Несмотря на впечатляющие успехи молекулярной биологии, которые позволили раскрыть основные механизмы и этапы канцерогенеза, создать ряд принципиально новых по механизму действия противоопухолевых препаратов, значительно улучшить возможности инструментальной диагностики, проблема онкологических заболеваний продолжает оставаться одной из самых серьезных проблем современности. Так, в структуре онкологической заболеваемости женского населения России за 2015 г. Рак шейки матки (РШМ) занимает 6-е место, его удельный вес в структуре составил 5,2% (16 710 случаев), в то время как в возрастной группе 25–49 лет он занял 2-е место (после рака молочной железы) и составил 16,8%. За этот период времени под диспансерным наблюдением в онкологических учреждениях России находились 174 822 больных РШМ, смертность от РШМ составила 6628 случаев [1].
В 96% случаев причиной развития РШМ являются вирусы папилломы человека (ВПЧ) высокого, вероятного и возможного канцерогенного риска (IARC, 2012) [2]. По данным ВОЗ, распространенность в мире цервикальных интраэпителиальных неоплазий (CIN) составляет 40 млн случаев: CIN 1-й степени – 30 млн, CIN 2–3-й степени – 10 млн случаев. Частота прогрессии CIN 2–3 в карциному in situ варьирует от 40 до 60%. Так, в структуре патологии шейки матки у женщин репродуктивного возраста CIN составляет от 17 до 20%. Согласно данным литературы, после инфицирования ВПЧ CIN 2–3 развивается уже через 3 года у 27% женщин [3].
В настоящее время для поиска биомаркеров СIN и РШМ широко исследуют геном и транскриптом человека, то есть поиск ведется «внутри» клетки. Полученные биомаркеры связаны непосредственно с опухолевой клеткой и продуктами ее жизнедеятельности [4].
Большой интерес представляют появившиеся в последние годы исследования в области геномики, транскриптомики, протеомики [5–7] и особенно метаболомики, в частности липидомики [8, 9].
Известно, что при канцерогенезе повышается число не только молекулярно-генетических, но и метаболических повреждений эпителия шейки матки. Доказано, что злокачественная трансформация цервикального эпителия сопровождается количественными и качественными изменениями показателей уровня синтеза не только белков, но и различных низкомолекулярных соединений, включая липиды, в пораженных клетках [10, 11].
Атипические (предраковые и раковые) клетки находятся в окружении различных факторов, которые формируют их микроокружение (различные клетки, простые химические вещества и сложные макромолекулы) [12].
На сегодняшний день данные по исследованию липидного состава тканей шейки матки при ее ВПЧ-ассоциированных поражениях различной степени тяжести, включая РШМ, методом масс-спектрометрии в мировой литературе отсутствуют.
Требуется поиск потенциальных биомаркеров, которые могут обладать диагностической и прогностической значимостью в скрининговых программах или при разработке таргетной терапии.
Целью данной работы были выявление и оценка изменений липидомного состава тканей шейки матки при ВПЧ-ассоциированных поражениях различной степени тяжести у пациенток репродуктивного возраста.
Материал и методы исследования
В одномоментное проспективное исследование была включена 41 женщина в возрасте от 21 до 45 лет (средний возраст 34 года), обратившаяся в научно-поликлиническое отделение ФГБУ Национальный медицинский исследовательский центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России по поводу наличия патологии шейки матки.
Критерии включения: папилломавирусная инфекция, ВПЧ-ассоциированные заболевания шейки матки, регулярный менструальный цикл, подписанное информированное согласие.
Критерии исключения: наличие хронических заболеваний в стадии обострения, беременность, период лактации, прием гормональной терапии, наличие инфекций, передающихся половым путем, нарушение функции почек, печени, легких в стадии декомпенсации.
Комплексное обследование женщин включало сбор клинико-анамнестических данных, определение гинекологического статуса, цитологическое исследование, ВПЧ-типирование, расширенную кольпоскопию, прицельную биопсию шейки матки, гистологическое исследование биопсийного материала, липидомный анализ тканей шейки матки. Для оценки кольпоскопической картины использовалась единая Международная кольпоскопическая классификация, одобренная на 14-м Всемирном конгрессе IFCPC в Рио-де-Жанейро (2011 г.). Цитологическая оценка мазков с шейки матки осуществлялась по системе Бетесда (2004 г.). Гистологические образцы классифицировали следующим образом: легкая дисплазия (CIN1), умеренная дисплазия (CIN2), тяжелая дисплазия (CIN3) и РШМ. Нами была использована двухуровневая гистопатологическая классификация предраковых процессов шейки матки, согласно которой используется термин цервикальная интраэпителиальная неоплазия легкой степени (CIN1), который соответствует термину LSIL (Low grade squamous intraepithelial lesion или низкой степени плоскоклеточное интраэпителиальное поражение) и термин цервикальная интраэпителиальная неоплазия умеренной и тяжелой степени (CIN 2,3), который соответствует термину HSIL (High grade squamous intraepithelial lesion или высокой степени плоскоклеточное интраэпителиальное поражение) (Lower Anogenital Squamous Terminology (LAST), 2012 г.) [13].
Участники исследования дали добровольное письменное согласие на его проведение, подробности проводимого исследования были им разъяснены.
Для выполнения исследования использовали ткани, полученные после прицельной биопсии шейки матки у женщин с ВПЧ-ассоциированными заболеваниями. Биопсию выполняли в поликлиническом отделении ФГБУ НМИЦ АГП им. академика В.И. Кулакова Минздрава РФ строго при наличии показаний. Полученные ткани замораживали в жидком азоте и хранили при -80°С до дальнейшего анализа.
Экстракты липидов получали в соответствии с модифицированным методом Фолча. Биоптат гомогенизировали в керамической ступке с добавлением жидкого азота и к получившемуся гомогенату добавляли 4 мл смеси хлороформ-метанол (2:1, об./об.), смесь инкубировали в течение 10 мин, фильтровали с использованием фильтровальной бумаги и в полученный раствор добавляли 800 мкл водного раствора NaCl (1 моль/л). Смесь центрифугировали при 3000 об./мин. в течение 5 минут при температуре окружающей среды. Органический нижний слой, содержащий липиды, отбирали и высушивали в потоке азота, затем повторно растворяли в смеси ацетонитрил-2-пропанол (1:1, об./об.) для последующего масс-спектрометрического анализа.
Определение молекулярного состава образцов проводили с помощью масс-спектрометрии с электрораспылительной ионизацией на масс-спектрометре Maxis Impact qTOF (Bruker Daltonics, Бремен, Германия). Масс-спектры получали в режиме положительных ионов в диапазоне m/z 400–1000 со следующими настройками: напряжение на капилляре 4,1 кВ, давление распыляющего газа 0,7 бар, скорость потока осушающего газа 6 л/мин, температура осушающего газа 200oC. Для идентификации соединений, входящих в состав образца, выполняли тандемную масс-спектрометрию.
Масс-спектрометрические данные анализировали с помощью многофакторного анализа OPLS-DA (дискриминантный анализ с помощью ортогональных проекций на скрытые структуры) [14]. Этот метод позволяет построить статистическую модель на основе многомерных данных для классификации исследуемых образцов. С помощью анализа вклада переменной в проекцию (variable influence on projection – VIP) выявляли липиды, наиболее значимые для классификации. Данные липиды можно рассматривать в качестве потенциальных биомаркеров. В дальнейшем их идентифицировали по точной массе с помощью базы данных Lipid Maps [15] и по характерным тандемным масс-спектрам.
Номенклатура липидов соответствует рекомендациям консорциума Lipid Maps [15].
Результаты и обсуждение
По результатам гистологического исследования были сформированы 4 группы: I группа – 8 (19%) пациенток с хроническим цервицитом в сочетании с ВПЧ, II группа – 9 (22%) с LSIL, III группа – 13 (32%) с HSIL, IV группа – 11 (27%) с РШМ. Основная часть обследуемых пациенток были репродуктивного возраста, соматически не отягощены и подходили под все критерии включения в исследование. Возраст менархе в среднем составил 13,3 года. Средний возраст начала половой жизни 18,7 года. Общее количество беременностей составило 76, из которых родами завершились 37 (49%), абортами – 39 (51%). У 12 (29%) пациенток было более 4 половых партнеров. Из перенесенных гинекологических заболеваний в анамнезе выявлена высокая частота инфекций, передающихся половым путем (51%). Анализ клинико-анамнестических данных показал, что более половины женщин инфицированных ВПЧ имели ранний половой дебют (до 18 лет в 58% случаев).
При изучении возрастного распределения выяснилось, что у женщин в возрасте до 30 лет чаще встречались ВПЧ высоко онкогенного типа (70%). У 85% пациенток вирусная нагрузка была более 3,2 log (в среднем – 5,2 (3,3 – 7,2 log) без существенных различий между группами.
Всем пациенткам была проведена расширенная кольпоскопия. Нормальная кольпоскопическая картина наблюдалась у 5 (12%) пациенток, слабовыраженные и выраженные изменения при кольпоскопии – у 36 (88%) включали наличие ацетобелого эпителия, интенсивность проявления которого коррелировала со степенью тяжести процесса: слабовыраженные изменения выявлялись у 16 (39%), выраженные – у 20 (49%).
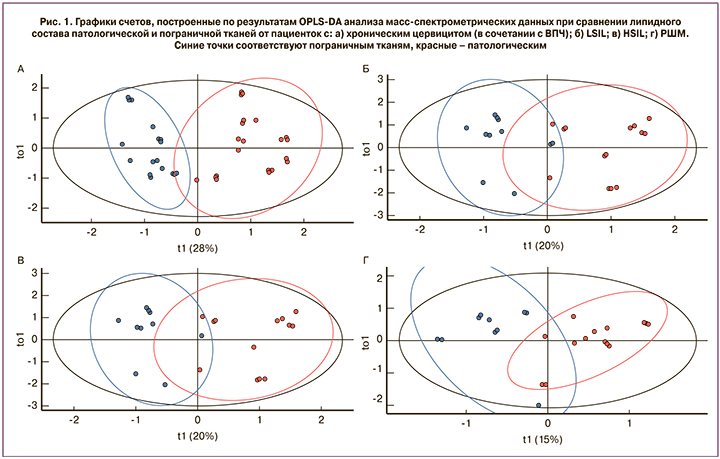
В ходе данного исследования определяли липидом неопластически измененных и пограничных тканей шейки матки посредством масс-спектрометрического анализа экстрактов липидов. Для выявления изменений липидного профиля при неопластической трансформации эпителия сравнивали патологически измененную и пограничную ткани. Также оценивали возможность классификации тканей по признаку норма-патология на основании липидного состава. Оценку проводили с помощью метода многофакторного анализа OPLS-DA. Результаты сравнительного анализа образцов с различными гистологическими диагнозами (хронический цервицит в сочетании с ВПЧ, LSIL, HSIL, РШМ) представлены на рис. 1 в виде графиков счетов, на которых каждая точка соответствует отдельному образцу. Цветом обозначены пограничная (синий) и патологическая (красный) ткани. В случае хронического цервицита в сочетании с ВПЧ в качестве патологической выступала ткань с воспалением и койлоцитозом, в случаях LSIL и HSIL – неопластически измененная ткань с койлоцитоатипией и дискариозом, в случае РШМ – биоптат злокачественной опухоли. Для всех четырех групп наблюдается хорошее разделение точек пограничных и патологических образцов, что свидетельствует о заметных отличиях липидного профиля сравниваемых образцов. Качество созданных методом OPLS-DA статистических моделей оценивали по значениям R2 и Q2. Первая из величин характеризует долю исходных данных, вошедших в модель, то есть насколько хорошо модель описывает экспериментальные результаты. Вторая величина показывает ожидаемую точность классификации модели при добавлении в нее данных по новым образцам и оценивается с помощью кросс-валидации. Значения R2 для групп с цервицитом (в сочетании с ВПЧ), LSIL, HSIL и РШМ составили 0,8130; 0,694; 0,553 и 0,673, соответственно. Величина Q2 в аналогичном ряду менялась следующим образом: 0,784; 0,620; 0,386; 0,575. Наибольшие значения R2 и Q2 наблюдаются в группе с цервицитом (в сочетании с ВПЧ), что связано, по всей видимости, не столько с более значительными, по сравнению с другими группами, различиями липидома клеток, пораженных ВПЧ, и прилежащих к ним нормальных клеток, сколько со сложностью получения здоровых пограничных тканей без примеси неопластически трансформированных клеток для групп LSIL, HSIL и РШМ. Некоторое повышение R2 и Q2 для образцов из группы с РШМ по сравнению с LSIL и HSIL может отражать выраженное изменение молекулярного состава малигнизированных клеток.
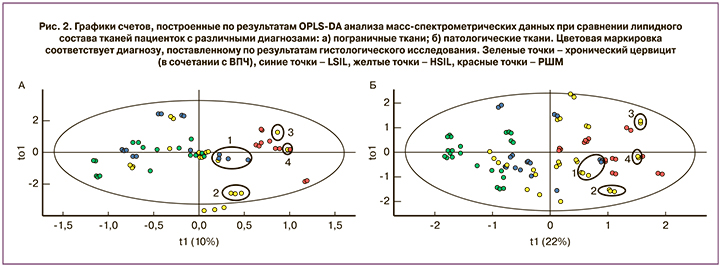
Представляет интерес сопоставление результатов гистологического исследования и липидного профиля исследуемых тканей. На рис. 2 представлены графики счетов OPLS-DA анализа данных отдельно для всех пограничных тканей (рис. 2а) и для всех патологических образцов (рис. 2б). Точки окрашены в соответствии с гистологическим диагнозом: зеленые – хронический цервицит (в сочетании с ВПЧ), синие – LSIL, желтые – HSIL, красные – РШМ. Для нормальных тканей группы точек, соответствующих цервициту (в сочетании с ВПЧ), LSIL и HSIL, пересекаются друг с другом, образуя достаточно равномерный кластер, а точки, соответствующие РШМ, группируются отдельно. Этот факт предполагает значительное отличие липидного состава тканей, граничащих со злокачественной опухолью, от тканей, граничащих с воспалительными и предраковыми поражениями эпителия. В случае патологически измененных тканей в отдельный кластер выделилась большая часть точек, соответствующих хроническому цервициту (рис. 2б). Точки HSIL и РШМ занимают перекрывающиеся участки графика, что свидетельствует о схожести липидного профиля соответствующих им образцов. Обращают на себя внимание выделенные на рис. 2 области 1–4. В области с одинаковыми номерами попали точки образцов, гистологически охарактеризованных как HSIL и LSIL на рис. 2б, и соответствующие им пограничные. Однако по липидному составу как патологические, так и пограничные образцы в указанных областях ближе к РШМ. Особенно ярко это сходство выражено для образцов, точки которых лежат в областях 3, 4.
В результате анализа масс-спектрометрических данных были выявлены статистически значимые отличия уровней липидов, относящихся к фосфатидилхолинам (PC 32:0, PC 34:1, PC 36:4, PC 34:0, PC 38:4), этаноламинам (PE O-46:0, LPE 46:0, PE O-48:0, LPE 48:0, PE O-46:1) и сфингомиелинам (SM 34:0, SM 42:2) в тканях, подвергшихся неопластической трансформации вследствие воздействия ВПЧ. Данные классы липидов, характерные для ВПЧ-ассоциированных поражений (LSIL, HSIL, РШМ), связаны с подавлением апоптоза, нарушением метаболизма клеток, стимуляцией пролиферативных процессов [16], что позволяет их рассматривать в качестве потенциальных биомаркеров для ранней и дифференциальной диагностики степени тяжести поражения эпителия. Таким образом, результаты исследования свидетельствуют о потенциальной возможности прогнозирования течения ВПЧ-ассоциированного заболевания либо в сторону прогресса, либо регресса.
Сходные изменения липидного профиля были обнаружены при исследовании липидного состава неопластически измененных тканей других репродуктивных органов. Например, некоторые из упомянутых липидов ответственны за разграничение нормальных и неопластически измененных тканей молочной железы и позволяют дифференцировать здоровую и опухолевую ткани молочной железы [17].
Группе проф. S. Kang удалось зафиксировать изменение уровня синтеза фосфатидилхолинов в тканях яичников, пораженных раком [18].
Аналогичные изменения уровней липидов обнаружены при изучении раковых и здоровых тканей эндометрия [19].
Фосфолипиды и сфинголипиды тесно связаны с подавлением апоптоза, нарушением метаболизма клеток, окислительным стрессом и малигнизацией, поэтому рассматриваются авторами в качестве потенциальных биомаркеров для дифференциальной диагностики доброкачественных и злокачественных заболеваний [20].
Известно, что атипические клетки при HSIL занимают всю толщу эпителия, что может быть связано с нарушением метаболизма атипической клетки, о чем свидетельствует изменение в патологических тканях уровня отдельных фосфатидилхолинов, которые способствуют пролиферативному росту и подавлению апоптоза [21, 22]. Повышенный синтез фосфатидилхолина необходим для быстрой пролиферации атипических клеток, чтобы постоянно обеспечивать их субстратом для синтеза мембран.
Заключение
Полученные результаты подчеркивают исключительную важность микроокружения пораженных клеток, указывают на его участие в модулировании липидного метаболизма атипических клеток и позволяют сформировать новый взгляд на регуляторные процессы данного метаболизма.



