Mitochondrial DNA copy number in sperm of hiv-infected men receiving antiretroviral therapy
Objective. To study mitochondrial DNA (mtDNA) content in sperm samples of HIV-infected men receiving antiretroviral therapy (ARVT).Perminova S.G., Mityurina Е.V., Selimova F.N., Burmenskaya О.V., Kozyrina N.V., Kravchenko А.V.
Materials and methods. This prospective clinical study included 190 patients who presented for the assessment of fertility and achieving conception. The main group consisted of 94 HIV-infected men; the control group included 96 patients without HIV infection. The quantitative assessment of mtDNA deletions (mtDNAdel-), total mtDNA copy number (mtDNAtotal) and their ratio (mtDNAtotal / mtDNAdel-) was carried out in 58 and 96 samples, respectively, using real-time polymerase chain reaction (PCR) method.
Results. The median age of HIV-infected men was 37 (33; 39) years, duration of the disease was 5 (2; 11) years. The median duration of receiving ARVT was 2 (1; 5) years. The patients of groups were similar in mtDNAdel- (1.9 (0.9; 5.9) and 2.4 (1.9; 5.6); р=0.54), mtDNAtotal (5.4 (3.7; 9.8) and 5.5 (3.5; 8.2); р=0.62) and their ratio mtDNAtotal /mtDNAdel- (2.4(1.5; 3.9) and 1.9 (1.3; 3.0); р=0.09), respectively. In the group with severe sperm abnormality there was a negative correlation between ARVT duration and copy number of mtDNAtotal (r=-0.627; р=0.01), as well as between ARVT duration and mtDNAdel- level (r=-0.542; р=0.04). The pronounced negative correlation was revealed between CD4+ level and ratio mtDNAtotal / mtDNAdel- (r=-0.29; р=0.03).
Conclusion. HIV-infected men did not show significant differences in sperm mtDNA copy number in comparison with the men of the control group. HIV-infected men with severe sperm abnormality had inverse relationship between the levels of mtDNAtotal, mtDNAdel- and ARVT duration; mtDNAdel- content was negatively correlated to the immune system state.
Keywords
HIV infection has spilled over from the vulnerable groups of people and is actively spreading to the general population. As at 31 October 2019, the cumulative number of registered HIV cases in the Russian Federation was 1,408,264 which included 347,711 HIV-related deaths. Currently, the highest level of HIV infection in Russia is observed in the age group of 30-44 years. Male dominance is observed in the gender structure (63%) including 3.28% of men aged 35-39 years with most of them planning to have a baby [1].
The administration of antiretroviral therapy (ARVT) allows the patients of this group to achieve parenthood, even without using assisted reproductive technologies (ART). However, there is an opinion that ARVT can have a negative impact on sperm indicators leading to a decrease in the concentration, motility of spermatozoa, an increase in the number of pathological forms, that eventually results in a decrease in the fertility of HIVinfected men [2]. Negative influence of ARVT, namely nucleotide reverse transcriptase inhibitors (NRTI), on the quality of sperm may be caused by the changes in the metabolism of spermatozoa due to the toxic damage to mitochondria [3, 4, 5]. A mature human spermatozoon is known to contain approximately 50-75 mitochondria and each of them has one copy of mitochondrial DNA (mtDNA). Mitochondria are the main source of energy for motility of spermatozoa in mammals [6]. The studies of Santos (2009) and Pelliccione (2011) showed that the relative amount of mtDNA is significantly reduced in the sperm of men with oligo- and asthenozoospermia [7, 8]. According to some authors, the adverse effects of ARVT are associated with the development of oxidative stress [9]. The formation of reactive oxygen species (ROS) increases lipid peroxidation, damages the sperm chromatin and impairs the integrity of the sperm DNA [10, 11]. In addition, ROS can damage the membrane of spermatozoa, which reduces their motility and ability to fertilize the egg [12]. On the contrary, Van Leeuwen E. et al. (2009) showed that the number of DNA copies in the spermatozoa in HIV-infected men receiving ARVT increases in comparison with HIV-infected men who have never taken ARVT and HIV-seronegative men [13].
Therefore, there is no sufficient data on the impact of ARVT treatment on the mtDNA copy number of spermatozoa in HIV-infected men.
The aim of this research was to study mtDNA content in sperm samples of HIV-infected men receiving antiretroviral therapy (ARVT).
Materials and Methods
This prospective clinical study included 190 men who presented for the assessment of fertility and achieving conception to the 1st Gynecological Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia (research supervision was performed by PhD A.N. Abubakirov). The main group consisted of 94 HIVinfected men; the control group included 96 patients without HIV infection. The quantitative assessment of mtDNA deletions (mtDNAdel-), total mtDNA copy number (mtDNAtotal) and their ratio (mtDNAtotal / mtDNAdel-) was carried out in 58 and 96 samples, respectively. The criteria for inclusion in the main group were as follows: HIV infection, 3rd subclinical stage, stages 4A, 4B, 4C, remission phase; administration of ARVT, undetectable viral load in two consecutive studies which were performed at least 3 months apart; informed consent to participate in this study. Exclusion criteria were viral hepatitis B, C and HIV coinfection, absence of ARVT. Criterion for inclusion in the control group was HIV-seronegative status.
The status of HIV-infected patients was assessed on the basis of the data on the stage and phase of the disease, the level of viral load, CD4+, CD8+ lymphocytes, its duration, as well as ARVT components.
The ejaculate was collected in a sterile container by masturbation after 3-4 days of sexual abstinence. Semen analysis parameters were evaluated in accordance with the standards of the World Health Organization (2010) [14].
Further, the sperm samples were divided into normozoospermia, subfertile and severe pathozoospermia groups. The criteria for severe sperm pathology were as follows: sperm concentration is up to 12 million/ml, spermatozoa with progressive movement (A+B) are less than 29%, the number of pathological forms is more than 97%. Subfertile sperm is characterized by sperm concentration of 12-15 million/ml, spermatozoa with progressive movement (A+B) is 30-32%, the number of pathological forms is not more than 97%.
The study of mtDNA copy number was performed using the following technique: spermatozoa were washed in a sterile phosphate-buffered saline and then placed in individual test tubes containing this buffer under aseptic conditions using an inverted microscope. The absolute mtDNA copy number was determined using the method of polymerase chain reaction (PCR) in real time. For this purpose, DNA was extracted from sperm sample. The cells were lysed in a buffer containing guanidine thiocyanate for 10 minutes at 65°C. Afterwards isopropanol precipitation of DNA was performed, the samples were centrifuged at 13000 rpm for 10 minutes (Kit Proba-NK-plus, DNA-Technology LLC, Russia). Then the precipitate was washed with two washing solutions, dried, and resuspended in 50 ml of eluting solution. mtDNA copies were counted using real-time PCR with oligonucleotides and TaqMan samples for amplification and quantification of specific mtDNA fragments (MTND2 gene - mitochondrially encoded NADH dehydrogenase 2 and MTND4 gene - mitochondrially encoded NADH dehydrogenase 4). The use of primers for the MTND2 gene allowed us to estimate the content of the total mtDNA pool (mtDNA total), and the primers for the MTND4 gene made it possible to evaluate the content of full-length mtDNA pool deprived of deletions (mtDNAdel-), particularly del mtDNA4977. Normalization was performed for genomic DNA (LTC4S gene - leukotriene C4-synthase). The ratio between mtDNA number and genomic DNA was determined by comparing threshold cycles (2∆Сt) and represented in relative amounts using formula 1: mtDNA/gDNA = 2Ct gDNA - Ct mtDNA, where Ct gDNA is the threshold cycle of amplification of genomic DNA, and Ct mtDNA is the threshold cycle of amplification of mitochondrial DNA. TaqMan samples for mitochondrial and genomic DNA fragments were labeled with different fluorophores (FAM and HEX) which allowed us to perform the reaction in one test tube (multiplex PCR) with two repeats for each sample. “Hot start” PCR was performed using paraffin. Reagents, oligonucleotides, TaqMan samples and detecting amplifiers “DTprime” (LLC “DNA-Technology”, Russia) were used in the study. Amplification was performed according to the following program: 80° C for 1 min, incubation at 95° C for 1 min followed by 50 cycles: 94° C for 15 sec and 64° C for 20 sec with the measurement of fluorescence level in each cycle.
Total mtDNA copy number (mtDNAtotal) in ejaculate and mtDNA deletions (mtDNAdel-) were assessed, as well as their ratio (mtDNAtotal / mtDNAdel-) was carried out. A higher value of the ratio indicated an increase in the content of mtDNA deletions.
Statistical processing of the data was performed using the IBM SPSS Statistics version 22 software package. Observed sample distribution was compared using the Kolmogorov-Smirnov test. Due to the fact that the data distribution in the study was different from the normal one, the median and quartiles (Me(Q1;Q3)) were determined. Nonparametric statistical methods (the Mann-Whitney U test) were used to assess differences in the groups. The χ2 test was used to compare categorical variables, as well as to evaluate significant differences between them. Dependent data were evaluated using the Pearson correlation coefficient. At a significance level of p<0.05 the results were considered statistically significant.
Results
The median age of HIV-infected men was 37 years (31;37). Duration of HIV infection in patients was 5 (2; 11) years. HIV-infection was diagnosed at the age of 29 (26; 32) years, third subclinical stage of the disease prevailed (64.9%). Patients with stages 4A (23.4%), 4B (4.3%) and 4C (7.5%) had a remission for at least 6 months. Combined ARVT was taken by 79 out of 94 patients (84%), including 38 (48.1%) patients who took NRTI medications in combination with non–nucleoside reverse transcriptase inhibitor, 35 patients (44.3%) took NRTI medications in combination with protease inhibitors, 5 (6.3%) patients took NTRI medications in combination with integrase inhibitors and 1 (1.3%) patient was administered three medications of NTRI group. The median duration of drug administration was 2 (1; 5) years. The immunological status of HIVseropositive patients is presented in Table 1.
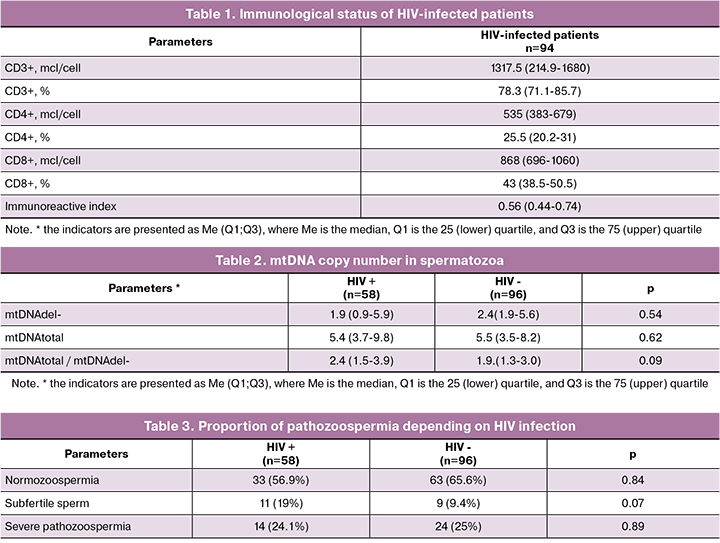
Concomitant hepatitis C was revealed in 36 out of 94 (38.2%) HIV-seropositive men. These patients were excluded from the study.
The analysis of mtDNA copy number in the ejaculate showed that the number of mtDNA deletions (1.9 (0.9; 5.9) and 2.4 (1.9; 5.6); p=0.54), the level of total mtDNA (5.4 (3.7; 9.8) and 5.5 (3.5; 8.2), p=0.62) and the ratio mtDNAtotal /mtDNAdel- (2.4 (1.5; 3.9) and 1.9 (1.3 and 3.0), p=0.09) were comparable between groups (Table 2).
Further, the sperm samples were divided into normozoospermia, subfertile and severe pathozoospermia (Table 3).
In the group of HIV-infected men, normozoospermia was detected in 56.9% of cases, subfertile sperm - in 19%, severe sperm pathology - in 24.1 %; in the control group these parameters were 65.6%, 9.4% and 25%, respectively (p=0.22). There were no statistically significant differences in the type of pathozoospermia; teratozoospermia prevailed in both groups (20.7% and 16.7%; p=0.56) (Table 4).
As can be seen from the data presented in Table 5, the content of total mtDNA, mtDNA deletions and their ratio were comparable between the groups.
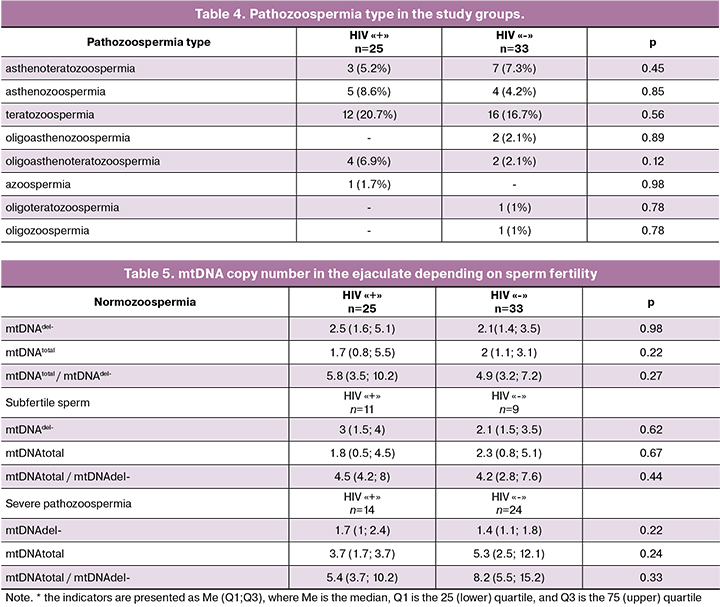
The assessment of the relationship in the group of severe sperm pathology revealed a negative correlation between the duration of ARVT and total mtDNA copy number (r=-0.627; p=0.01), as well as between the duration of ARVT and the level of mtDNA deletions (r=-0.542; p=0.04). An expressed negative correlation was found between the CD4+ level and the ratio of mtDNAtotal / mtDNAdel- (r=-0.629; p=0.03).
Discussion
The fact that HIV-infected men receiving ARVT are able to achieve parenthood without using ART treatment makes a strong case for studying the characteristics of fertility in this group of patients. However, most studies indicate a decrease in the ability to conceive in HIVinfected men [2, 4]. Particular attention is given to the role of the virus and ARVT in the genesis of reproductive disorders in HIV–infected patients. It should also be noted that HIV-positive patients often experience a number of other factors that negatively affect fertility, such as depression, anxiety, the use of psychotropic drugs, inflammatory diseases of the genitals, sexually transmitted diseases. In addition, ARVT can result in serious side effects, some of them are due to the negative effects of medications on mitochondria. NRTI medications may have a great influence on mitochondria, they can negatively affect mtDNA copy number mainly by suppressing the replication enzyme, namely mtDNApolymerase gamma, causing mtDNA depletion [13]. In this regard, we evaluated total DNA, mtDNA deletions and their ratio in HIV-infected men who receive ARVT in comparison with HIV-seronegative patients. The limitation of this study was the inability to assess the mtDNA content in the semen of HIV-infected men who do not receive ARVT.
This study included men aged 37 years (33; 39); their disease was not long-term, namely 5 (2;11) years, ARVT was taken for 2 (1; 5) years. The semen analysis showed that more than half of HIV-infected men (56.9%) had normozoospermia, subfertile sperm and severe pathozoospermia were revealed in 19% and 24.1% of patients, respectively. The obtained results are consistent with the literature data. A number of studies also found no significant differences in the sperm samples of HIV-positive and HIV-negative men [15, 16], except those cases where the general condition of patients deteriorated suddenly due to progressive immunosuppression [17]. In this study, all men had an undetectable viral load in the blood, the level of CD4 + lymphocytes (535 (383-679)) indicated quite “satisfactory” condition of the immune system in most cases, though low immunoreactive index (IU 0.56) and low relative indicators of CD4+ lymphocytes (25.5 (20.2-31) should be noted.
Among sperm pathology types, teratozoospermia prevailed (20.7%). A decrease in the percentage of morphologically normal spermatozoa was shown in the studies of Dondero et al., (1996), Muller et al., (1998), Nicopoullos et al., (2004), Bujan et al., (2007) [2, 4, 18, 19]. And on the contrary, Dulioust E. et al. (2002) noted that the percentage of morphologically normal spermatozoa in patients with HIV infection is comparable to one in HIV–seronegative patients [20].
The analysis of mtDNA copy number in the ejaculate of HIV-infected patients showed that the number of mtDNA deletions (1.9 (0.9; 5.9) and 2.4 (1.9; 5.6); (p=0.54)), the level of total mtDNA (5.4 (3.7; 9.8) and 5.5 (3.5; 8.2); (p=0.62)) and the ratio mtDNAtotal / mtDNAdel- (2.4 (1.5; 3.9) and 1.9 (1.3; 3.0), (p=0.09)) were comparable to those of HIV-seronegative patients in the control group. The absence of changes in the mtDNA content in HIV-infected patients may be due to the fact that this study included the patients who received ARVT not so long, namely for 2 (1; 5) years. Since the study included patients with different parameters of semen analysis, we divided the sperm samples into normozoospermia, subfertile sperm, and severe sperm pathology. Differences in the content of total mtDNA and mtDNA deletions were not found. However, in case of severe pathozoospermia in HIVinfected men, it was found that the longer the patients were administered ARVT, the lower the content of total mtDNA (r=-0.627; p=0.01) and mtDNA deletions (r=0.542; p=0.04) was. Moreover, there was a correlation between the low level of CD4 + lymphocytes in the blood which reflects the condition of the immune system and the increasing number of mtDNA deletions in the sperm, which was determined by the ratio mtDNAtotal / mtDNAdel- (r=-0.629; p=0.03). In other words, the longer the patient was administered ARVT, the lower the quality of mitochondria in spermatozoa was. Ratio mtDNAtotal/mtDNAdel- increases with the development of pathological process and a decrease in the CD4+ content which is suggestive of appearance of mtDNA deletions. Wu H. et al. (2019) believed that an increase in the number of mtDNA deletions in spermatozoa is a marker of pathozoospermia and reflects negative changes in sperm parameters [21]. Thus, in our study, the decrease in total mtDNA and increase in mtDNA deletions reflected the pronounced changes in sperm of HIV-infected men who took ARVT for a long time.
Unlike genomic DNA, mtDNA does not have protective histones and an antioxidant-rich cytoplasm [22], which contribute to DNA repair, making mtDNA more vulnerable to deletions and damage [23]. ARVT medications can inhibit the repair of defects that occur in the mtDNA structure, thereby leading to an increase in the number of copies of mtDNA and mtDNAdel+ [24]. ARVT medications also contribute to the formation of ROS and increase lipid peroxidation resulting in the development of oxidative stress and damage to the chromatin of spermatozoa and spermatogenesis, which in turn causes an increase in mtDNAdel+ and the development of mitochondrial dysfunction in spermatozoa [9]. Different studies present conflicting evidence. For example, research by Diehl S. et al. (2003) indicates that there is no difference in mtDNA content between HIV-infected patients who receive and do not receive ARVT in comparison with HIVnegative men [25]. On the contrary, White D. J. et al. (2010) demonstrated that patients who take ARVT for a long time have mtDNA deletions in spermatozoa compared to HIV-infected patients with who never received therapy [26].
Conclusion
This study has shown that there were no statistically significant differences in mtDNA copy number in spermatozoa in HIV-infected men compared with the controls with normozoospermia, as well as men with subfertile sperm and severe pathozoospermia. However, in cases of severe sperm pathology, the levels of total mtDNA and mtDNA deletions in spermatozoa of HIVinfected semen were inversely related to the duration of ARVT, and the content of mtDNA deletions negatively correlated with the conditions of the patient’s immune system. Therefore, since HIV-infected patients are expected to receive ARVT for the whole life, they are recommended to undergo ART and/or cryopreservation of sperm after reaching undetectable viral load in blood.
References
- Федеральный научно-методический центр по профилактике и борьбе со СПИДом ФБУН Центрального НИИ эпидемиологии Роспотребнадзора. Доступно по: http://www.hivrussia.info [Federal’nyi nauchno-metodicheskii tsentr po profilaktike i bor’be so SPIDom FBUN Tsentral’nogo NII epidemiologii Rospotrebnadzora. Available at: http://www.hivrussia.info (in Russian).]
- Nicopoullos J.D., Almeida P., Vourliotis M., Gilling-Smith C. A decade of the sperm-washing programme: correlation between markers of HIV and seminal parameters. HIV Med. 2011; 12(4): 195-201. https://dx.doi.org/10.1111/j.1468-1293.2010.00868.x.
- Kehl S., Weigel M., Müller D., Gentili M., Hornemann A., Sütterlin M. HIV-1 infection and modern antiretroviral therapy impair sperm quality. Arch. Gynecol. Obstet. 2011; 284(1): 229-33. https://dx.doi.org/10.1007/s00404-011-1898-6.
- Bujan L., Sergerie M., Moinard N., Martinet S., Porte L., Massip P. et al. Decreased semen volume and spermatozoa motility in HIV-1-infected patients under antiretroviral treatment. J. Androl. 2007; 28(3): 444-52. https://dx.doi.org/10.2164/jandrol.106.001529.
- Lewis W., Dalakas M.C. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1995; 1(5): 417-22. https://dx.doi.org/10.1038/nm0595-417.
- Michaels G.S., Hauswirth W.W., Laipis P.J. Mitochondrial DNA copy number in bovine oocytes and somatic cells. Dev. Biol. 1982; 94(1): 246-51. https://dx.doi.org/10.1016/0012-1606(82)90088-4.
- Ramalho-Santos J., Varum S., Amaral S., Mota P.C., Sousa A.P., Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. 2009; 15(5): 553-72. https://dx.doi.org/10.1093/humupd/dmp016.
- Pelliccione F., Micillo A., Cordeschi G., D’Angeli A., Necozione S., Gandini L. et al. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011; 95(2): 641-6. https://dx.doi.org/0.1016/j.fertnstert.2010.07.1086.
- Hulgan T., Morrow J., D’Aquila R.T., Raffanti S., Morgan M., Rebeiro P., Haas D.W. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin. Infect. Dis. 2003; 37(1): 1711-7. https://dx.doi.org/10.1086/379776.
- Aitken R.J., Koppers A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011; 13(1): 36-42. https://dx.doi.org/10.1038/aja.2010.68.
- Blumer C.G., Restelli A.E., Giudice P.T., Soler T.B., Fraietta R., Nichi M. et al. Effect of varicocele on perm function and semen oxidative stress. BJU Int. 2012; 109(2): 259-65. https://dx.doi.org/10.1111/j.1464-410X.2011.10240.x.
- Божедомов В.А., Торопцева М.В., Ушакова И.В., Спориш Е.А., Ловыгина Н.А., Липатова Н.А. Активные формы кислорода и репродуктивная функция мужчин: фундаментальные и клинические аспекты (обзор литературы). Андрология и генитальная хирургия. 2011; 12(3): 15-21. [Bozhedomov V.A., Toroptseva M.V., Ushakova I.V., Sporish E.A., Lovygina N.A., Lipatova N.A. Aktivnye formy kisloroda i reproduktivnaya funktsiya muzhchin: fundamental’nye i klinicheskie aspekty (obzor literatury). Andrology and Genital Surgery/Andrologiya i genital’naya khirurgiya. 2011; 12(3): 15-21. (in Russian).]
- Van Leewen L., Repping S., de Baar M.P., Wit F.W., Prins J.M., van der Veen F., Reiss P. Ch.6: Increase of spermatozoal mtDNA copy numbers during first twelve weeks of first-line HAART. In: van Leeuwen E. Male reproduction and HIV-1 infection. 2009: 75-86.
- ВОЗ, Медико-генетический научный центр Российской академии медицинских наук. Руководство ВОЗ по исследованию и обработке эякулята человека. Пер. с англ. 5-е изд. Курило Л.Ф., ред. М.: Издательство «КАПИТАЛ ПРИНТ»; 2012. [WHO laboratory manual for the examination and processing of human semen. 5th ed. WHO; 2010.]
- van Leeuwen E., Cornelissen M., de Vries J.W., Lowe S.H., Jurriaans S., Repping S., van der Veen F. Semen parameters of a semen donor before and after infection with human immunodeficiency virus type 1: case report. Hum. Reprod. 2004; 19(12): 2845-8. https://dx.doi.org/10.1093/humrep/deh510.
- van Leeuwen E., Wit F.W., Prins J.M., Reiss P., van der Veen F., Repping S. Semen quality remains stable during 96 weeks of untreated human immunodeficiency virus-1 infection. Fertil. Steril. 2008; 90(3): 636-41. https://dx.doi.org/10.1016/j.fertnstert.2007.06.102.
- Drobnis E., Nangia K. Antivirals and male reproduction Adv. Exp. Med. Biol. 2017; 1034: 163-8. https://dx.doi.org/10.1007/978-3-319-69535-8_11.
- Muller C.H., Coombs R.W., Krieger J.N. Effects of clinical stage and immunological status on semen analysis results in human immunodeficiency virus type 1-seropositive men. Andrologia. 1998; 30(Suppl. 1): 15-22. https://dx.doi.org/10.1111/j.1439-0272.1998.tb02821.x.
- Dondero F., Rossi T., D’Offizi G., Mazzilli F., Rosso R., Sarandrea N. et al. Semen analysis in HIV seropositive men and in subjects at high risk for HIV infection. Hum. Reprod.1996; 11(4): 765-8. https://dx.doi.org/10.1093/oxfordjournals.humrep.a019251.
- Dulioust E., Du A.L., Costagliola D., Guibert J., Kunstmann J.M., Heard I. et al. Semen alterations in HIV-1-infected men. Hum. Reprod. 2002; 17(8): 2112-8. https://dx.doi.org/10.1093/humrep/17.8.2112.
- Wu H., Huffman A.M., Whitcomb B.W., Josyula S., Labrie S., Tougias E. et al. Sperm mitochondrial DNA measures and semen parameters among men undergoing fertility treatment. Reprod. Biomed. Online. 2019; 38(1): 66-75. https://dx.doi.org/10.1016/j.rbmo.2018.10.004.
- Kao S.H., Chao H.T., Wei Y.H. Multiple deletions of mitochondrial DNA are associated with the decline of motility and fertility of human spermatozoa. Mol. Hum. Reprod. 1998; 4(7): 657-66. https://dx.doi.org/10.1093/molehr/4.7.657.
- Lee H.C., Yin P.H., Lu C.Y., Chi C.W., Wei Y.H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J. 2000; 348(Pt 2): 425-32.
- Maisonneuve C., Igoudjil A., Begriche K., Lettéron P., Guimont M.C., Bastin J. et al. Effects of zidovudine, stavudine and beta-aminoisobutyric acid on lipid homeostasis in mice: possible role in human fat wasting. Antivir. Ther. 2004; 9(5): 801-10.
- Diehl S., Vernazza P., Trein A., Schnaitmann E., Grimbacher B., Setzer B. et al. Mitochondrial DNA and sperm quality in patients under antiretroviral therapy. AIDS. 2003; 17(3): 450-1. https://dx.doi.org/10.1097/00002030-200302140-00025.
- White D.J., Mital D., Taylor S., St John J.C. Sperm mitochondrial DNA deletions as a consequence of long term highly active antiretroviral therapy. AIDS. 2001; 15(8): 1061-2. https://dx.doi.org/10.1097/00002030-200105250-00017.
Received 04.02.2020
Accepted 07.02.2020
About the Authors
Svetlana G. Perminova, Cand. Med. Sci.; Leading Researcher, Gynecology Department One, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology; Ministry of Health of Russia. Tel.: +7(916)202-16-87. E-mail: perisvet@list.ru Address: 4, Oparin St., Moscow 117997Elena V. Mityurina, Cand. Med. Sci.; Researcher, Gynecology Department One, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology; Ministry of Health of Russia. Tel.: +7(964)796-74-65. E-mail: mity-elena@yandex.ru.
Address: 4, Oparin St., Moscow 117997
Fatima N. Selimova, Postgraduate Student, Gynecology Department One, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology; Ministry of Health of Russia. Tel.: +7(926)888-77-55. E-mail: doc.fselimova@mail.ru. Address: 4, Oparin St., Moscow 117997
Olga V. Burmenskaya, BD; Head, Laboratory of Cancer Genetics, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology; Ministry of Health of Russia. Tel.: +7(495)438-22-92. E-mail: o_bourmenskaya@oparina4.ru. Address: 4, Oparin St., Moscow 117997
Nadezhda V. Kozyrina, Cand. Med. Sci., Senior Researcher, Central Research Institute of Epidemiology, Federal Service for Supervision of Consumer Rights Protection and Human Well-Being. Tel.: +7(916)715-10-18. E-mail: nad-kozyrina@yandex.ru. Address: 3a, Novogireevskaya St., Moscow 111123
Aleksey V. Kravchenko, MD; Professor, Leading Researcher, Federal Research and Guidance Center for AIDS Prevention and Control, Central Research Institute of Epidemiology, Federal Service for Supervision of Consumer Rights Protection and Human Well-Being. Tel.: +7 (495) 366-05-18.
E-mail: alexey-kravtchenko@yandex.ru. Address: 3a, Novogireevskaya St., Moscow 111123
For citation: Perminova S.G., Mityurina Е.V., Selimova F.N., Burmenskaya О.V., Kozyrina N.V., Kravchenko А.V. Mitochondrial DNA copy number in sperm of hiv-infected men receiving antiretroviral therapy.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 120-126. (In Russian).
https://dx.doi.org/10.18565/aig.2020.3.120-126



