Five-year use of robotic surgery in operative gynecology: summary and prospects
Krasnopolsky V.I., Popov A.A., Krasnopolskaya K.V., Fedorov A.A.
Objective. To study the experience with robot-assisted surgical interventions performed at the Moscow Regional Research Institute of Obstetrics and Gynecology in 2013 to 2018.
Subjects and methods. A total of 594 operations were performed during the reporting period. The patients were divided into 3 groups. Group 1 consisted of patients with benign gynecological diseases (infiltrative endometriosis and recurrent genital prolapse) who had been operated on using the surgical complex. Groups 2 and 3 included patients with obvious morbid obesity and malignant endometrial diseases, who were at high surgical risk.
Results. In patients with infiltrative endometriosis, the pregnancy rate was 45.16%, which was comparable with that in the control laparoscopic group. In patients with prolapse, the disadvantage of robotic sacrocolpopexy was only additional time required to install the patient’s console (docking). Anatomical and functional results were comparable with those in the laparoscopic group. In patients with morbid obesity, the complication rate in the robot-assisted laparoscopy group was 3.8%, which was significantly lower than that in the laparoscopic group.
Conclusion. Robot-assisted surgery has obvious advantages in the surgical treatment of complex gynecological diseases, not only malignant, but also benign, primarily the concomitant forms of severe infiltrative endometriosis (in certain situations that substantially affect the restoration of reproductive function in the patient).
Keywords
One of the fastest-growing fields of contemporary medicine is minimally invasive surgery, which emerged in the early 1940s when surgeons began using surgical access through the posterior vaginal fornix to visualize the abdominal cavity. D.O. Ott was the first who proved the feasibility of this surgical access and described it at the beginning of the 20th century [1]. Medical-technological achievements in minimally invasive surgical techniques and advances in imaging technology have contributed to a significant increase in the use of endoscopic interventions. The first successful laparoscopic hysterectomy was reported by H. Rich [2] in 1989, and already in 1993 D. Nichols [3] presented the results of a laparoscopic pelvic lymphadenectomy in patients with cervical cancer. The first endoscopic uterine reconstruction was described by K. Semm and L. Mettler [4]. In the early ’90s, A. Watties [5] proposed a laparoscopic version of abdominal sacrovaginopexy, later recognized as the “gold standard” in the surgical management of apical forms of genital prolapse [6]. Along with the achievements of endoscopic surgery that are significant for practical medicine, the beginning of the 21st century has witnessed a revolution in the field of surgery, including gynecological surgery, with the introduction of robotic surgery.
The worldwide success of robot-assisted surgery began in 1999 when Intuitive Surgical Inc. (USA) launched the da Vinci Surgical System (DV) [7].


Currently, there are more than 4400 da Vinci units in 64 countries around the world. Since this surgical complex is designed to be used for various surgical procedures, more than 43 000 console surgeons have been trained and certified. In the Russian Federation, 29 DV systems have been installed, and the first robotic surgery using the da Vinci robotic surgical system was performed on November 2007 at the Sverdlovsk Regional Clinical Hospital No. 1 (Yekaterinburg) [8]. The total number of operations performed using the robotic complex both in the world and in the Russian Federation has been increasing every year. Robotic surgery statistics are presented in Fig. 1.
The data presented by the manufacturer of DV demonstrate the prevalence of surgical interventions in various areas of surgery. Fig. 2 shows the distribution of surgical procedures.
Material and methods
As shown in the diagram, the most active utilization of DV has been in urology, accounting for 68.1% of all procedures. In the Russian Federation in 2018, gynecological surgery was the top second surgical subspecialty by volume of robot-assisted procedures comprising 13.1% of the total number of interventions. Almost half of all gynecological robot-assisted surgeries (200 out of 401) were performed at the MRRIOG. The Institute’s surgical experience related to robot-assisted surgery includes a total of 594 interventions. Table 1 summarizes the distribution of surgical procedures. In the structure of endoscopic interventions performed at the MRRIOG, robotic operations account for 14.8%.
Based on the indications for the surgery, we identified three main categories of operations.
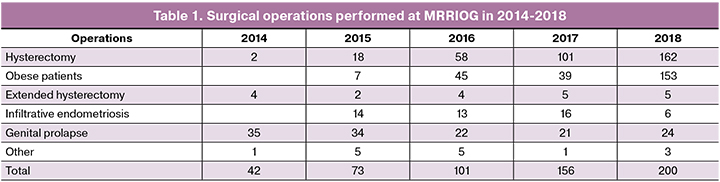
The first category included patients with benign gynecological conditions including infiltrative forms of endometriosis and complicated forms of genital prolapse with or without urinary incontinence, especially in patients with morbid obesity or chronic obstructive pulmonary disease (COPD) or other severe comorbidities, as well as patients with recurrent genital prolapse after undergoing transvaginal repair procedures using mesh implants.
Surgical treatment of patients suffering from infiltrative forms of endometriosis in combination with infertility was carried out jointly with the staff of the Department of Reproductology of the MRRIOG. Analysis of pelvic endometriotic lesions in 146 operated patients showed that in 77.6%, 50.3%, 23.7%, 6.9%, and 5.5% of patients endometriotic infiltrates were located in the retrocervical region, intestinal wall, rectovaginal septum, urinary bladder, and ureter, respectively. Based on the infiltrate location, surgical history, and body mass index, a total of 115 patients were selected for laparoscopic surgery; 31 patients underwent robot-assisted surgery. Data on the operative time, blood loss, and complication rates are summarized in table. 2.

The complications of surgical interventions included intraoperative bowel injury (enterotomy), pelvic hematoma, postoperative urinary retention, and the need for ureteral stenting.
An absolute criterion for successful surgery in this patient category is both spontaneous pregnancy rate and pregnancy rate in an IVF program. Our data showed that the total rate of spontaneous pregnancy and pregnancy in an IVF program among patients undergoing laparoscopic surgery was 49.56% (57 out of 115 patients). Patients who were operated using the robotic complex had a comparable rate of 45.16% (14 patients out of 31).
As a result of the study, we determined the criteria for using the surgical complex to perform reconstructive surgery in patients with endometriotic lesions infiltrating the bowel wall. According to our data, an indication for this treatment modality is the presence of retrocervical infiltrate with parametrial invasion or partial invasion of the rectum and sigmoid colon without mucosal involvement, which allows for “shaving” the infiltrate without opening the intestinal lumen. In patients with the total involvement of the rectum and sigmoid colon, the optimal treatment is circular bowel resection using endoscopic stapling device using laparoscopic access, since this type of intervention is technically challenging during robot-assisted laparoscopy.
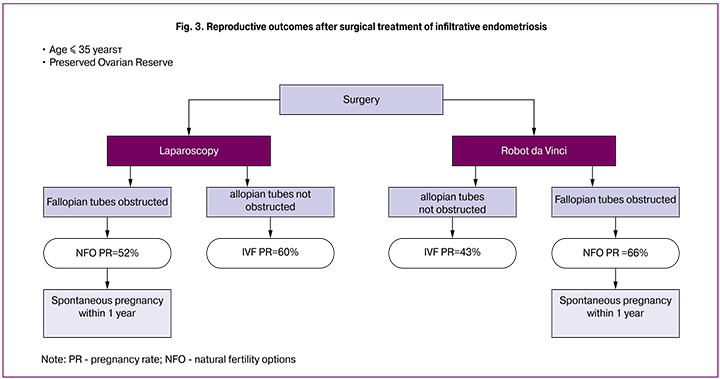
Important factors affecting the reproductive prognosis were patients’ age, the presence of the lesions in the Fallopian tubes, and the state of the ovarian reserve. We found that the best results were achieved in a group of patients younger than 35. The results of surgical treatment are presented in Fig. 3.
 As seen from this diagram, the study included patients with preserved ovarian reserve, who were younger than 35. In both the laparoscopic and robotic group, all spontaneous pregnancies occurred during the first year after surgery, while patients with normal fallopian tubes in the robotic group had higher pregnancy rate than in the laparoscopic group and reached 66%. All patients with compromised fallopian tubes underwent IVF.
As seen from this diagram, the study included patients with preserved ovarian reserve, who were younger than 35. In both the laparoscopic and robotic group, all spontaneous pregnancies occurred during the first year after surgery, while patients with normal fallopian tubes in the robotic group had higher pregnancy rate than in the laparoscopic group and reached 66%. All patients with compromised fallopian tubes underwent IVF.
Some patients with benign conditions of the female pelvic organs had complex, recurrent forms of genital prolapse, requiring sacrocolpopexy (SCP) to eliminate both pelvic organ prolapse and the concurrent urinary incontinence. The study included 173 patients with stage II-IV pelvic organ prolapse (POP-Q), who underwent SCP from January 2010 to February 2015. In group 1, 115 patients underwent LsSCP. Since 2013, RASCP has been used, which was performed in 58 patients who made up group 2. SCP was performed using the standard surgical technique, which was standardized. After dissection of paravaginal tissues and isolation of the longitudinal presacral ligament, a posterior graft sized 15x3 cm and anterior graft sized 3x5 cm were cut from the type 1 polypropylene mesh (index soft). The grafts were fixed to m. Pubococcygeus on both sides, the sacro-uterine ligaments and the posterior surface the cervix or the vaginal fornix, the anterior vaginal wall, the anterior surface of the cervical stump or the vaginal fornix (Fig. 4). The free end of the graft I was attached to the longitudinal presacral ligament with moderate tension.
 In addition to SCP, patients with the complicated forms of genital prolapse underwent several simultaneous operations, which are summarized in Fig. 5.
In addition to SCP, patients with the complicated forms of genital prolapse underwent several simultaneous operations, which are summarized in Fig. 5.
Results
Clinical data on patients operated on laparoscopically (group 1) and robotically (group 2) are presented in the table. 3. Patients in both groups were comparable in age, but in group 1 there were more sexually active women. No differences in parity were observed in body mass index (BMI), and the number of recurrent forms of genital prolapse.
In most cases, a supracervical hysterectomy was performed in both groups. Every fifth patient (19% and more than 15% in groups 1 and 2, respectively) also had a cervical amputation due to elongated cervix. The main objective was to fix the mesh to a remaining cervical stump, thus avoiding colpotomy as a stage of total hysterectomy and reducing the risk of vaginal erosion [9,10].
The patients in group 2 had longer time spent in the operating room, anesthesia and operation time, and SCP time (Table 4). They also reported a higher level of postoperative pain intensity, evaluated on a 10-point visual analog scale (VAS) (Table 4).
Long-term outcomes were followed for 18 ± 9 and 12 ± 6 months in groups 1 and 2, respectively. Follow-up examinations were performed at 3, 6, 12, and 24 months after the operation. The anatomical results of surgical treatment at 12 months after surgery are presented in table. 5. Recurrence of genital prolapse corresponding to stages III and IV (POP-Q) was observed in 3 (5.2%) patients in group 1. One patient required re-operation.

When evaluating the results of the operations, we concluded that the only disadvantage of RASСP was the need for robot docking time. Some literature sources have reported a significant difference in operation and anesthesia times between LsSСП and RASСP (+ 67 min. difference; 95% CI 43–89; p <0.001 *) [11].
The most important advantages of RASСP include ergonomic settings at the surgeon console, three-dimensional imaging, the ability to work in hard-to-reach areas of the small pelvis, and a simplified surgical suture technique. The disadvantages of RASСP include a longer operation and anesthesia time, and higher postoperative pain intensity compared to LsSСP. RASCP should be used mainly in patients with apical prolapse, for which this operation is the most effective and physiological, especially in patients with recurrent prolapse.
Among patients operated on using the surgical complex, there were patients with severe morbid obesity, who were assigned to groups 2 and 3. Group 2 included patients with morbid obesity, who had endometrial malignancies. The main indications for surgery were invasive endometrial cancer and cervical cancer. All patients were consulted by the Onco-consilium. Based on its recommendations, they underwent a hysterectomy with pelvic lymphadenectomy. For a surgeon, operation settings were comfortable to perform lymph node dissection in obese patients. Postoperatively, these patients continued their treatment in oncology hospitals.
 Group 3 included patients with high surgical risk and morbid obesity; the main indication for surgery were large genital tumors and their atypical location.
Group 3 included patients with high surgical risk and morbid obesity; the main indication for surgery were large genital tumors and their atypical location.
Since patients groups 1 and 2 had similar somatic state, it is necessary to identify the main parameters affecting the choice of surgical access in these patients. First of all, attention should be paid to morbid obesity, since the highest BMI was 75.1, and the greatest patient body weight was 228 kg. The second factor was the presence of COPD in combination with other severe comorbidities. These operations require the highest qualification of the anesthesia team and the need to use expert class anesthetic equipment.
Morbidly obese patients present numerous challenges to the safe endotracheal anesthesia. Formation of end-expiratory airway closure (EEAC), the so-called “gas trap” is associated with compromised gas exchange due to airway compression (Fig. 6). The phenomenon of “gas trap” results in low ventilation-perfusion ratio and an increase in intrapulmonary shunting and hypoxemia.
Mechanical ventilation in morbidly obese patients is also complicated by the need for at least 20-degree Trendelenburg positioning of the patient. Extremely obese patients require a specific choice of the operating table, which can tolerate the patient’s weight. The use of positive end-expiratory pressure (PEEP) during endotracheal anesthesia in morbidly obese patients prevents end-expiratory airway closure (EEAC) and pneumothorax.
We examined 82 patients with benign, malignant, and precancerous diseases of the internal genital organs to investigate the effect of obesity on the outcomes of endoscopic operations. The criteria for the presence of metabolic syndrome were BMI of more than 40 kg/m2 or more than 35kg/m2 in combination with diseases associated with obesity including coronary heart disease (CHD), arterial hypertension (AH), heart failure (HF), diabetes mellitus (DM), cholelithiasis, obstructive sleep apnea syndrome, and osteoarthritis.
The study group (group 1) comprised 52 patients, who underwent robot-assisted surgery complex; the comparison group( group 2) consisted of 30 patients with diseases of the internal genital organs who had normal body weight (BMI ranging from 18.5 to 25 kg / m2).
There was a statistically significant difference in body weight and height of patients in groups 1 and 2. Most patients with morbid obesity had a bodyweight in the range from 100 to 130 kg (n = 35, 67.3%) with a mean of 127.1 ± 19.7 kg and BMI ranging from 40 to 50 kg/m2 (n = 34, 65.4%) with a mean of 48.82 ± 8.0 kg/m2. In the comparison group, the mean bodyweight of the patients was 64.7 ± 6.4 kg, while more often the weight was in the range from 60 to 70 kg (n = 19.63.3%), and the mean BMI was 23.1 ± 1. 9 kg/m2, with a predominance of values in the range from 23 to 25 kg/m2 (n = 19.63.3%).
An important analysis is the structure of extragenital comorbidities, which were markedly more prevalent in patients with obesity. All women in group 1 had cardiovascular diseases, such as AH (100%), coronary heart disease (53.8%), varicose veins of the lower extremities (55.8%). Every second obese patient had endocrine diseases, mainly type 2 diabetes (42.3%), every 3rd patient had respiratory diseases mainly, bronchial asthma and COPD (30.8%). Among patients with normal body weight, the most common comorbidity was anemia of various severity (33.3%), which was attributed to symptomatic uterine fibroids.
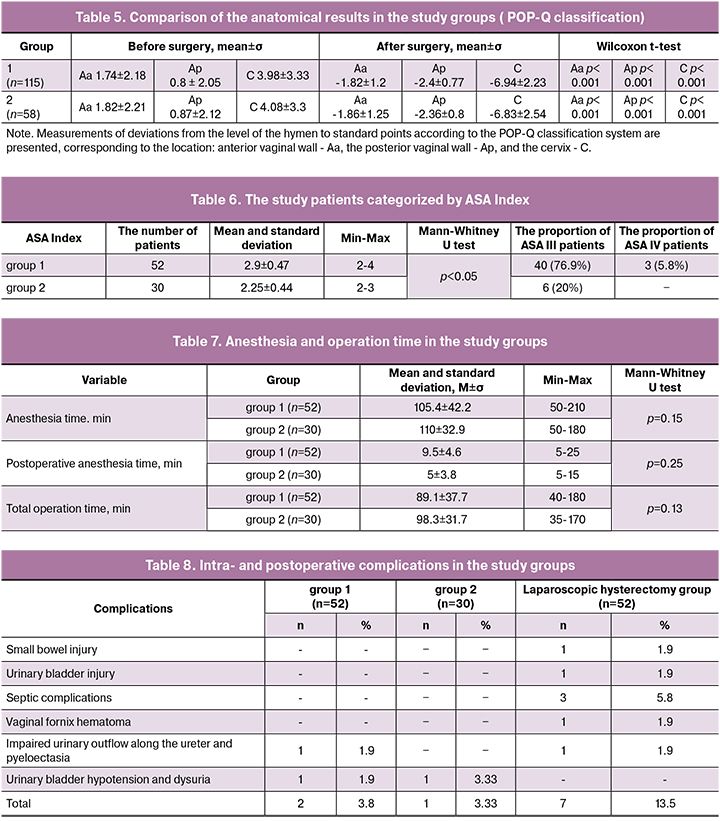
Preoperative evaluation of patients for anesthesia risk assessment showed significantly higher ASA scores and higher comorbidity rate in obese patients than in patients with normal body weight. The results of anesthetic risk assessment are presented in the table. 6.
The results of a comparative assessment of anesthesia time and operation time in patients with obesity and normal body weight are presented in the table. 7.
As seen from the table, no correlation was observed between anesthesia time, operation time, and the patient’s body mass index. Blood loss also did not differ significantly between obese and normal-weight patients.
To evaluate the hysterectomy complication rate in patients with morbid obesity, we compared the results of our study with the data of the MRRIOG endoscopic surgery department for a similar period. Data on intra- and postoperative complication rates are presented in table. 8.
The patients with morbid obesity undergoing laparoscopic surgery had a higher complication rate (13.5 %, n = 7) than patients with normal body weight (3.33%, n = 1). At the same time, the complication rate in obese patients undergoing robot-assisted surgery was 3.8%, which was significantly lower than in the laparoscopic group. Complications in obese patients undergoing robot-assisted surgery were also associated with a greater extent of surgery. These indicators were comparable with the complication rate in patients with normal BMI. However, in all groups, complications were only mild and moderate. No severe complications or respiratory and cardiovascular dysfunction were observed.

The analysis of anesthetic or non-surgical complications is presented in the table. 9.
 The range of robot-assisted surgeries can be broadened by adding reconstructive uterus operations. First of all, they include myomectomy (11 operations in total), repair of uterine scar dehiscence at the stage of pregnancy planning (5 operations in total), and pre-pregnancy transabdominal cerclage. Our experience shows that robot-assisted pre-pregnancy transabdominal cerclage does not have advantages over a laparoscopic procedure. Analysis of myomectomy and metroplasty showed that robot-assisted intracorporeal suturing allows precise myorraphy, especially in hard-to-reach anatomical locations. No patients undergoing robot-assisted myomectomy and after metroplasty had uterine scar dehiscence postoperatively. All operated patients gave birth at full-term. Fig. 7 shows a uterus after fetus extraction in a patient undergoing robotic removal of an atypical fibroid measuring 8 cm in diameter at the pre-pregnancy stage.
The range of robot-assisted surgeries can be broadened by adding reconstructive uterus operations. First of all, they include myomectomy (11 operations in total), repair of uterine scar dehiscence at the stage of pregnancy planning (5 operations in total), and pre-pregnancy transabdominal cerclage. Our experience shows that robot-assisted pre-pregnancy transabdominal cerclage does not have advantages over a laparoscopic procedure. Analysis of myomectomy and metroplasty showed that robot-assisted intracorporeal suturing allows precise myorraphy, especially in hard-to-reach anatomical locations. No patients undergoing robot-assisted myomectomy and after metroplasty had uterine scar dehiscence postoperatively. All operated patients gave birth at full-term. Fig. 7 shows a uterus after fetus extraction in a patient undergoing robotic removal of an atypical fibroid measuring 8 cm in diameter at the pre-pregnancy stage.
Based on our surgical experience, we formulated relative contraindications to the use of robot-assisted surgery in gynecology. They include a history of multiple surgical interventions and extensive adhesions in the abdominal cavity. The use of robotic access in patients with low body weight is debatable due to possible instrument crowding and collisions because robotic ports should have at least 8 cm of distance between them.
Probably, the next generation of tools and the updated da Vinci surgical system will have instruments with a smaller diameter and different docking techniques, combined with single-port access, which will allow similar interventions in patients with low BMI. According to the literature, these operations may not be appropriate in patients with cervical cancer and ovarian cancer.
Conclusion
Robot-assisted surgery has obvious advantages in the management of complex gynecological diseases, of both benign and malignant nature, primarily combined forms of severe infiltrative endometriosis and in certain situations can significantly affect the recovery of reproductive function;
The contemporary system of surgical care in combination with the da Vinci robotic system and advanced anesthetic management allows successful surgical treatment of patients with severe extragenital comorbidities including morbid obesity, chronic obstructive pulmonary disease, and cardiovascular disease, especially in combination with a high BMI;
In patients with malignant neoplasm of internal genital organs, the use of the da Vinci complex is an advisable option for patients with endometrial cancer. However, in patients with cervical cancer open surgery is still preferable, while endoscopic methods are informative for staging the disease;
Robot-assisted surgery has obvious advantages over all available treatments of severe genital prolapse with or without urinary incontinence, especially in women with recurrent forms of prolapse.
The da Vinci complex should be used in combination with high-tech anesthesia equipment. This combination ensures favorable outcomes for both surgery and anesthesia.
References
- Отт Д.О. Результаты, достигнутые применением при операциях и в целях распознавания непосредственного освещения брюшной полости, толстой кишки и мочевого пузыря // Русский врач (СПб). 1908;43:3–11. [Ott D. O. Rezul’taty, dostignutye primeneniem pri operatsiyakh i v tselyakh raspoznavaniya neposredstvennogo osveshcheniya bryushnoi polosti, tolstoi kishki i mochevogo puzyrya // Russkii vrach (SPb). 1908;43:3–11.(In Russian)]
- Mori K.N. Minimally Invasive Surgery in Gynecologic Oncology // Obstetrics and Gynecology . 2013: 11. Doi: 10.1155 / 2013/312982
- Nichols D.H., ed. Gynecologic and obstetric surgery. St. Louis: Mosby Year Book, 1993:1167–82.
- Semm K., Mettler L. Technical progress in pelvic surgery via operative laparoscopy. Am J Obstet Gynecol. 1980 Sep 15;138(2):121-7.
- Wattiez A., Boughizane S., Alexandre F., Canis M., Mage G., Pouly JL., Bruhat M.A. Laparoscopic procedures for stress incontinence and prolapse.// Curr Opin Obstet Gynecol. 1995 Aug;7(4):317-21.
- Краснопольский В.И., Буянова С.Н., Щукина Н.А., Петрова В.Д., Попов А.А., Чечнева М.А., Кашина Е.А., Краснопольская И.В., Муравьева Т.Г., Путиловский М.А., Хайруллина Д.М. Хирургическое лечение больных с опущением и выпадением внутренних половых органов и профилактика опущения купола влагалища после гистерэктомии. Российский вестник акушера-гинеколога 2006; 4: 66. [Krasnopol’skii V.I., Buyanova S.N., Shchukina N.A., Petrova V.D., Popov A.A., Chechneva M.A., Kashina E.A., Krasnopol’skaya I.V., Murav’eva T.G., Putilovskii M.A., Khairullina D.M. Khirurgicheskoe lechenie bol’nykh s opushcheniem i vypadeniem vnutrennikh polovykh organov i profilaktika opushcheniya kupola vlagalishcha posle gisterektomii. Rossiiskii vestnik akushera-ginekologa 2006; 4: 66.(In Russian)]
- Ballantyne G.H. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Surg Endosc. 2002;12(1):6-16.
- Федоров А.В, Кригер А.Г., Берелавичус С.В., Ефанов М.Г., Горин Д.С. Робот-ассистированные операции в абдоминальной хирургии. Хирургия. Журнал им. Н.И. Пирогова. 2010; 1:16-19. [Fedorov A.V, Kriger A.G., Berelavichus S.V., Efanov M.G., Gorin D.S. Robot-assistirovannye operatsii v abdominal’noi khirurgii. Khirurgiya. Zhurnal im. N.I. Pirogova. 2010; 1:16-19.(In Russian)]
- Culligan P.J., Murphy M., Blackwell L., Hammons G., Graham C., Heit M.H. Long-term success of abdominal sacral colpopexy using synthetic mesh. J Obstet Gynecol 2002; 187: 1473– 80; discussion 1481-2.
- Imparato E., Aspesi G., Rovetta .E, Presti M. Surgical management and prevention of vaginal vault prolapse. Surg. Gynecol Obstet. 1992; 175: 233–7.
- Paraiso M.F., Jelovsek J.E., Frick A., Chen C.C., Barber M.D. Laparoscopic Compared With Robotic Sacrocolpopexy for Vaginal Prolapse: A Randomized Controlled Trial. Obstetrics & Gynecology.2011;118(5):1005–1013. doi: 10.1097/AOG.0b013e318231537c
Received 16.04.2019
Accepted 19.04.2019
About the Authors
Krasnopolsky Vladislav I., professor, academician of RAS, president of Moscow Regional Research Institute Obstetrics and Gynecology. Phone +7 (495) 6231054.E-mail: gyn_endoscopy@mail.ru 101000 Russia, Moscow, Pokrovka st. 22A.
Popov Alexander A., professor, chef of endoscopyc department of Moscow Regional Research Institute Obstetrics and Gynecology. Phone +7 (495) 6257332.
E-mail: gyn_endoscopy@mail.ru 101000 Russia, Moscow, Pokrovka st. 22A.
Krasnopolskaya Ksenia V., professor, member of RAS, chef of reproduction department of Moscow Regional Research Institute Obstetrics and Gynecology.
Phone +7 (495) 6257332. E-mail: gyn_endoscopy@mail.ru 101000 Russia, Moscow, Pokrovka st. 22A.
Fedorov Anton A., PhD, endoscopyc department of Moscow Regional Research Institute Obstetrics and Gynecology. Phone +7 (495) 6257332. E-mail: gyn_endoscopy@mail.ru
101000 Russia, Moscow, Pokrovka st. 22A.
For citation: Krasnopolsky V.I., Popov A.A., Krasnopolskaya K.V., Fedorov A.A. Five-year use of robotic surgery in operative gynecology: summary and prospects .
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (9): 92-101.(in Russian)
https://dx.doi.org/10.18565/aig.2019.9.92-101



