The volume of samples after isolation was 50 μl. The reverse transcription reaction was performed in the volume of 40 μl (33 μl of the isolated RNA solution was taken into the reaction). Specific oligonucleotides were used as primers for reverse transcription. The reaction was carried out at a temperature of 40°C for 30 minutes, followed by inactivation of reverse transcriptase at 95°C for 5 minutes.
Expression levels of the following genes responsible for production were measured: cytokines (IL1b, IL6, IL8, IL10, IL12a, IL15, IL18, TNFa, TGFb1), transcription factors (TBX21, GATA3, RORC2), cell surface structures (CD45, CD68, CD69), TLR2, TLR4, TLR9, matrix metalloproteinase (MMP8). Commercial reagents were used in the study (DNA-Technology, Russia). The manufacturer guaranteed the absence of amplification on the genomic DNA matrix of the test and reference genes. Therefore, it was possible to avoid the additional stage of processing nucleic acids with DNase. Amplification was carried out in real time using the DT-384 equipment in the volume of 12.5 μl according to the following program: 1 cycle was done at 80°C for 30 sec, at 94°C for 1 min 30 sec; 50 cycles - at 94°C for 20 sec, at 62°C for 20 sec; storage was at 10°C. The fluorescence level was measured at each cycle at a temperature of 64° C using the FAM fluorescence channel. The reaction was repeated twice at each point. Normalization was performed for four reference genes HPRT1, TBP, B2M, and GUSB. Indicator cycles were compared using ΔΔCq method. The expression level was measured in relative amounts which reflect the ratio of the number of mRNAs of the analyzed gene to the geometric average number of mRNAs of reference genes.
Statistical analysis
Statistical analysis of the data was performed using software package IBM SPSS Statistics version 23 (USA). Normality of distribution was assessed using the Kolmogorov-Smirnov test together with the Lilliefors and Shapiro-Wilk tests. Excess kurtosis and asymmetry were also assessed.
Abnormal distribution was detected in all the groups. In order to find out statistical significance of differences among groups, the Mann–Whitney U-test was used. The results are shown in a median line (Ме) and interquartile range of the 25–75 percentile (Q1–Q3). Taking into account the specific characteristics of the patients (newborns in NICU), in particular, significant differences in weight and length parameters, as well as the practical focus of the study, we present the minimum (min) and maximum (max) values for each variable [min-max] in order to provide a more detailed clinical description of the groups. The differences were considered as verifiable at the significance level of p<0.05. To assess the correlational relationship, Spearman’s correlation coefficient was used. In the analysis of nominal variables, Pearson’s χ2-test together with Yates’ correction as well as Fisher’s exact test were used for a small number of observations. The quality of the diagnostic model was evaluated using ROC analysis (Receiver Operating Characteristic) on the area under the characteristic curve in Se and 1-Sp coordinates. The area value is given with a 95% confidence interval. When calculating operational characteristics, the threshold level was chosen on the basis of the requirement of maximum total sensitivity and specificity. The 95% confidence intervals for operational characteristics were calculated based on the chi-squared distribution.
Results
During the study, 25 out of 590 newborns with different GA (4.2%) developed NEC of various severity. After completing the clinical part of the study and technical evaluation of the biomaterial, samples of 172 infants were excluded from the study, among them there were 7 infants whose neonatal period was complicated by the development of NEC. The diagnosis of NEC was made in 18 out of 418 newborns, 16 of them were born earlier than 33 weeks’ gestation. In order to exclude confounding factors, the biological samples of only 130 preterm infants born at 24–32 weeks’ gestation were compared and analyzed.
GA of newborns with NEC ranged from 26 to 31 weeks (Me=29 weeks, Q1–Q3: 26.5–30), and body weight ranged from 485g to 1230g (Me=900g, Q1–Q3: 760–987).
The level of expression of immune response genes in the cells of biological samples of 16 very preterm infants whose neonatal period was complicated by the development of NEC (the main group) was compared with similar indicators of 114 very preterm infants who did not develop NEC during the neonatal period (the control group). GA, Apgar score at 1 minute, frequency of antenatal steroid prophylaxis, and rate of birth by cesarean section were compared in the infants of the main and control groups.
There was a higher incidence of intrauterine growth retardation syndrome among the patients with NEC (9/16, 56%) than in the infants of the control group (34/114, 29%), as well as a lower Apgar score at 5 minutes, namely [4–8] scores (Me=7; Q1–Q3: 5.5–7) compared to [4–9] scores (Me=7; Q1–Q3: 7–7) in the control group (p=0.02).
NEC stage I was observed in 1 patient out of 16 (6.3%), IIa stage – in 11 newborns (69%), IIb stage – in 3 infants (18.8%). NEC stage III developed in 1 child who underwent surgery (6.3%).
All newborns of the main group (16/16) who developed NEC were diagnosed with an infection (early-onset neonatal sepsis (n=5), congenital pneumonia (n=11)); this parameter differed from the one in the control group, 102/114 (early-onset neonatal sepsis (n=14), congenital pneumonia (n=88)), but the difference did not reach the statistical significance (p=0.37).
Fatal outcome occurred in two cases in the main group, the newborns had NEC (in the first case there was a multiorgan failure and disseminated intravascular coagulation syndrome at 26 weeks’ gestation in the infant with early-onset neonatal sepsis, in the second case there was a multiorgan failure, disseminated intravascular coagulation syndrome and intrauterine growth retardation at 26 weeks gestation in the infant with congenital pneumonia). The clinical characteristics of the patients are shown in the Table.
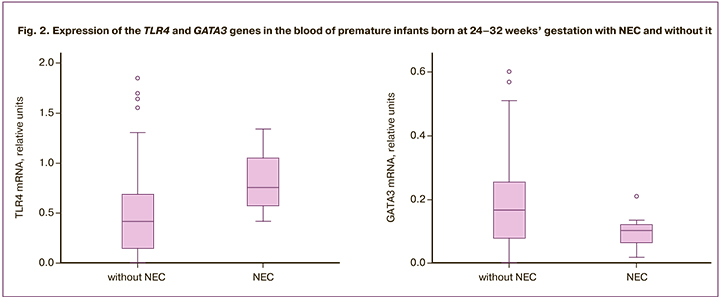
The comparison of the expression profile of the studied genes in blood cells in premature newborns born at 24–32 weeks’ gestation revealed a statistically significant increase in TLR4 expression in newborns with NEC: (0.8; Q1–Q3: 0.6–1.0 versus 0.4; Q1–Q3: 0.1–0.7, p=0.012) and a tendency to decrease in GATA3 (0.10; Q1–Q3: 0.70–0.12 versus 0.17; Q1–Q3: 0.08–0.26, p=0.08) compared to a group of infants of similar GA without signs of NEC (Fig. 2). The expression level of other genes did not differ significantly between the groups. The study of the expression of the proper genes involved in the immune response in the buccal epithe lium of premature newborns showed no statistically significant differences between the groups.
Taking into account various changes in the expression of TLR4 and GATA3 in blood cells, the decimal logarithm of the ratio of the expression levels of these genes was analyzed as a diagnostic criterion. In practice, the implementation of the ratio of the expression levels of key genes makes it possible to abandon the use of reference genes. The ROC analysis showed that the logarithm (TLR4/GATA3) has a good diagnostic value: the area under the ROC curve was 0.78; (95% CI: 0.64–0.91, p=0.011). At a threshold value of 0.74, the sensitivity of the model was 88% (47–100%), and specificity was 77% (63–88%); positive predictive value was 39% (95% CI: 17–64%), negative predictive value was 97% (95% CI: 86–100%).
Discussion
Toll-like receptors (TLR) play a primary role in the immune defense of the body. TLRs are involved in the induction and modulation of innate and adaptive immune responses, acting as their integrators [13]. These proteins are present on the surface of various cells and are able to identify standard molecular structures (patterns) specific to different groups of pathogens. Compared to the adaptive (acquired) immune system, such receptors and their associated immune defense mechanisms are evolutionarily older.
If TLRs are inactive, they are in the membrane in a monomeric state. TLR activation occurs when ligands are bound, and certain structures of bacteria, viruses, and fungi act as ligands for them [14]. In humans, it was TLR4 that was first discovered, and a total of 13 TLRs have been described so far, each of them binding to its own molecular pattern. TLR4 selectively reacts to lipopolysaccharides (LPS), which are a part of the cell wall of gram-negative bacteria, lipooligosaccharides, and bacterial endotoxin [15]. Moreover, TLR4 identifies a wide range of proteins of viruses, fungi, and mycoplasmas. TLR4 can also be activated by endogenous factors called DAMP (danger associated molecular patterns which are molecular structures associated with damage). Typical DAMPs acting as TLR4 agonists are extracellular matrix components such as fibronectin (more precisely, its extracellular domain), hyaluronic acid or heparan sulfate, hyaluronic acid oligosaccharides and β-defensin, high mobility group box 1 protein (HMGB1), heat shock proteins (HSP), substance P; they are released during cell damage and inflammation and activate TLR4 and TLR2. However, these endogenous TLR4 ligands activate immune cells only at high concentrations, unlike LPS, which trigger immune responses even at low concentrations [14].
TLR activation triggers the intracellular signaling pathway NF-κB (nuclear factor-kappa B) which is a protein complex, and NF-κB, in turn, plays a key role in the development of the immune response to infection, regulating DNA transcription, pro-inflammatory cytokine production, and cell survival [16, 17].
TLR4, like TLR2, takes an active part in initiating the inflammatory response, as well as cell apoptosis [18]. In addition, TLR4 is the only receptor in the TLR family that is expressed on the cell surface and is capable of inducing IFNα production.
The role of TLR in the development of obstetric pathology has now been established. Clinical studies with pregnant women and women in labor confirm the key role of TLR in the development of infection-associated preterm birth. It has been shown that the expression of TLR2 and TLR4 in the decidual membrane and placenta increases in preterm birth associated with the development of chorioamnionitis. TLR2 expression was proposed to be used as a marker of preterm birth in patients with intrauterine infection and in pregnant women with a high risk of intrauterine infection [19]. In addition, it has been shown that the termination of pregnancy in the first trimester can lead to apoptosis of trophoblast cells which is caused by activation of TLR4 when it binds to the chlamydia HSP [20, 21].
The role of TLR in the development of neonatal pathology has also been studied. The experiments on mice showed that damage to the white matter of the brain of premature mice may be associated with the activation of TLR4 in the fetus. It was found that low doses of LPS that do not cause severe pregnancy complications, however, can significantly increase fetal brain damage associated with hypoxia in newborn mice [22].
At the same time, the results of studies on the expression of TLR in newborns exposed to the septic process are quite contradictory. The research conducted by Redondo et al. showed that the expression of TLR4 on peripheral blood monocytes increases in newborns with the developing late neonatal sepsis [23]; but this study had limitations: it included only full-term children (n=27), the total sample size was relatively small, and the control group included healthy adults. Silveira-Lessa et al. studied the expression of TLR and cytokines in 44 newborns of different GA, both with early and late neonatal sepsis. The study was performed using umbilical cord blood samples obtained from patients with early neonatal sepsis and peripheral blood samples obtained from newborns with late neonatal sepsis. The indicators were compared among adults and newborns. There were no differences in TLR4 expression on monocytes in patients of the study groups [7]. It is obvious that the small number and heterogeneity of the groups do not allow us to extrapolate the obtained data to the entire population.
There was a study of the mechanism of TLR4 involvement in the development of NEC in newborns [24]. Activation of TLR4 leads to a damage to the mucous membrane and a decrease in the rate and ability to restore the epithelium of the intestinal wall. It is worth noting that the level and function of TLR4 in the intestinal mucosa are increased in preterm infants compared to full-term infants [25]. TLR4 plays an important role in regulating normal development of the intestines in the fetus. Due to colonization by numerous gram-negative bacteria, premature infants experience excessive stimulation of TLR4 signaling, which causes the release of pro-inflammatory cytokines, increased enterocyte apoptosis, and impaired mucosal healing. Moreover, bacterial translocation through the intestinal mucosa activates TLR4 on the intestinal vascular endothelium, which leads to a decrease in blood flow and the development of intestinal ischemia and necrosis [26]. The study conducted by Hui et al. demonstrated an increase in pro-inflammatory cytokine levels and an increase in TLR4 expression in resected intestinal samples in preterm infants with NEC who were born at 28–29 weeks’ gestation [27].
Nowadays, neonatologists are increasingly interested in non-invasive methods for diagnosing infectious and inflammatory diseases (in saliva or buccal epithelial cells), which could help to avoid stress, pain and patient’s anemisation caused by phlebotomy losses. Recent studies have shown that saliva can serve as an equally informative substrate for detecting cytokines as blood [28, 29]. The published data on the study of TLR expression in saliva of one-day-old newborns are not available.
This research showed that determining the expression of the TLR4 gene in the BS of premature newborns with NEC is less informative than in the blood, but there is a similar tendency to its increase that is confirmed by the data of our study. Apparently, this may be due to the characteristics of the main TLR4 localization, namely on the membrane of monocytes and macrophages, in liver cells, myeloid, dendritic cells and in intestinal epithelial cells, and due to the development of a systemic inflammatory response during translocation of the pathogen from the mucous membranes to the blood. Transcription factor GATA3 belongs to a family of six transcription factors containing a common DNA fragment (A/G) GATA(A/G) and a terminal zinc-containing domain [30]. Knockdown of the GATA3 gene in mice leads to the death of the embryo which results from defects in hematopoiesis and the central nervous system. Transcription factor GATA3 regulates the proliferation and differentiation of many types of cells and tissues, it plays a crucial role in the development of diseases and participates in the Th2 polarized immune response. In addition, the action of GATA3 causes changes in histones (H3K4, H3K14) located in the loci of genes encoding the production of IL-5 (eosinophil colony-stimulating factor), IL-4 and IL-13, which are structurally related to each other and involved in powerful immunosuppressive and anti-inflammatory activity [31]. The present study revealed a decrease in the expression of the transcription factor GATA3, which is responsible for the differentiation of naive Th lymphocytes into Th2 cells in the VB of very preterm infants with NEC born at 24–32 weeks’ gestation; this decrease can be suggestive of a remarkable pro-inflammatory profile of the immune response and insufficient activity of the anti-inflammatory link in this category of patients. The high incidence of infectious pathology in newborns with NEC is associated with similar changes in the expression profile, which apparently explains the low diagnostic value of increased ratio TLR4/GATA3 and the high value of reducing this ratio.
The impaired blood flow in the basin of the celiac trunk in newborns with severe intrauterine growth retardation is known to cause ischemia of the intestinal wall. Together with colonization of the intestine by opportunistic microflora, especially gram-negative one, ischemia leads to an increase in TLR4 expression and to the launch of a cascade of pathological reactions, which play a key role in NEC genesis in very preterm infants.
Conclusion
Our study showed a statistically significant increase in the level of TLR4 expression and a tendency to a decrease in GATA3 expression in the VB of premature infants born at 24–32 weeks’ gestation. One should continue further studies of the expression of key immune response genes in the VB of premature newborns with NEC, since a larger sample of patients may show a statistically significant decrease in the level of expression of the transcription factor GATA3.
To date, it becomes obvious that the systemic inflammatory response of the newborn does not depend only on the pathogen, but it can be considered as a normal reaction of the patient’s body to the infection. In this regard, an increase in the level of TLR4 expression in the VB of very preterm infants characterizes their systemic inflammatory response, which is produced during the local inflammation in the intestinal wall in infants with NEC.
Therefore, determining TLR4 expression in the VB of premature infants born at 32 weeks’ gestation and earlier can be a useful marker of predicting the further development and early diagnosis of NEC at its subclinical stage, which makes it possible to take preventive measures in due time and minimize possible complications.
References
- Дорофеева Е.И., Подуровская Ю.Л., Буров А.А., Рюмина И.И., Нароган М.В., Грошева Е.В., Ионов О.В., Балашова Е.Н., Киртбая А.Р., Дегтярев Д.Н., Хаматханова Е.М. Диагностика и консервативное лечение новорожденных с некротизирующим энтероколитом (проект клинических рекомендаций). Неонатология: новости, мнения, обучение. 2014; 2: 84-92. [Dorofeeva E.I., Podurovskaya Yu.L., Burov A.A., Ryumina I.I., Narogan M.V., Grosheva E.V., Ionov O.V., Balashova E.N., Kirtbaya A.R., Degtyarev D.N., Khamatkhanova E.M. Diagnosis and conservative treatment of necrotizing enterocolitis in newborn (project of clinical practice guidelines). Neonatology: News, Opinions, Training. 2014; 2: 84-92. (in Russian)].
- Hull M.A., Fisher J.G., Gutierrez I.M., Jones B.A., Kang K.H., Kenny M. et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J. Am. Coll. Surg. 2014; 218(6): 1148-55. https://dx.doi.org/10.1016/j.jamcollsurg.2013.11.015.
- Kastenberg Z.J., Sylvester K.G. The surgical management of necrotizing enterocolitis. Clin. Perinatol. 2013; 40(1): 135-48. https://dx.doi.org/ 10.1016/j.clp.2012.12.011.
- Biouss G., Antounians L., Li B., O'Connell J.S., Seo S., Catania V.D. et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J. Neuroinflammation. 2019; 16(1): 97. https://dx.doi.org/10.1186/s12974-019-1481-9.
- Tremblay É., Thibault M.P., Ferretti E., Babakissa C., Bertelle V., Bettolli M. et al. Gene expression profiling in necrotizing enterocolitis reveals pathways common to those reported in Crohn’s disease. BMC Med. Genomics. 2016; 9: 6. https://dx.doi.org/10.1186/s12920-016-0166-9.
- Yin Y., Liu F., Li Y., Tang R., Wang J. mRNA expression of TLR4, TLR9 and NF-κB in a neonatal murine model of necrotizing enterocolitis. Mol. Med. Rep. 2016; 14(3): 1953-6. https://dx.doi.org/10.3892/mmr.2016.5455.
- Silveira-Lessa A.L., Quinello C., Lima L., Redondo A.C.C., Ceccon M.E.J.R., Carneiro-Sampaio M. et al. TLR expression, phagocytosis and oxidative burst in healthy and septic newborns in response to Gram-negative and Gram-positive rods. Hum. Immunol. 2016; 77(10): 972-80. https://dx.doi.org/10.1016/j.humimm.2016.07.230.
- Fenton T.R., Kim J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013; 13: 59. https://dx.doi.org/10.1186/1471-2431-13-59.
- Wynn J.L. Defining neonatal sepsis. Curr. Opin. Pediatr. 2016; 28(2): 135-40. https://dx.doi.org/10.1097/MOP.0000000000000315.
- Weiss S.L., Peters M.J., Alhazzani W., Agus M.S.D., Flori H.R., Inwald D.P. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med. 2020; 21(2): e52-106. https://dx.doi.org/10.1097/PCC.0000000000002198.
- Голубцова Ю.М., Дегтярев Д.Н. Современные подходы к профилактике, диагностике и лечению раннего неонатального сепсиса. Неонатология: новости, мнения, обучение. 2014; 2: 15-25. [Golubtsova Yu.M., Degtyarev D.N. Modern approaches to preventing, diagnosing, and treating early-onset neonatal sepsis. Neonatology: News, Opinions, Training. 2014; 2: 15-25. (in Russian)].
- Антонов А.Г., Байбарина Е.Н., Балашова Е.Н., Дегтярев Д.Н., Зубков В.В., Иванов Д.О., Ионов О.В., Карпова А.Л., Киртбая А.Р., Крохина К.Н., Крючко Д.С., Ленюшкина А.А., Ли А.Г., Малютина Л.В., Мебелова И.И., Никитина И.В., Петренко Ю.А., Рындин А.Ю., Рюмина И.И., Романенко А.В. и др. Врожденная пневмония (клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4: 133-48. [Antonov A.G., Baybarina E.N., Balashova E.N., Degtyarev D.N., Zubkov V.V. et al. Congenital pneumonia (clinical practice guidelines). Neonatology: News, Opinions, Training. 2017; 4: 133-48. (in Russian)].
- Lemaitre B., Nicolas E., Michaut L., Reichhart J.M., Hoffmann J.A. Pillars article: the dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996. 86: 973-83. J. Immunol. 2012; 188(11): 5210-20.
- Майданник В.Г. Toll-подобные рецепторы и почки. Международный журнал педиатрии, акушерства и гинекологии. 2014; 6(1): 98-108. [Maidannik V.G. Toll-like receptors and kidneys. International Journal of Pediatrics, Obstetrics and Gynecology. 2014: 6(1): 98-108. (in Russian)].
- Rosadini C.V., Kagan J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017; 44: 14-9. https://dx.doi.org/10.1016/j.coi.2016.10.005.
- Никитина И.В., Донников А.Е., Крог-Йенсен О.А., Ленюшкина А.А., Быстрицкий А.А., Крючко Д.С., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Генетические полиморфизмы у детей, ассоциированные с развитием врожденных инфекций. Акушерство и гинекология. 2019; 11: 175-85. https://dx.doi.org/10.18565/aig.2019.11.175-185. [Nikitina I.V., Donnikov A.E.,Krogh-Jensen O.A., Lenyushkina A.A., Bystritsky A.A., Kryuchko D.S.,Ionov O.V., Zubkov V.V., Degtyarev D.N. Congenital infection-associated genetic polymorphisms in children. Obstetrics and Gynegology. 2019; 11: 175-85. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.175-185.
- Yamamoto Y., Gaynor R.B. Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001; 1(3): 287-96. https://dx.doi.org/ 10.2174/1566524013363816.
- Wu H., Chen G., Wyburn K.R., Yin J., Bertolino P., Eris J.M. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 2007; 117(10): 2847-59. https://dx.doi.org/10.1172/JCI31008.
- Robertson S.A., Hutchinson M.R., Rice K.C., Chin P.Y., Moldenhauer L.M., Stark M.J. et al. Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury. Clin. Transl. Immunology. 2020; 9(4): e1121. https://dx.doi.org/10.1002/cti2.1121.
- Helmo F.R., Alves E.A.R., De Andrade Moreira R.A., Severino V.O., Rocha L.P., Monteiro M.L.G.D.R. et al. Intrauterine infection, immune system and premature birth. J. Matern. Fetal Neonatal Med. 2018; 31(9): 1227-33. https://dx.doi.org/10.1080/14767058.2017.1311318.
- Equils O., Lu D., Gatter M., Bertolotto C., Arditi M., McGregor J.A. et al. Chlamydia heat shock protein 60 induces trophoblast apoptosis through TLR4. J. Immunol. 2006; 177(2): 1257-63. https://dx.doi.org/10.4049/jimmunol.177.2.1257.
- Flemming A. Deciphering the gut-brain link in NEC. Nat. Rev. Immunol. 2019; 19(2): 70-1. https://dx.doi.org/10.1038/s41577-018-0115-2.
- Redondo A.C.C., Ceccon M.E.J.R., Silveira-Lessa A.L., Quinello C., Palmeira P., Carvalho W.B. et al. TLR-2 and TLR-4 expression in monocytes of newborns with late-onset sepsis. J. Pediatr. (Rio J). 2014; 90(5): 472-8. https://dx.doi.org/ 10.1016/j.jped.2013.12.012.
- Denning N.L., Prince J.M. Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol. Med. 2018; 24(1): 4. https://dx.doi.org/10.1186/s10020-018-0002-0.
- Hackam D.J., Afrazi A., Good M., Sodhi C.P. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin. Dev. Immunol. 2013; 2013: 475415. https://dx.doi.org/10.1155/2013/475415.
- Jia H., Sodhi C.P., Yamaguchi Y., Lu P., Martin L.Y., Good M. et al. Pulmonary epithelial TLR4 activation leads to lung injury in neonatal necrotizing enterocolitis. J. Immunol. 2016; 197(3): 859-71. https://dx.doi.org/10.4049/jimmunol.1600618.
- Hui L., Dai Y., Guo Z., Zhang J., Zhen F., Bian X. et al. Immunoregulation effects of different γδT cells and toll-like receptor signaling pathways in neonatal necrotizing enterocolitis. Medicine (Baltimore). 2017; 96(8); e6077. https://dx.doi.org/10.1097/MD.0000000000006077.
- Su T.Y., Chen I.L., Huang H.C. ID: 373. Salivatory cytokine - a non-invasive predictor for the development of bronchopulmonary dysplasia in premature neonates. Pediatr. Res. 2019; 86(Suppl. 1: The 3rd Congress of Joint European Neonatal Societies. 17-21 September 2019 Maastricht, the Netherlands.)
- Никитина И.В., Непша О.С., Донников А.Е., Трофимов Д.Ю., Милая О.В., Дегтярева А.В., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Современные возможности молекулярно-генетических методов в диагностике раннего неонатального сепсиса у недоношенных новорожденных. Акушерство и гинекология. 2016; 12: 106-13. https://dx.doi.org/10.18565/aig.2016.12.106-13. [Nikitina I.V., Nepsha O.S., Donnikov A.E., Trofimov D.Yu., Milaya O.V., Degtyarev A.V., Ionov O.V., Zubkov V.V., Degtyarev D.N. Modern possibilities of molecular genetic techniques in the diagnosis of early neonatal sepsis in preterm neonates. Obstetrics and Gynecology. 2016; 12: 106-13. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.12.106-13.
- Takaku M., Grimm S.A., Shimbo T., Perera L., Menafra R., Stunnenberg H.G. et al. GATA3-dependent cellular reprogramming requires activation-domain dependent recruitment of a chromatin remodeler. Genome Biol. 2016; 17: 36. https://dx.doi.org/10.1186/s13059-016-0897-0.
- Минеев В.Н., Сорокина Л.Н., Еремеева А.В. Транскрипционные факторы gata-3, Foxp3 и их кооперативные взаимодействия при бронхиальной астме. Вестник Санкт-Петербургского университета. Медицина. 2012; 4: 23-31. [Mineev V.N., Sorokina L.N., Eremeeva A.V. Transcription factors gata-3, Foxp3 and their cooperative interactions in bronchial asthma. St. Petersburg University Bulletin. 2012; 4: 23-31. (in Russian)].
Received 21.09.2020
Accepted 06.11.2020
About the Authors
Irina V. Nikitina, MD, Ph.D., Leading Researcher of the Neonatal Intensive Care Unit №2 of the Institute of neonatology and pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Associate Professor of Neonatology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)531-44-44, ex. 2700, 2697.Е-mail: i_nikitina@oparina4.ru. ORCID: 0000-0002-1103-1908; Reasearcher ID: AAH-3465-2019; SCOPUS Author ID: 57189233499. 117997, Russia, Moscow, Oparina str., 4.
Andrey E. Donnikov, M.D., Ph.D., Head of laboratory of molecular genetic methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-49-51. Е-mail: a_donnikov@oparina4.ru. ORCID: 0000-0003-3504-2406; Reasearcher ID: E-7178-2015; Scopus ID: 6505485697. 117997, Russia, Moscow, Oparina str., 4.
Olga A. Krogh-Jensen, M.D., Ph.D., neonatologist of the Neonatal Intensive Care Unit No. 2 of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Department of pediatrics and neonatology; Associate Professor at I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), Pediatric faculty, neonatal department. Tel.: +7(926)014-01-35. E-mail: o_krogh@oparina4.ru.; olgaborisevich@gmail.com. ORCID: 0000-0002-5178-5659; SCOPUS Author ID: 57214220453.
117997, Russia, Moscow, Oparina str., 4.; 119991, Russia, Moscow, Trubetskaya str., 8/2.
Regina V. Krasheninnikova, MD, Geneticist of laboratory of molecular genetic methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-49-51, ex. 3101. E-mail: r_krasheninnikova@oparina4.ru. SPIN-код: 1565-0791;
AuthorID: 1082698. 117997, Russia, Moscow, Oparina str., 4.
Oksana S. Nepsha, Ph.D., Researcher of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: o_nepsha@oparina4.ru. ORCID: 0000-0002-9988-2810;
Reasearcher ID: H-3489-2018; ScopusID: 55534915100. 117997, Russia, Moscow, Oparina str., 4.
Anna A. Lenyushkina, MD, Ph.D., Head of the Neonatal Intensive Care Unit №2 of the Institute of neonatology and pediatrics, Associate Professor of Neonatology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)531-44-44, ex. 2700, 2697. E-mail: a-lenushkina@yandex.ru. ORCID: 0000-0001-8929-2991. 117997, Russia, Moscow, Oparina str., 4.
Dmitriy N. Degtyarev, MD, PhD, Vice director of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Professor of Neonatology Department; Professor, Head of neonatal department at Pediatric Faculty, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-25-33. E-mail: d_degtiarev@oparina4.ru. ORCID: 0000-0001-8975-2425.
117997, Russia, Moscow, Oparina str., 4.; 119991, Russia, Moscow, Trubetskaya str., 8/2.
For citation: Nikitina I.V., Donnikov A.E., Krogh-Jensen O.A., Krasheninnikova R.V., Nepsha O.S., Lenyushkina A.A., Degtyarev D.N. Genetic predictors of necrotizing enterocolitis in neonates.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 150-158 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.150-158



