Uterine microbiota and implantation failure: is there a link?
Aim. To investigate the impact of uterine microbiota on the success of embryo implantation in assisted reproductive technologies. Materials and methods. The study included 130 infertile women, who were divided into three groups. Group I (n=39) included women with the first IVF attempt. Group II (n=27) comprised patients with recurrent implantation failure following embryo transfer in the ovarian stimulation cycle. Group III (n=64) included patients with recurrent implantation failure following embryo transfer in a cryocycle. All patients underwent microbiological examination of the cervical canal discharge and samples obtained from embryo transfer catheter tips after an embryo transfer into the uterine cavity. Also, 30 samples were taken from the uterine cavity for high-throughput sequencing studies. Results. The study findings suggest that the uterine cavity is not sterile. Forty-four types of microorganisms were identified, including 26 types of opportunistic microorganisms (OpM) and 18 types of commensals (14 types of lactobacilli and four types of bifidobacteria). Obligate anaerobic microorganisms and Gardnerella vaginalis culture positivity rates were statistically significantly higher in group I compared with group III (strict anaerobes15.4 and 1.6%; G. vaginalis 12.8 and 1.6%, respectively) (p<0.05). However, this fact did not negatively affect the pregnancy rate, which was highest in group I (51.3%). Among women with recurrent implantation failure, it was 29.6 and 35.9%, respectively. Conclusion. The presence of low and moderate titers (103–105 CFU/ml) of OpM in the uterine cavity and cervical canal did not affect pregnancy rates in the study cohort. In 87.9% of patients, the microflora of the uterine cavity and cervical canal had different compositions. This observation suggests the possibility of the formation of an independent uterine microbiota. The uterine microbiota is characterized by less species diversity compared to the microbiota of the cervical canal.Keburiya L.K., Smol’nikova V.Yu., Priputnevich T.V., Murav’eva V.V., Trofimov D.Yu., Shubina E.S., Kochetkova T.O.
Keywords
Currently, infertility is one of the most serious medical problems in developed countries because of unfavorable demographic trends in populations. According to the ART Register of the Russian Association for Human Reproduction, pregnancy rates per embryo transfer after vitro fertilization (IVF) and intracytoplasmic sperm injection programs was 38.4% and did not show a tendency to increase [1].
One of the reasons for low IVF success rates is implantation failures that can be caused by:
- impaired endometrial receptivity caused by gynecological diseases (endometriosis, endometrial polyps, the consequences of inflammatory diseases, hormonal regulation disorders) [2–4];
- embryo chromosomal abnormalities [5, 6];
- the zona pellucida thickening and hardening, leading to failure of IVF embryo attachment to the endometrium, which is more common in women of older reproductive age [7, 8].
In recent years, studies investigating this problem have been increasingly focused on uterine microbiota and its influence on implantation success.
The human microbiota is a complex combination of the microbes inhabiting many sites in the human body and living in symbiosis with the host organism. Although these symbiotic relationships have developed evolutionarily, our understanding of the physiological and pathophysiological role of the microbiota remains incomplete [9–15].
Until now, it remains unclear whether opportunistic microorganisms (OpM) identified in the endometrium have a negative effect on implantation; what composition of uterine microbiota is considered the norm, and what is associated with dysbiosis, which can impair embryo implantation, and where is the line between norm and pathology in quantitative and qualitative proportions.
The present study aimed to investigate the impact of uterine microbiota on the success of embryo implantation in women undergoing assisted reproductive technology.
Materials and methods
The study included 130 infertile women of reproductive age managed at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Inclusion criteria for the study were age from 23 to 37 years, regular menstrual cycle, absence of endometrial pathology according to ultrasound examination, embryo transfers (ET) with good quality embryos.
Exclusion criteria were interstitial and/or subserous uterine fibroids >4 cm deforming the uterine cavity, stage III–IV extragenital endometriosis, intrauterine pathology (intrauterine septum, endometrial polyp, chronic endometritis), severe pathozoospermia in the spouse (grade 3–4).
The patients were divided into three groups. Group I (n=39) included women with the first IVF attempt. Group II (n=27) comprised patients with recurrent implantation failure following embryo transfer in the ovarian stimulation cycle. Group III (n=64) included patients with recurrent implantation failure following embryo transfer in a cryopreserved cycle.
Before ET, all women underwent microbiological examination of the cervical canal microbiota. To prevent contamination of the cervical canal with vaginal microflora, the vaginal portion of the cervix was cleansed with a sterile cotton swab. The cervical canal discharge was sampled using a Dacron swab with Amies liquid transport medium (Medical Wire, England). The microbiota composition was investigated by culture-based techniques using an extended set of selective and non-selective culture media. The cervical canal discharge was cultured on selective and non-selective agar solid nutrient media. After ET, the embryo transfer catheter tips were cut off with sterile scissors and placed in a tube with a storage medium (1 ml) used for blood cultures (Oxoid, UK).
Obligate anaerobes were cultured for 48 hours in an anaerobic box (Jouan; France) filled with a three-component gas mixture (N2 – 80%; CO2 – 10%; Н2 – 10%). Microbiota composition was investigated culturomic technique. To culture facultative anaerobic microorganisms, we used Columbia agar (Oxoid, Great Britain), mannitol salt agar (Himedia, India), medium for the identification and differentiation of Streptococcus agalactiae (CHROMagar, France), enterococcal agar, and Endo-GRM agar (FBIS SRCAMB, Obolensk, Russia), Sabouraud dextrose agar (Oxoid, Great Britain). Lactobacilli were cultivated on Lactobacagar medium (FBIS SRCAMB, Obolensk, Russia), strict anaerobes on agar for bifidobacteria (Himedia, India), pre-reduced Shedler agar with the necessary additives, basic agar for anaerobes, and perfringens agar (Oxoid, the UK).
The isolated microorganisms were identified by MALDI time-of-flight mass spectrometry (MALDI-TOF MS) using an AutoFlex III mass spectrometer with Maldi BioTyper software (Bruker Daltonics, Germany) version 3.0.
All women underwent selective transfer of 1 good-quality embryo. Patients of groups I and II underwent controlled ovarian hyperstimulation from the 2nd or 3rd day of the menstrual cycle according to the protocol with gonadotropin-releasing hormone antagonists. Human chorionic gonadotropin (10,000 IU) was used as an ovulation trigger.
ET in cryopreserved cycle was performed without hormone replacement therapy in a natural ovulatory cycle. The post-transfer period was managed according to the generally accepted technique with oral 30 mg/day dydrogesterone from the day of transvaginal ovarian puncture in controlled ovarian hyperstimulation cycles.
A comparative analysis of the results was carried out in women in controlled ovarian hyperstimulation cycles and cryopreserved cycles, depending on pregnancy onset or failure.
At the second stage of the study, 30 samples were taken from the uterine cavity (a fragment of the embryo transfer catheter in the storage medium) for next-generation sequencing to compare with microbiological data.
Variable regions V3–V4 were selected to determine uterine microbiota species composition by next-generation sequencing (NGS). The amplification of the selected region was carried out using the published primers 357F and 806R, DNA isolation from the samples using the MCh-Rapid v2 DNA reagent kit, and the amplification of the 16S rRNA gene sequence was performed using the DT-Prime detecting amplifier (DNA Technology, Russia). The quality of the obtained amplicons was assessed in a 2% agarose gel. The quality and concentration of DNA libraries for NGS sequencing were checked using a Bioanalyzer. Sequencing was performed on a MiSeq device (Illumina, USA) on v2 wells according to the manufacturer's protocol. Data were analyzed using the QIMME software.
Statistical analysis
Statistical analysis was performed using the IPM SPSS Statistics software, version 22. Quantitative variables were expressed as means (M) and standard deviation (SD). Categorical variables were summarized using frequency counts (n) and percentages (%). Normally distributed continuous variables were compared between two groups with a Student’s t-test. Categorical variables were compared between groups with the χ2 test. Differences between the groups were considered statistically significant at p<0.05. Bonferroni correction was applied for multiple comparisons; p-values < 0.017 were considered significant.
Results
The age of the patients in the study groups ranged from 22 to 37 years; no statistically significant differences in age were found between the groups (p> 0.017). Mean ages in groups I, II, and III were 30.4 (3.3), 32.3 (3.5), and 32.0 (3.0) years, respectively.
Reproductive outcomes in the study groups are presented in the table.
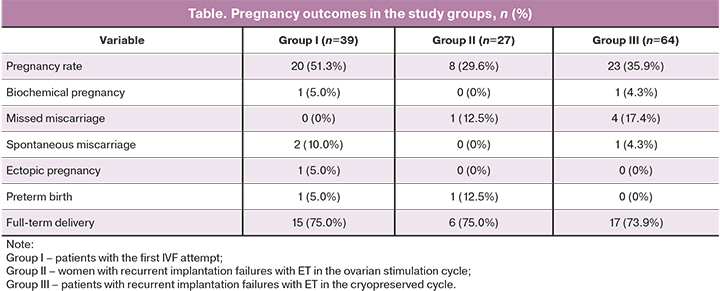
As shown in the table, the pregnancy rate in group I [51.3% (20/39)] was 1.7 times higher than in group II [29.6% (8/27)] and 1.4 times higher than in group III [35.9% (23/64)]. However, the differences were statistically insignificant (p> 0.017). There were no missed miscarriages in group I (0%), while patients in groups II and III 12.5% and 17.4% of patients had missed miscarriages, respectively; the differences were statistically insignificant (p> 0.017). The rates of full-term delivery in groups I, II, and II were 75% 1 (15/20), 75% (6/8), and 73.9% (17/23), respectively.
A quantitative and qualitative analysis of the composition of microflora isolated from the cervical canal in the study participants was carried out.
Patients with the first IVF attempt most often had moderate (104–105 CFU/ml) or high (106 CFU/ml and more) colony counts of lactobacilli, more often in women with than without pregnancy [90.0% (18/20) vs. 73.7% (14/19)]; however, no statistically significant difference was found (p>0.05). OpMs were isolated mainly in low colony counts (103 CFU/ml or less). The exceptions were Gardnerella and obligate anaerobic microorganisms. Thus, G. vaginalis was found primarily in moderate or high colony counts. In pregnant women, it was isolated only in the medium [15% (3/20)] or high [5% (1/20)] counts, but among those who did not achieve pregnancy, it was isolated in high [5.3% (1/19)], and moderate [10.6% (2/19)] counts.
Obligate-anaerobic microorganisms, on the contrary, were more often isolated in low [15.8% (3/19)] or moderate [10.5% (2/19)] counts in women achieving pregnancy. In pregnant women, they were found with similar rates 5% (1/20) with low and moderate counts and without a statistical difference. Other OpMs such as enterococci, enterobacteria, and actinomycetes colonized the cervical canal only at low concentrations. A complete picture of microbial colonization, taking into account the isolation rates and quantitative assessment of microorganisms in group I, is shown on the heat map (Fig. 1).
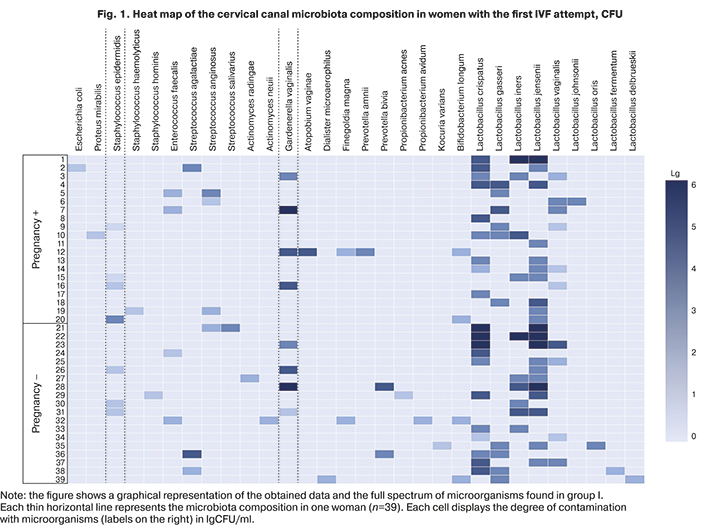
Thus, in patients of group I who achieved and did not achieve pregnancy, there was no statistically significant difference in isolation rates of lactobacilli and OpM in the cervical samples, both in low, moderate, or high counts. More frequent colonization with lactobacilli in moderate or high concentration and more frequent culture positivity of OpM (G. vaginalis) with moderate or high titer in patients of group I with the onset of pregnancy did not significantly affect embryo implantation.
In women with recurrent implantation failures and ET in the ovarian stimulation cycle, compared with patients of group I, moderate or large amounts of lactobacilli were more often isolated in non-pregnant women [63.2% (12/19)], than in women who became pregnant [50.0% (4/8)], however, no statistically significant difference was found (p>0.05). OpM was isolated at low, moderate, or high titers. Thus, enterococcus and coagulase-negative staphylococci were found only in low concentrations; streptococci in both subgroups were isolated mainly in moderate titer with predominance in the group of pregnant women [12.5% (1/8)] and [5.3% (1/19)], respectively. Gardnerella was found in both subgroups in a moderate titer more often in women not achieving pregnancy [21.1% (4/19)], and a high titer in one pregnant woman [12.5% (1/8)]. Strict anaerobes in low [12.5% (1/8)] and moderate [12.5% (1/8)] titers were also isolated more often in women who became pregnant than in those who did not [10.5% (2/19)]. A complete picture of microbial colonization, taking into account the isolation rates and quantitative assessment of microorganisms in group II, is shown in Figure 2.
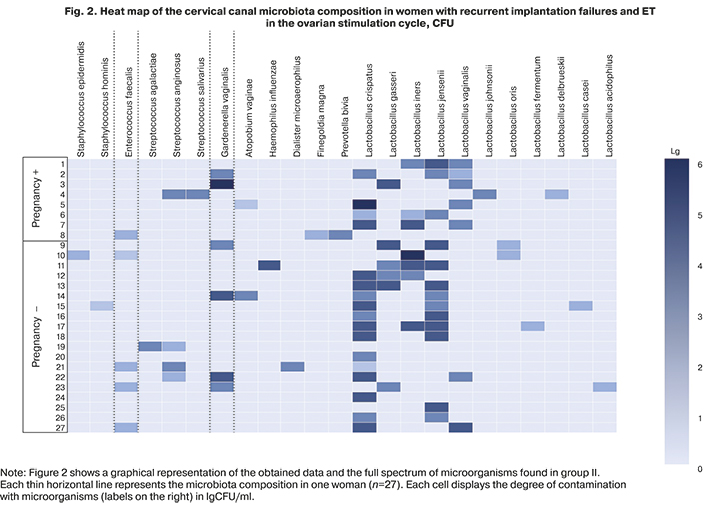
In group II, pregnant women with recurrent implantation failures and ET in the controlled ovarian hyperstimulation showed lower isolation rates of lactobacilli, which determine the colonization resistance of the biotope, and a lower degree of their colonization of the cervical canal and, on the contrary, more frequent colonization of the cervical canal OpM in low and moderate titers. However, this did not negatively affect the onset of pregnancy, which indicates that the presence of microorganisms in the cervical canal, including OpM in a low and moderate titer, does not necessarily affect implantation processes and the onset of pregnancy.
In patients with recurrent implantation failures and ET in the cryopreserved cycle, moderate or high counts of lactobacilli were most often isolated, more often in women with pregnancy [60.9% (12/23)] than in those not achieving pregnancy [51.2% (21/41)]; however, no statistically significant difference was found (p> 0.05). OpM was isolated predominantly in a low titer. The exceptions are Gardnerella, which was found in both subgroups only in moderate counts [4.3% (1/23) and 7.3% (3/41)], respectively; severe anaerobes, which in pregnant women were detected only in low concentrations [13.0% (3/23)], and among women without pregnancy with the same frequency in low and moderate titers [9.75% each (4/41)]. Coagulase-negative staphylococci and enterococci were almost always present in low titers.
It should be noted that there was no statistically significant difference in the cervical canal colonization rate by the mentioned groups of microorganisms in a low and, more importantly, in a moderate count between women with the onset of pregnancy and non-pregnant women (p>0.05). The complete picture of microbial colonization, taking into account the isolation rates and quantitative assessment of microorganisms in group III, is shown in Figure 3.
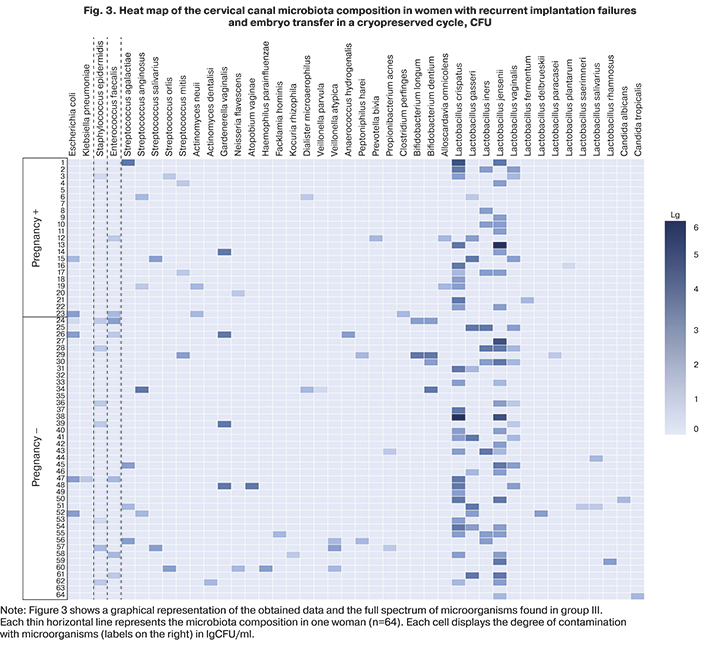
In group III, pregnant women with a missed miscarriage, recurrent implantation failures, and ET in a cryopreserved cycle, colonization of the cervical canal with high or moderate titers were noted more often than in women achieving pregnancy. Conversely, they more often had OpM (streptococci, enterobacteria, and especially strict anaerobes, including Gardnerella) in moderate titers. However, the emerging trend in the absence of a statistically significant difference in these indicators does not suggest that a decrease in the titer of lactobacilli, as well as an increase in the number of OpM to moderate values, as a risk factor for non-pregnancy.
A comparative assessment of the cervical canal microbiota showed that the discharge of the cervical canal was sterile only in 7.8% of patients with recurrent implantation failures and ET in the cryopreserved cycle (group III). The species composition of cervical canal communities varied. Dominant in all groups were lactobacilli (16 species), found in patients of groups I, II, and III with detection rates of 94.9% (37/39); 92.6% (25/27), and 81.3% (52/64), respectively.
The second most abundant bacteria of the cervical canal microbiota were OpMs, representing 66.6% (26/39), 55.5% (15/27), and 54.7% (35/64) in groups I, II, and III. Among facultative anaerobic OpMs enterobacteria were more common in women with recurrent implantation failures and ET in the cryopreserved cycle (group III) [9.4% (6/64)], compared with group I [5.1% (2/39)] and II (0%). Streptococci were also isolated more often in group III [23.4% (15/64)] compared with women with the first IVF attempt [18.0% (7/39)] and group II [14.8% (4/27)]. Staphylococci were isolated more often in group I [23.1% (9/39)] compared with patients in group II [7.4% (2/27)], and group III [12.5% (8/64)], respectively. However, isolation rates of enterococci were higher in women in group II [18.5% (5/27)] compared with patients in group I [10.3% (4/39)] and in group III [10.9% (7/64)].
The isolation rate of obligate anaerobic bacteria was approximately the same in all groups, including 17.9% (7/39) in group I, 14.8% (4/27) in group II, and 17.2% in group III (11/64). The isolation rate of G. vaginalis was noticeably higher in women in groups I [20.5% (8/39)] and in groups II [22.2% (6/27)] compared with patients in group III [6.3% (4/64)]; however, the difference was statistically insignificant (p>0.017).
 The most abundant microorganisms of the cervical canal microbiota in all groups of women were lactobacilli, which were somewhat more common in women with ET in the ovarian stimulation cycle (groups I and II) compared with women with ET in the cryopreserved cycle (group III), but without statistically significant difference. In almost 40.0% of women of all groups, the cervical canal was inhabited only by lactobacilli [33.3% (13/39); 44.4% (12/27) and 36.0% (23/64), respectively], mainly by associations of two or three types. OpM was found in every second woman in all groups. There was no statistically significant difference in all groups regarding the colonization of the cervical canal by facultative and obligate anaerobes. The isolation rates of G. vaginalis were higher in women in groups I (20.5%) and II (22.2%) compared with III (6.3%), but the difference was statistically insignificant (p>0.017).
The most abundant microorganisms of the cervical canal microbiota in all groups of women were lactobacilli, which were somewhat more common in women with ET in the ovarian stimulation cycle (groups I and II) compared with women with ET in the cryopreserved cycle (group III), but without statistically significant difference. In almost 40.0% of women of all groups, the cervical canal was inhabited only by lactobacilli [33.3% (13/39); 44.4% (12/27) and 36.0% (23/64), respectively], mainly by associations of two or three types. OpM was found in every second woman in all groups. There was no statistically significant difference in all groups regarding the colonization of the cervical canal by facultative and obligate anaerobes. The isolation rates of G. vaginalis were higher in women in groups I (20.5%) and II (22.2%) compared with III (6.3%), but the difference was statistically insignificant (p>0.017).
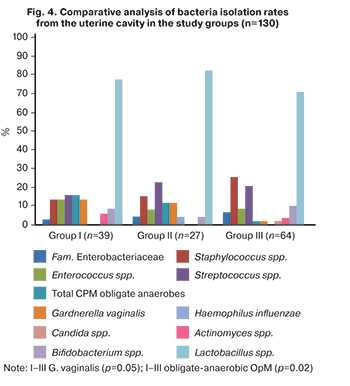
In the study, the analysis of biomaterial from the uterine cavity was carried out. Results of comparative analysis of the uterine microbiota in the study groups are presented in Figure 4.
According to a culture study of the uterine cavity samples of 130 women, microflora was found in 89.2% of cases. It was found that the uterine cavity in women of groups I, II, and III is sterile only in 10.3% (4/39), 7.4% (2/27), and 12.5% (8/64) of cases, respectively. The species composition of the uterine cavity communities varied. Lactobacilli (14 species) were dominant in all groups, of which L. jensenii, L. crispatus, and L. vaginalis were more common. There was no statistically significant difference in uterine cavity colonization rate with lactobacilli in total and isolation rate of certain species among the patients of the compared groups (p>0.017). OpMs in groups I–III were isolated with almost the same rate – 46.1% (18/39), 44.4% (12/27), and 48.4% (31/64), respectively (p>0.017).
It is noteworthy that staphylococci were isolated more often in group III [25.0% (16/64)] compared with group I [12.8% (5/39)] and group II [14.8% (2/27)]. Streptococci were more common in group II [22.2% (6/27)] and III with ET in the cryopreserved cycle [20.3% (13/64)], compared with women with the first IVF attempt [15.4% (6/39)]. However, the isolation rate of enterococci was higher in group I [12.8% (5/39)] compared to women in group II [7.4% (2/27)] and group III [7.8% (5/64)]. It should be noted that the isolation rate of obligate-anaerobic microorganisms was statistically significantly higher in group I [15.4% (6/39)] compared to group III [1.6% (1/64)], (p <0.001). The isolation rate of G. vaginalis was also higher in group I [12.8% (5/39)] than in group III [1.6% (1/64)], (p=0.02).
The sequencing findings of uterine microbiota are shown in Figure 5. The most abundant (more than 5.0%) bacterial genera are displayed according to the bacterial 16S ribosomal RNA gene sequencing data.
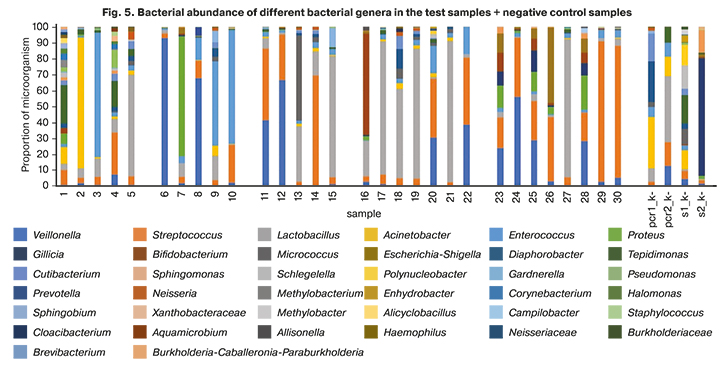
All samples from a sterile uterine cavity had extremely low concentrations of bacterial DNA, comparable to the concentration of DNA in negative control samples. These samples contained the genera Lactobacillus, Tepidimonas, Streptococcus, Acinetobacter, Proteus, Acinetobacter, Enterococcus, Pseudomonas, and Veillonella, which are also detected in negative control samples. These findings may be due to trace amounts of bacterial DNA in some of the reagents used and indicate that the sequencing method is not specific enough to work with samples with extremely low DNA concentration.
In most cases, the data on the presence of microorganisms in the uterine cavity according to microbiological studies were similar to those according to sequencing. Some detected bacterial genera were not identified by microbiological analysis. These results can be explained by the presence in the sample of microorganisms that, for various reasons, did not grow on the nutrient medium, but their DNA is present in the sample.
Sequencing allowed us to determine the quantitative ratio of microorganisms; however, this data should be treated with caution since the samples of the culture medium are examined, and not the sample itself, and the ratio may depend on the growth rate of various microorganisms.
Thus, using this method for assessing the uterine microflora at the time of ET is questionable. However, sequencing may help assess the endometrial microbiota and receptivity when obtaining biological material using pipelle endometrial sampling, given the greater sample biomass than on embryo transfer catheter tips.
Discussion
In recent years, studies investigating the human microbiome have been increasingly focused on uterine microbiota and its influence on implantation success.
There remains much debate concerning the sterility of the uterine cavity. However, there is still no clear understanding of the effect of endometrial microflora on implantation processes. Besides, it has not been studied what should be understood by the uterine cavity eubiosis and what by dysbiotic disorder; there is no clear line between these two concepts. Moreover, the detection of OpMs in the reproductive tract and antibiotic therapy is often unsuccessful.
It should be noted that the uterine microflora was found in 87.7% of womenб suggesting that the uterine cavity is not free from microorganisms, and our findings are consistent with the results of other authors [16–23].
After ET, the highest pregnancy rate was in the group I (51.3%) compared with patients with recurrent implantation failures (29.6% and 35.9%, respectively). Although the isolation rate of obligate anaerobic microorganisms and G. vaginalis was statistically significantly higher in the group, I compared with group III [strict anaerobes 15.4 and 1.6% (p<0.001)]; G. vaginalis 12.8% and 1.6%, respectively (p=0.02), this fact did not negatively affect the implantation process in this group of women. In patients with recurrent implantation failures (groups II and III), the missed miscarriage incidence was 12.5% and 17.4%, respectively, while in women with the first IVF attempt, it was 0%. The rates of full-term delivery in groups I, II, and II were 75% 1 (15/20), 75% (6/8), and 73.9% (17/23), respectively.
Moreno I. et al. [16] evaluated the effect of the uterine microbiota on implantation. They found that a non–Lactobacillus dominated (<90%) endometrial microbiota significantly correlates with adverse reproductive outcomes compared with subjects presenting a Lactobacillus-dominated (>90%) endometrial microbiota, including a lower of implantation rate (60.7% versus 23.1%, p=0.02), pregnancy rate (70.6% versus 33.3%, p=0.03), ongoing pregnancy rate (58.8% versus 13.3%, p=0.02), and birth rate (58.8% versus 6.7%, p=0.002).
In a study by Kyono K. [24, 25], pregnancy rates were analyzed in patients with lactobacilli dominance (lactobacilli abundance > 80%) and a group without lactobacilli dominance (lactobacilli abundance <80% and OpMs >20%). The pregnancy rate was higher in women with lactobacillus dominance (>80%) – 61.3% of women compared with patients without lactobacillus dominance (<80%) – 40% of women.
Some other researchers have suggested otherwise. So, in a study by Kitaya K. et al. [26], on the contrary, the prevalence of lactobacilli in the uterine cavity (> 90%) was more common in women with repeated implantation failures [64.3% (18/28)] than in the control group [38.9% (7/18)]. Similar results were obtained from vaginal samples, where lactobacilli dominated in women with repeated implantation failures [67.9% (19/28)], in contrast to patients with the first IVF attempt [44.4% (8/18)]. The isolation rate of Gardnerella was 39.3% (11/28) in women with repeated implantation failures and 27.7% (5/18) in the control group.
In addition, Wang R. et al. (2016) reported a greater abundance of lactobacilli in patients with chronic endometritis (33.2%) compared with healthy women (6.2%) [27]. Thus, the pathogenetically significant interaction between the endometrial microbiota and immunity is becoming a considerable direction of scientific research, not just confirmation of the presence of microorganisms in the endometrium.
The results of our study are consistent with the results of other researchers [26, 27]. There is no clear evidence of an advantage of lactobacillus-dominated endometrial composition in terms of pregnancy outcomes. But the restoration of the endometrial microbiota aiming to lactobacilli predominance is favorable for embryo implantation. The presence of OpMs in the uterine cavity did not affect the pregnancy rate.
Probably, the endometrial microbiota is a collection of functionally related microorganisms. And, obviously, in every healthy individual, the homeostasis of the uterine cavity is supported by "their" certain microorganisms. Biofilms, which represent microbial communities, play an essential role. Bacteria in biofilms have specific physiological properties. Normal biofilms in the human body are characterized by microbial communities that form the physiological microflora of the skin, mouth, vagina, intestines, etc. But there are also pathological biofilms that are often associated with chronic inflammatory processes.
However, one cannot exclude the negative impact on the onset of pregnancy of such microorganisms as Haemophilus influenzae and Haemophilus parainfluenzae, found in moderate quantities in patients with repeated implantation failures. These OpMs have a high pathogenic potential and are atypical for the biotope of the reproductive tract, more often entering it through the hematogenous route from the oral cavity.
When comparing the microbiota of the uterine cavity and cervical canal, it was noted that in 87.9% of patients, these two biotopes differed in qualitative composition, which does not exclude the possibility of the formation of an independent microbiota in the uterine cavity, which is less species-rich compared to the cervical canal.
Comparison of the microbiota of the upper and lower parts of the genital tract causes numerous discussions. In a study by Moreno I. et al. in approximately 20% of the women analyzed, the bacteria community identified in the endometrium varied greatly from that in the vagina, suggesting that the endometrial and vaginal microbiota are not identical [16]. Differences between these biotopes were found in Wee B.A. et al., who examined the vaginal, cervical, and endometrial microbiota in women with a history of infertility compared to fertile women [28]. These results indicate that the upper and lower reproductive tract microbiota may be similar but not always identical.
A separate stage of the work was the metagenomic sequencing of the uterine microbiota (embryo transfer catheter tips in the storage medium). This method of studying the microbiota is described in the studies of other authors [16, 19, 20]. In most cases, uterine cavity microorganisms detected by culture and metagenomic studies are identical. Some detected bacterial genera were not identified by microbiological analysis. These findings can be explained by the presence in the sample of microorganisms that, for various reasons, did not grow on the nutrient medium, but their DNA is present in the sample. These findings may be due to trace amounts of bacterial DNA in some of the reagents used and indicate that the sequencing method is not specific enough to work with samples with extremely low DNA concentration. It can be assumed that, in addition to the presence of microorganisms in the uterine cavity, it is essential to quantitatively assess the composition of the microflora, which is problematic at the time of transfer, given the low potential for obtaining a sufficient amount of a sample using an embryo transfer catheter. The second important point is the state of the macro-organism in terms of protection from the destructive effect of the microbial factor on the endometrium. It is known that under physiological conditions, microorganisms are inactivated by the system of innate and adaptive immunity. This process is carried out in the early stages by TOLL-NOD and TOLL-RIG-like receptors located on the surface of the immune system cells, epithelial cells, endothelial cells, and fibroblasts. Microbial colonization of the endometrium is also inhibited by TOLL-like receptors, which, interacting with the microbial cell structures, stimulate the mechanisms of innate antimicrobial resistance.
Conclusion
The lack of statistical confirmation of the impact of uterine cavity microorganisms on reproductive outcomes suggests the need for further studies on the endometrial microbiota. The presence of bacteria is important, but also the individual characteristics of the macro-organism and the interaction between microorganisms and macro-organism. A clear understanding of the mechanisms of dysbiotic processes of the development of a chronic inflammation in the uterine cavity and its timely diagnosis and treatment are fundamental issues in the management of patients with a history of implantation failures.
References
- Регистр ВРТ. Отчет за 2017 год. [Register of ART. Report for 2017. (in Russian)].
- Савельева Г.М., Сухих Г.Т., Серов В.Н., Радзинский В.Е., Манухин И.Б., ред. Гинекология. Национальное руководство. 2-е изд. М.: ГЭОТАР-Медиа; 2019. [Savelieva G.M., Sukhikh G.T., Serov V.N., Radzinsky V.E., Manukhin I.B., ed. Gynecology. National leadership. M.: Geotar-Media, 2019. (in Russian)].
- Унанян А.Л., Сидорова И.С., Коган Е.А., Белогубова С.Ю., Демура Т.А., Елисаветская А.М., Сизова Н.М. Эндометриоз, аденомиоз, хронический эндометрит: клинико-патогенетические взаимоотношения и репродуктивные неудачи. Акушерство и гинекология. 2018; 10: 136-40. [Unanyan A.L., Sidorova I.S., Kogan E. A., Belogubova S.Yu., Demura T.A., Elisavetskaya A.M., Sizova N.M. Endometriosis, Adenomiosis, Chronic endometritis: clinical and pathogenetic relationships and reproductive failure. Obstetrics and Gynecology, 2018; 10: 136-40. (in Russian)].
- Тихончук Е.Ю., Асатурова А.В., Адамян Л.В. Молекулярно-биологические изменения эндометрия у женщин с наружно-генитальным эндометриозом. Акушерство и гинекология. 2016; 11: 42-8. https://dx.doi.org/10.18565/aig.2016.11.42-48. [Tikhonchuk E.Yu., Asaturova A.V., Adamyan L.V. Endometrial molecular biological changes in woman with external genital endometriosis». Obstetrics and Gynecology, 2018;10: 136-40. (in Russian)].
- Сафронова Н.А., Калинина Е.А., Донников А.Е., Бурменская О.В., Макарова Н.П., Горшинова В.К. Уровень экспрессии гена кальмодулина в кумулюсных клетках как маркер наличия хромосомных аномалий в эмбрионах в программах экстракорпорального оплодотворения. Акушерство и гинекология. 2016; 10: 64-72. [Safronova N.A., Kalinina E.A., Donnikov A.E., Burmenskaya O.V., Makarova N.P., Gorshinova V.K. Calm 2 gene expression levels in the cumulus cells as a marker for chromosomal abnormalities in the embryo in in vitro fertilization programs. Obstetrics and Gynecology. 2016; 10: 64-72. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.10.64-72.
- Кулакова Е.В., Калинина Е.А., Трофимов Д.Ю., Макарова Н.П., Хечумян Л.Р., Дударова А.Х. Вспомогательные репродуктивные технологии у супружеских пар с высоким риском генетических нарушений. Преимплантационный генетический скрининг. Акушерство и гинекология. 2017; 8: 21-7. [Kulakova E.V., Kalinina E.A., Trofimov D.Y., Makarova N.P., Khechumyan L.R., Dudarova A.K. «Assisted reproductive technologies in married couples at high risk for genetic disorders. Preimplantation genetic screening». Obstetrics and Gynecology. 2017; 8: 21-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.8.21-27.
- Шафеи Р.А., Сыркашева А.Г., Романов А.Ю., Макарова Н.П., Долгушина Н.В., Семенова М.Л. Хетчинг бластоцисты у человека. Онтогенез. 2017; 48(1): 8-20. [Shafei R.A., Semenova M.L., Syrkasheva A.G., Romanov A.Y., Makarova N.P., Dolgushina N.V. «Blastocyst hatching in humans». Russian Journal of Developmental Biology. 2017; 48(1): 8-20. (in Russian)].
- Ибрагимова Э.О., Долгушина Н.В., Сыркашева А.Г., Романов А.Ю., Языкова О.И., Макарова Н.П. Роль вспомогательного хетчинга в программах лечения бесплодия методами вспомогательных репродуктивных технологий: обзор литературы. Гинекология. 2016; 18(2): 44-7. [Ibragimova E.O., Dolgushina N.V., Syrkasheva A.G., Romanov A.Yu., Yazikova O.I., Makarova N.P. The role of assisted hatching in in vitro fertilization cycles: a literature review. Gynecology. 2016; 18(2): 44-7. (in Russian)].
- Perez-Muñoz M.E., Arrieta M.C., Ramer-Tait A.E., Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017; 5(1): 48. https://dx.doi.org/10.1186/s40168-017-0268-4.
- D’Ippolito S., Di Nicuolo F., Pontecorvi A., Gratta M., Scambia G.., Di Simone N. Endometrial microbes and microbiome: Recent insights on the inflammatory and immune “players” of the human endometrium. Am. J. Reprod. Immunol. 2018; 80(6): e13065. https://dx.doi.org/10.1111/aji.13065.
- Benner M., Ferwerda G., Joosten I., van der Molen R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update. 2018; 24(4): 393-415. https://dx.doi.org/10.1093/humupd/dmy012.
- Walther-Antonio M.R., Chen J., Multinu F., Hokenstad A., Distad T.J., Cheek E.H. et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016; 8(1): 122. https://dx.doi.org/10.1186/s13073-016-0368-y.
- Miles S.M., Hardy B.L., Merrell D.S. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingooopherectomy. Fertil. Steril. 2017; 107(3): 813-20. e81. https://dx.doi.org/10.1016/j.fertnstert.2016.11.028.
- Chen C., Song X., Wei W., Zhong H., Dai J., Lan Z. et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017; 8(1): 875. https://dx.doi.org/10.1038/s41467-017-00901-0.
- Fang R.L., Chen L.X., Shu W.S., Yao S.Z., Wang S.W., Chen Y.Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016; 8(3):1581-92.
- Moreno I., Franasiak J.M. Endometrial microbiota – new player in town. Fertil. Steril. 2017; 108(1): 32-9. https://dx.doi.org/10.1016/j.fertnstert.2017.05.034.
- Franasiak J.M., Scott R.T. Endometrial microbiome. Curr. Opin. Obstet. Gynecol. 2017; 29(3): 146-52. https://dx.doi.org/10.1097/GCO.0000000000000357.
- Mitchell C.M., Haick A., Nkwopara E., Garcia R., Rendi M., Agnew K., Fredricks D.N., Eschenbach D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obstet. Gynecol. 2015; 212(5): 611. e1-9. https://dx.doi.org/10.1016/j.ajog.2014.11.043.
- Moreno I., Codoñer F.M., Vilella F., Valbuena D., Martinez-Blanch J.F., Jimenez-Almazán J. et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016; 215(6): 684-703. https://dx.doi.org/10.1016/j.ajog.2016.09.075.
- Tao X., Franasiak J.M., Zhan Y., Scott R.T., Rajchel J., Bedard J. et al. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microb. J. 2017; 3(12): 15-21. https://dx.doi.org/10.1016/j.humic.2017.01.004.
- Franasiak J.M., Werner M.D., Juneau C.R., Tao X., Landis J., Zhan Y. et al. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016; 33(1): 129-36. https://dx.doi.org/10.1007/s10815-015-0614-z.
- Zygmunt M., Muzzio D.O. Microorganisms in the healthy upper reproductive tract: from denial to beneficial assignments for reproductive biology. Reprod. Biol. 2019; 19(2): 113-8. https://dx.doi.org/10.1016/j.repbio.2019.04.001.
- Verstraelen H., Vilchez-Vargas R., Desimpel F., Jauregui R., Vankeirsbilck N., Weyers S. et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ. 2016; 4: e1602. https://dx.doi.org/10.7717/peerj.1602.
- Kyono K., Hashimoto T., Kikuchi S., Nagai Y., Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: An analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod. Med. Biol. 2018; 18(1): 72-82. https://dx.doi.org/10.1002/rmb2.12250.
- Hashimoto T., Kyono K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J. Assist. Reprod. Genet. 2019; 36: 2471-9. https://dx.doi.org/10.1007/s10815-019-01630-7.
- Kitaya K., Nagai Y., Arai W., Sakuraba Y., Ishikawa T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediators Inflamm. 2019 May 21; 2019: 4893437. https://dx.doi.org/10.1155/2019/4893437.
- Wee B.A., Thomas M., Sweeney E.L., Frentiu F.D., Samios M., Ravel J. et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. N. Z. J. Obstet. Gynaecol. 2018; 58(3): 341-8. https://dx.doi.org/10.1111/ajo.12754.
Received 23.04.2021
Accepted 14.05.2021
About the Authors
Lela K. Keburiya, Ph.D. Student at the Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(903)965-55-47. E-mail: tati-keburiya@yandex.ru. 117997, Russia, Moscow, Oparina str., 4.
Veronika Yu. Smol’nikova, Dr. Med. Sci., Leading Researcher at the Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: v_smolnikova@oparina4.ru. 117997, Russia, Moscow, Oparina str., 4.
Tatiana V. Priputnevich, Dr. Med. Sci., Director of the Institute of Microbiology, Antimicrobial therapy and Epidemiology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. Tel.: +7(495)438-25-10. E-mail: priput1@gmail.com. 117997, Russia, Moscow, Oparina str., 4.
Vera V. Murav’eva, Ph.D. (bio), Researcher at the Institute of Microbiology, Antimicrobial therapy and Epidemiology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. E-mail: v_muravieva@oparina4.ru. 117997, Russia, Moscow, Oparina str., 4.
Dmitry Yu. Trofimov, Dr. Bio. Sci., Director of the Institute of Reproductive Genetics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: d_trofimov@oparina4.ru. 117997, Russia, Moscow, Oparina str., 4.
Ekaterina S. Shubina, Ph.D. (bio), Head of the Laboratory of Genomic Analysis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: e_shubina@oparina4.ru. 117997, Russia, Moscow, Oparina str., 4.
Taisya O. Kochetkova, Biologist at the Laboratory of Molecular Genetics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
E-mail: t_kochetkova@oparina4.ru. 117997, Russia, Moscow, Oparina str., 4.
For citation: Keburiya L.K., Smol’nikova V.Yu., Priputnevich T.V., Murav’eva V.V., Trofimov D.Yu., Shubina E.S., Kochetkova T.O. Uterine microbiota and implantation failure: is there a link?
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 133-143 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.133-143



