Uterine fibroids as a risk factor for tubal infertility
Objective. To identify the regularities of macro/microscopic changes in the uterine tube wall in uterine fibroids with a submucosal and/or intramural nodule.Brodsky G.V., Adamyan L.V., Sukhikh G.T.
Subjects and methods. A retrospective, comparative study was conducted to examine the morphological status of histological specimens of 100 fallopian tubes in 50 reproductive-aged women through histological, immunohistochemical, and morphometric examinations.
Results. The findings were analyzed taking into account the assessment of structural elements in different parts of the fallopian tube, the thickness of its wall layers, the characteristics of arterial and venous blood supply and microarchitecture of the fallopian tube wall in different phases of the ovarian and menstrual cycle in concomitant submucosal and intramural uterine fibroids. The findings were compared with the histological and morphometric data of the control group, without indicating the presence of concomitant genital pathology in the clinical protocol.
Conclusion. The finding may lead to the conclusion that the observed changes in the fallopian tube wall in uterine myoma play an important role as a morphological substrate for the clinical data related to the low efficiency of assisted reproductive technologies. This study makes it possible to clarify a diagnostic algorithm and management tactics for patients with uterine myoma in an infertility clinic.
Keywords
One of the current problems in modern gynecologic practice is therapy of impairment of anatomic integrity and functional activity of the fallopian tubes [1-4]. One of the reasons reducing efficiency of treatment is pathology of the uterus and adnexa [1-3]. According to literature, uterine fibroid, is associated with female infertility in 46% of cases [4]. The influence of uterine myoma with submucosal and/or intramural localization of uterine fibroid on the condition of the fallopian tube is a major area of interest in relation to performing microsurgical operations, reproductive technologies and tubal patency treatment.
The aim of the investigation is to identify regularities of macro/microscopic changes in the fallopian tube wall in uterine fibroid with submucosal and/or intramural localization of fibroid.
Materials and Methods
The retrospective, comparative research was conducted on archival histological specimen of 100 fallopian tubes of 50 women of reproductive age from 25 to 40 years old. Uterine fibroid was found in 25 women, and hysterectomy with adnexectomy was performed. The samples of 50 fallopian tubes from 25 women without genital pathology were obtained during tubal sterilization with the excision of the part of the uterine tube. They served as the control group. For studying microtopography and macro/microscopic anatomy of the fallopian tube, the serial hystotopograms stained by hematoxylin-eosin and by van Gizon methods were used. There was hystotopography investigation of the following parts of the fallopian tube: the middle of the ampulla, the part between the ampulla and isthmus of the fallopian tube, the middle of the isthmic part of the tube, the utero-tubal junction and the intramural part of the fallopian tube. The quantitative assessment of the lumen, wall, various parts of the fallopian tube, its coats and layers was carried out. There was also an evaluation of the quality of arterial and venous vessels, the measurement of the diameter, and the distribution of the vessels within the fallopian tube wall. Calculations of distances, perimeters and areas were carried out using Magicscan image analysis system.
Results and Discussion
The changes in thickness of the wall of the fallopian tube, its coats and layers, as well as blood vessels in uterine fibroid could be revealed using the morphometric investigation (Figures 1, 2 and 3).
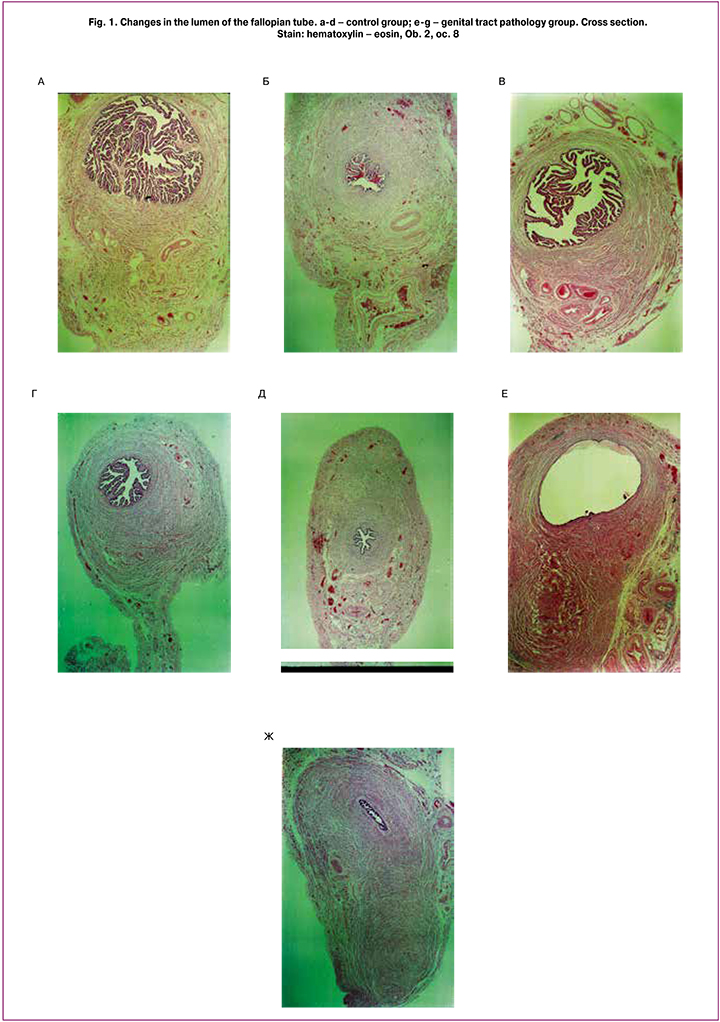
In uterine fibroid some changes (increase) in thickness of the wall of the fallopian tube are noted. Thus, in patients with uterine fibroid, thickness of anterior, superior and posterior walls of the fallopian tube in the isthmic part is 1.74±0.25 mm, 2.3±0.3 mm and 2.03±0.28 mm, whereas normal thickness of the walls of the fallopian tube, is equal to 1.69±0.13 mm, 2.03±0.13 mm and 1.89±0.14 mm, respectively. Such changes, namely increase, in thickness occurr due to the increase of thickness of the muscular layer of the fallopian tube, especially longitudinal muscular layer. This process is most evident along the isthmus of the uterine tube. In its turn, there is a tendency to reduction of thickness of the mucous membrane along the isthmus and ampulla of the fallopian tube. Reducing the quantity of folds of the mucous membrane in all parts of the fallopian tube is characteristic of patients with uterine fibroid, in comparison with the control group. In patients with uterine fibroid, the thickness of membrane is 28±4, 8.2±1.1, 4.6±0.6 in the ampulla, isthmus and intramural part, respectively. In the control group the data are equal to 34±5, 11.6±1.6, 6.5±0.9, respectively. The relief of the mucous membrane is the most complicated in the ampulla of the fallopian tube; primary, secondary and tertiary folds in the lumen can be found there. In uterine fibroid, reduction or total absence of tertiary folds, reduction in the quantity of secondary folds in ampullary part of the fallopian tube can be noted. Increase in thickness of the folds with reduction of its ramification is noted. The patients with uterine fibroid show increase in the lumen of the ampulla (2.6±0.4 mm²) and decrease in the lumen of the isthmic part of the fallopian tube (0.150±0.020 mm²). The lumen becomes oval in the ampullary part and slotted in the isthmic part of the fallopian tube. In the control group the cross-sectional area of the lumen decreases from the ampulla to the isthmus, respectively 2.5±0.3 mm² and 0.18±0.03 mm². The lumen size in the isthmic part and intamural part are approximately equal. In the ampulla the lumen is oval, in the isthmus and intramural part it becomes rounded.
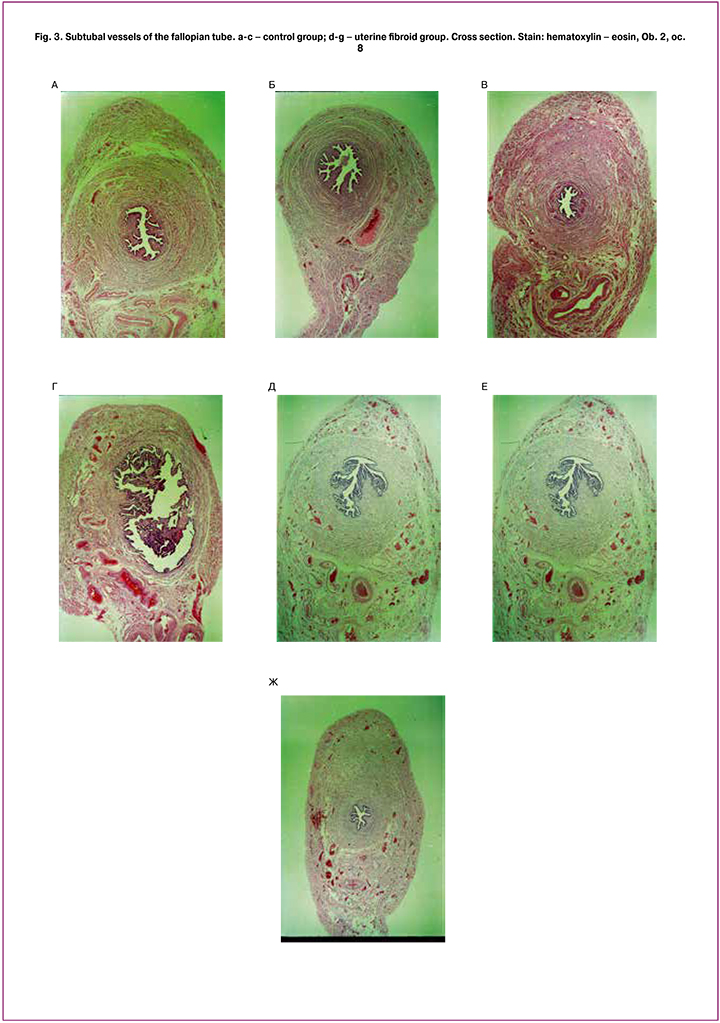
In uterine fibroid group, there is a loose type of vasculature without any regularities in distribution of vessels within the wall of the fallopian tube. There is a redistribution of the arterial blood supply of the fallopian tube wall. In the control group, small arteries and capillaries are mainly concentrated in the subtubal vascular bunch on the anterior and posterior walls of the fallopian tube In uterine fibroid group, small arterial vessels and capillaries are irregularly distributed on the anterior, posterior and superior walls of the fallopian tube, the expressiveness of the subtubal vascular bunch and its sizes is reduced. The processes of neo-angiogenesis and formation of the collateral blood supply in the wall of the fallopian tube are noted. Along with this, the stasis of the vessels from the venous system in the wall of the fallopian tube was noted. In this group, the most frequent are the vessels with the diameter of 0.3-0.79 mm. In addition to multiple blood vessels in the inferior wall of the fallopian tube, the large vessels in the posterior and superior walls can also be found. In the control group, the analysis of blood vessels diameter revealed that the vessels with the diameter of 0.1-0.39 mm can be found more frequently in the ampulla and isthmus of the fallopian tube. The largest vessels in these parts of the fallopian tube are located in the inferior part of the fallopian tube wall.
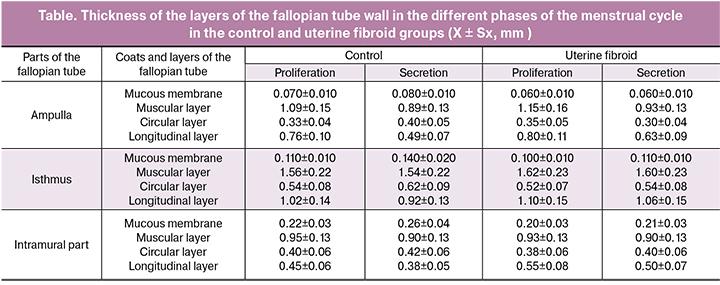
Morphometric characteristics of the lumen and wall of the fallopian tube dynamically change depending on the phase of the menstrual cycle. In the control group, the ampullary lumen which is the largest in the proliferation phase (0.35±0.5 mm²) decreases in the secretion phase (2.5±0.4 mm²). The lumen in the isthmic and intramural parts is the largest in the proliferation phase (0.230±0.020 mm² and 0.27±0.05 mm², respectively); it considerably decreases in the secretion phase (0.13±0.4 mm² and 0.10±0.04mm², respectively). In uterine fibroid group, there is a significant increase in the lumen of the ampulla and isthmus of the fallopian tube in the proliferation phase (3.5±0.8 mm², 0.23±0.01 mm²) and in the secretion phase (2.4±0.5 mm², 1.64±0.23 mm²). The reduction of the lumen of the intramural part in the phase of proliferation and secretion is noted (0.080±0.010 mm², 0.050±0.010mm², respectively). In the control group, thickness of the mucous membrane is the smallest in the phase of proliferation and it gradually increases in all parts in the secretion phase.
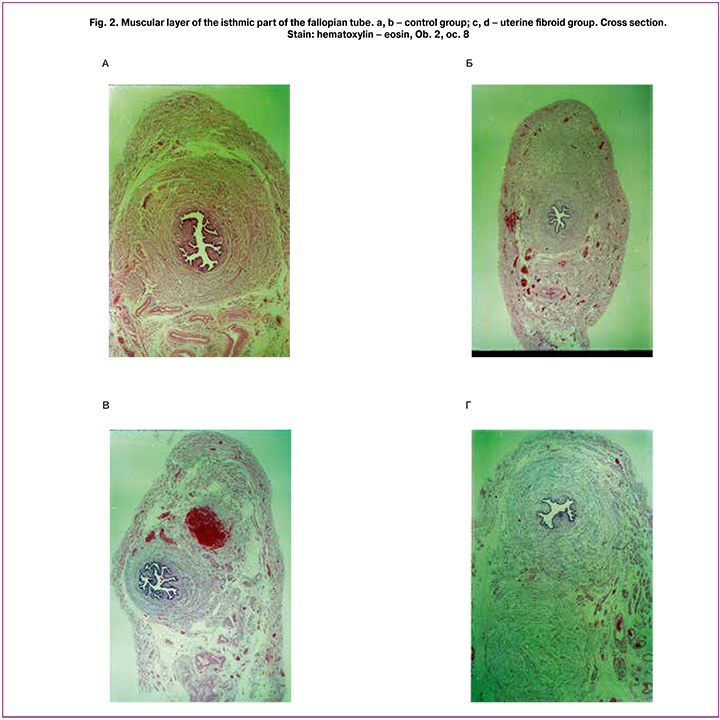
Uterine fibroids are characterized by minor dynamic changes in thickness of the fallopian tube wall depending on the phase of the menstrual cycle, while there is general increase of thickness of the wall, in comparison with that in the control group (Table). In the control group, thickness of the muscular layer in the ampullary part decreases in the secretion phase in comparison with the proliferation phase. In the isthmic intramural parts, it is possible to note the reduction of muscular layer thickness in the secretion phase in comparison with the proliferation phase (Table). In uterine fibroid group, the circular muscular layer of the fallopian tube wall has the morphometric characteristics inherent to those in the proliferation phase in the control group, without any dynamic changes that can be found in the secretion phase of the menstrual cycle. In the control group, increase in thickness of the circular muscular layer in the secretion phase is characteristic of the ampolla, isthmus and intramural part. In uterine fibroid group, increase in thickness of the longitudinal muscular layer and minor changes depending on the phase of the menstrual cycle is noted. In control group, thickness of the longitudinal muscular layer of the ampolla, isthmus and intramural part decreases in the secretion phase (Table).
The serous layer covering the anterior, superior and posterior walls of the ampulla and isthmus has identical thickness along the fallopian tube. Thickness of the subserous layer slightly increases in the isthmic part mainly due to the thickening of the muscular layer which is its part.
Conclusion
This research has shown the comparative analysis of morphological changes of the fallopian tubes in reproductive-aged women with non-tubal genital tract pathology, in particular uterine fibroid. The results of the research are the most important for assessment of the condition of the fallopian tubes in patients with idiopathic infertility. Practical application of our research can be recommended for surgical removal or reduction of uterine fibroid by iatrogenic interventions (resectoscopic surgery of submucosal uterine fibroids, laparoscopic myomectomy, uterine artery embolization, FUS ablation) in combination with the therapy of the fallopian tubes (local medicinal and/or recovery physiotherapy) in patients with intramural and/or submucosal forms of uterine fibroid. These recommendations are especially important for those patients who cannot conceive after surgical intervention, or artificial reproductive technologies and who have no other obvious cause for infertility except fibroid.
The participation of the fallopian tube in the processes of fertilization and transportation of the fertilised ovum into the uterine cavity allows to consider the changes in micro-architecture and vasculature of the fallopian tube wall in patients with uterine fibroid as the morphological substrate. It explains the increase in frequency of tubal pregnancy in performing reconstructive plastic surgeries or transferring gametes or zygotes into the lumen of the fallopian tubes in the IVF procedure. Besides, these changes can determine low efficiency of uterine pregnancy after reconstructive plastic surgeries of the fallopian tubes or IVF procedures followed by transferring gametes or zygotes into the lumen of the fallopian tube in the presence of uterine fibroid.
The results of the morphological research indicate that structural changes in the fallopian tube in patients with uterine fibroid can play a significant role as a morphological substrat. It can be used for clinical data associated with significantly low outcome of assisted reproductive technologies in uterine fibroid case records.
Our research allows us to define the algorithm of making a diagnosis and management of patients with uterine fibroid in an infertility clinic.
References
1. Адамян Л.В., Брагина Е.Е., Арсланян К.Н., Харченко Э.И. Возможности электронной микроскопии в оценке состояния маточных труб при бесплодии. В кн.: Адамян Л.В., ред. Современные технологии в диагностике и лечении гинекологических заболеваний. Руководство. М.: ФГУ НЦ АГиП Росмедтехнологий; 2007: 133-4. [Adamyan L.V., Bragina E.E., Arslanyan K.N., Kharchenko E.I. Possibilities of electron microscopy in assessing the state of fallopian tubes in infertility. In: Adamyan L.V., ed. Modern technologies in the diagnosis and treatment of gynecological diseases. Guideline. Moscow: FGU NC AGIP of Rosmedtechnologies; 2007: 133-4. (in Russian)]
2. Краснопольская К.В., Штыров С.В., Бугеренко А.Е. Чеченова Ф.К. Хирургическое лечение трубного бесплодия (обзор литературы). Проблемы репродукции. 2000; 6(4): 31-5. [Krasnopolskaya K.V., Shtyrov S.V., Bugerenko A.E. Chechenov F.K. Surgical treatment of tubal infertility (literature review). Problemy reproduktsii. 2000; 6(4): 31-5. (in Russian)]
3. Кулаков В.И., Селезнева Н.Д., Краснопольский В.И. Оперативная гинекология. Руководство. Кулаков В.И., ред. М.: Медицина; 1990. 464с. [Kulakov V.I., Selezneva N.D., Krasnopolsky V.I. Operative gynecology. Guideline. Kulakov V.I., ed. Moscow: Meditsina; 1990. 464p. (in Russian)]
4. Rabe T., Ahrend H.-J., Römer T., Bohlmann M.K., Wallwiener M., Fehr P.M. et al. Myomsprechstunde-Teil 2: Kinderwunsch. Neue diagnostische und therapetische Optionen bei Patientinnen mit Myomen. Gyn Praktische Gynäkologie. 2013; 18(2): 120-32.
5. Бродский Г.В., Адамян Л.В., Кондриков Н.И. Морфологические изменения маточной трубы при бесплодии, обусловленном миомой матки. Вопросы реконструктивной и пластической хирургии. 2010; 12(3): 42-7. [Brodsky G.V., Adamyan L.V., Kondrikov N.I. Morphological changes of the fallopian tube with infertility caused by uterine myoma. Voprosy rekonstruktivnoy i plasticheskoy khirurgii. 2010; 12(3): 42-7. (in Russian)]
Received 16.01.2018
Accepted 02.03.2018
About the Authors
Brodsky, Gregory V., MD, Specialist Obstetrics and Gynaecology, Head of Practice – Clinic for Gynaecology and Obstetrics, Munich, Germany.80807, Germany Munich, Oberhofer Platz 4. E-mail: 150665@gmx.net
Adamyan, Leila V., MD, professor, academician of RAS; deputy director on science, head of department of operative gynecology, National Medical Research Center
of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4
Sukhikh, Gennady T., MD, PhD, Professor, Academician of Russian Academy of Sciences, Director of National Medical Research Center of Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia. 117997, Russia Moscow, Ac. Oparina str. 4. Tel.: +74954381800. E-mail: gtsukhikh@mail.ru
For citations: Brodsky G.V., Adamyan L.V., Sukhikh G.T. Uterine fibroids as a risk factor for tubal infertility. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (10): 92-8. (in Russian)
https://dx.doi.org/10.18565/aig.2018.10.92-98



