Clinical and immunological characteristics in the newborn infants of mothers with organ transplants
Objective. To assess the health status of newborn infants of mothers with solid organ transplants who have received immunosuppressive therapy during pregnancy and to characterize the immune status of the infants.Shatalova E.A., Matveeva N.K., Vanko L.V., Kravchenko N.F., Zhukova A.S., Makieva M.I., Krechetova L.V., Zubkov V.V.
Subjects and methods. General clinical examination was made in pregnant women with solid organ transplants (n = 16) and their newborns (n = 16). Ultrasonography of the brain, abdominal organs, kidney, and heart were performed in all the infants. Flow cytometry was used to assess the subpopulation composition of lymphocytes in the umbilical and venous blood of newborns and in the venous blood of their mothers.
Results. The babies born to women with organ transplants, who had received immunosuppressive therapy during pregnancy, had more frequently lower height and weight, lower gestational age, and increased frequency of early neonatal complications than the control group; however, the differences were statistically insignificant. As compared with the control group, the pregnant women with organ transplants during immunosuppressive therapy showed the signs of cellular immunity imbalance, which were manifested by decreases in the immunoregulatory index and the number of B lymphocytes and natural killer cells, by increases in the content of activated T lymphocytes and lymphocytes, ready for apoptosis. The umbilical cord blood of newborns in the study group displayed a decline in the relative content of B lymphocytes with no decreased immunoregulatory index, despite a significant increase in the percentage of CD3+CD4+ T lymphocytes.
Conclusion. The infants born to women with organ transplants, whose pregnancy occurred while receiving immunosuppressive therapy, generally have a satisfactory health status during the early neonatal period and much less pronounced changes in their immune status, compared with those observed in their mothers. The absence of substantial health problems and changes in the immune status of the newborns of mothers with organ transplants makes it possible to count on a good quality of life in these children. However, further observations of a larger sample of infants are required to assess the long-term effects of intrauterine immunosuppressive therapy.
Keywords
The implementation of reproductive function is one of the important aspects of quality of life in young patients. Transplantation of donor organs to save a patient’s life may be the only treatment for many serious diseases of internal organs. The number of transplantations of organs and tissues is constantly increasing and, according to forecasts, will increase further [1].
A history of a transplanted organ has long been considered as a contraindication to pregnancy. Pregnancy in women with transplanted organs is often accompanied by the development of complications, such as hypertension, gestational diabetes, preeclampsia, transplant rejection [2-5]. In this case, intrauterine growth of the fetus and premature birth of babies occur more frequently [6].
Modification of surgical methods and especially tactics of immunosuppressive therapy improved the survival rate and quality of life of women with organ transplants. It has allowed many patients who were previously infertile due to chronic illness to become pregnant. At present, childbirth has become possible thanks to modern immunosuppressive drugs, the use of which minimizes the risk of transplant rejection during pregnancy. It is very important to plan pregnancy, the selection of the scheme and dosage of immunosuppressive therapy, necessary for the normal functioning of the graft and having a minimal risk for the developing fetus [3, 7-10].
The condition for the existence and functioning of the transplanted organ (if it is not from an identical twin) is the suppression of developing immunological rejection reactions, since the antigens of the transplanted foreign organ inevitably cause an immune response. Therefore, it is not possible to abandon immunosuppressive drugs during pregnancy. Conception of children and the development of the fetal immune system occur under the action of substances that suppress the immune response. Pregnant women proceed to use immunosuppressive drugs during gestation, and the outcome of pregnancy depends both on the severity of the immune response and on the effect of immunosuppressive drugs on the development of the fetus. Inevitably, the question arises about the degree of influence of immunosuppressive drugs on the formation of the fetal immune system, its functioning and the state of health of the child after birth.
The aim of this study is to assess the health and immune status of newborns born to mothers with a transplanted solid organ who received immunosuppressive therapy during pregnancy.
Materials and Methods
The prospective cohort study included 16 women with a transplanted parenchymal organ and 20 women with a normal course of pregnancy (control group) who gave birth to 16 and 20 children, respectively, in National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia, Moscow (2015-2017). The somatic, obstetric and gynecological history, the course of current pregnancy, childbirth, the time of pregnancy after transplantation, the scheme of immunosuppressive therapy during pregnancy and its changes during pregnancy, and the mode of delivery were taken into account.
Newborns were divided into the following groups: the first main group consisted of children (n = 16), born to mothers (n = 16) with a transplanted solid organ (14 had the kidney transplantation, 2 had the liver transplantation). The second (control) group included the children (n = 20), born to healthy women (n = 20) with a normal course of pregnancy. An assessment of the course of the early neonatal period was carried out. Children were assessed by gestation age, anthropometric indicators and Apgar score at 1 and 5 minutes. In order to diagnose neutropenia, anemia, and thrombocytopenia, the clinical analysis of blood was performed on the 1st and 3rd or 4th day of the child’s life. An ultrasound study of the newborn’s brain, heart, abdominal organs and kidneys was performed; the assessment of the course of the early neonatal period was carried out. To determine the effect of the treatment received by the mother during pregnancy on the immune system of both mother and fetus, venous blood was collected before delivery in women, umbilical blood of their newborns and venous blood in children was obtained on the 3rd or 4th day and a month after birth.
Phenotyping of peripheral blood lymphocytes was performed by flow cytometry on a FACSCanto II device (Becton Dickinson, USA) using monoclonal antibodies to surface markers CD3, CD16, CD25, CD95, labeled with FITC (fluorescein isothiocyanate) and against CD4, CD8, CD19, CD56, CD7, HLA-DR, labeled with PE (phycoerythrin), CD4, labeled with APC (allophycocyanin) (BD Biosciences, USA). To assess positive stained subpopulations, we used the corresponding isotypic IgG labeled with FITC-, PE-, and APC. The leukocyte gate, which enables to exclude other blood cells from the analysis, was detected using monoclonal antibodies to CD45 labeled with PerCP (peridinine-chlorophyll protein). The data were analyzed using the FACSDiva program (Becton Dickinson, USA). The absolute lymphocyte content of the studied subpopulations was calculated based on the results of a clinical analysis of blood. The content of IgM, IgA and IgG in the serum was determined by the turbidimetric method using commercial kits (Human, Germany). Together with the scientific advisory pediatric department, children were followed-up.
Statistical data processing was carried out using generally accepted methods of variation statistics using statistical analysis packages. Compliance with the calculated samples of the normal distribution parameters was estimated using the Shapiro–Wilk criterion with the statistical package MedCalc12 (Belgium) for Windows 7. To assess the differences, we used the Student’s criterion for data with a normal distribution. Results were presented as mean ± standard deviation (Mean ± SD). To assess differences in the samples, where the distribution of data differed from the normal, nonparametric criteria were used: the Mann-Whitney U-test for independent samples and the Wilcoxon test for dependent samples. In this case, the results were presented as a median (25th - 75th percentile). Differences were considered significant at a significance level of p ˂ 0.05.
The study was approved by the Ethical Committee of National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia, Moscow.
Results
The women who gave birth to the children included in the study were comparable in age. The average age of women in the main group was 32.2±5.7 years, the age of women in the control group was 31.8±5.5 years. The diseases that caused organ transplantation were chronic pyelonephritis (n = 4), glomerulonephritis (n = 7), bilateral hydronephrosis (n = 1), systemic lupus erythematosus (n = 2), viral liver damage (n=1), Wilson’s disease (n = 1).
Pregnancy in 30% of cases occurred between 2 and 4 years after transplantation. The average time of pregnancy after transplantation was 5.5 years (the minimum time of pregnancy is 2 years, the maximum is 14 years). The history of five women included pregnancies: among them there were two artificial abortions, two missed miscarriages and one spontaneous miscarriage.
All women of the main group received various combinations of drugs during pregnancy: prednisolone (n = 14), cyclosporine (n = 6), azathioprine (n = 7), tacrolimus (n = 10). In 50% of cases, three-component therapy was carried out, in 31% of cases there was two-component therapy and in 19% it was one-component therapy. It should be noted that the scheme of immunosuppressive therapy changed with the course of pregnancy: thus, in the second and third trimesters of pregnancy, 12.5% of women received a three-component scheme of immunosuppressive therapy; two-component scheme was received by 69% of patients. The most frequent combinations of drug are tacrolimus and prednisone, tacrolimus, azathioprine and prednisone.
In this group, such complications of pregnancy as arterial hypertension, threatened miscarriage, anemia of mild to moderate severity, and acute respiratory viral infections were the most frequent.
Delivery in the group of women with a transplanted organ in three cases occurred through the natural birth canal (two women with a transplanted liver, one with a transplanted kidney and a stable course of pregnancy), in 13 cases there was cesarean section. In the control group, 13 pregnancies ended in spontaneous delivery, 7 patients were performed cesarean section.
Newborns from the main group had lower mass growth rates compared to newborns from the control group. However, no significant differences were found between the groups. The children from the main group had an average birth weight of 2729.12 ± 595.6 g, in the control group it was 3256.85 ± 347.24 g. Their height was 48.19 ± 4.5 cm and 51.0 ± 1.9 cm, respectively (p > 0.05). The children from the study and control groups were comparable in the Apgar score at 1 and 5 minutes: a 1-minute Apgar score was 8.0 (7.0-8.0) and 8.5 (8.0-9.0); a 5-minute Apgar score was 9.0 (8.0-9.0) and 9.5 (8.0-9.0), respectively (p > 0.05).
The average gestational age of the newborns in the main group was 36.5 ± 2.7 weeks, in the control group it was 39.1 ± 0.8 weeks. In the main group, four (25%) children were born prematurely, and one child was born at a gestation period of less than 32 weeks (6.25% of the total number of children). Compared with full-term neonates, these children had a higher risk of developing such pathological conditions as respiratory disorders, the implementation of intrauterine infection, disorders of thermoregulation, problems of nutritional absorption, hematological changes, hyperbilirubinemia and metabolic disorders.
The following features were noted in the morbidity of newborns from mothers with transplanted organs. According to neurosonography, a choroid plexus cyst was found in three children; according to echocardiography, persistent fetal circulation of the newborn was revealed in four children on the third day of life. Congenital pneumonia was diagnosed in one newborn, one child had neonatal jaundice, another one had transient tachypnoea.
Examination of children at birth did not reveal statistically significant differences between the groups, which may be due to an insufficient number of children in the main group. Therefore, it is not possible to make an unambiguous conclusion about the presence or absence of the harmful effects of immunosuppressive drugs on the development and condition of the fetus.
There were no statistically significant differences in the content of the total number of lymphocytes in the peripheral blood of the mothers of the main group compared with the control group. But changes in the subpopulation composition of lymphocytes were detected (Table 1).
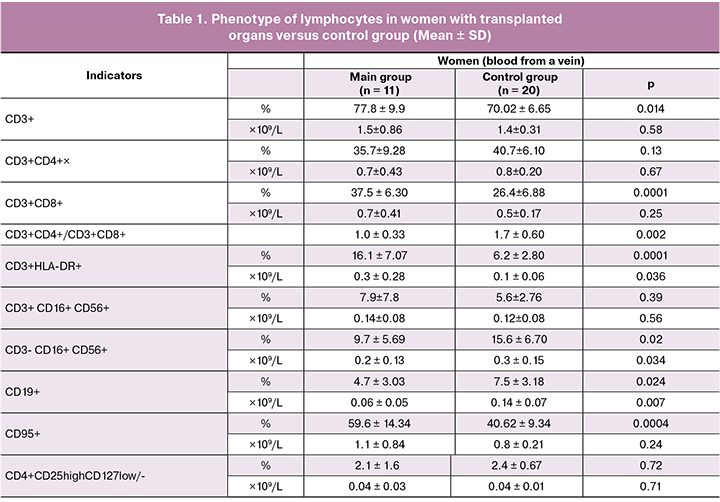
In the group of pregnant women with organ transplants who received chemotherapy, there was a significant change in the subpopulation composition of lymphocytes in peripheral blood. Reduced proportion of CD3+ T lymphocytes (p < 0.05). The decrease in the ratio of CD3+CD4+ and CD3+CD8+ subpopulations of T cells when compared with that in the control group (1.0±0.3 versus 1.7±0.6, respectively, p < 0.05) occurred due to a decrease in CD3+CD4+ T cells and a statistically significant increase in CD3+CD8+ T cells. The number of activated CD3+HLA-DR+ T lymphocytes was increased more than two times. There was a significant decrease in CD3-CD16+CD56+ -NK-cells and CD19+ B lymphocytes. Significant differences in the content of CD4+CD25highCD127low/- cells (T-regulatory) were not detected. There was an increase in the proportion of lymphocytes bearing the Fas antigen (CD95), a marker of cell readiness for apoptosis. When determining the level of IgG, IgM and IgA in the serum of peripheral blood, no significant difference was found in their concentration in women of the main group compared to the control (9.2 ± 3.1 g / L versus 11.4 ± 2.77 g / L, 1.7 ± 1.1g / L versus 1.9 ± 0.26 g / L and 1.7 ± 0.8 versus 1.8 ± 0.8 g / L, respectively, p > 0.05).
There was no significant depression of the hemopoietic sprouts and pronounced hematological disorders in the group of children whose mothers during pregnancy received immunosuppressive therapy, necessary to prevent rejection of the transplanted organ. The content of the total number of leukocytes, as well as the ratio of lymphocytes, neutrophils and monocytes, in the umbilical cord blood of newborns of the main group did not differ from the content in the control group.
When comparing the subpopulation composition of the umbilical cord blood of newborns in the main and control groups, there were no changes in the ratio of T lymphocytes, similar to those observed in their mothers (Table 2). There was an increase in the percentage of CD3+ T lymphocytes (75.5 ± 9.7% versus 64.2 ± 12.1%, p < 0.05) due to an increase in the relative number of CD3+CD4+ T cells (54.2 ± 10.9% versus 46.0 ± 12.2%, p < 0.05). However, there was no significant change in the ratio of CD3+CD4+ and CD3+CD8+ subpopulations of T cells, when compared with that in the control group (2.9 ± 1.2% versus 2.4 ± 0.6%, p > 0.05). There was a decrease in the relative number of B lymphocytes in newborns from mothers with a transplanted organ who received immunosuppressive therapy during pregnancy (10.0 ± 4.8% versus 15.5±6.1%, p < 0.05) and CD3+CD16+CD56+ - TNK cells (0.42 ± 0.34% versus 0.85 ± 0.49%, p < 0.01). Changes in the content of cells of other subpopulations of lymphocytes when compared with the control group of newborns were not revealed. Thus, children whose mothers received immunosuppressive therapy during gestation period showed less pronounced differences in the indicators of the control group than their mothers.
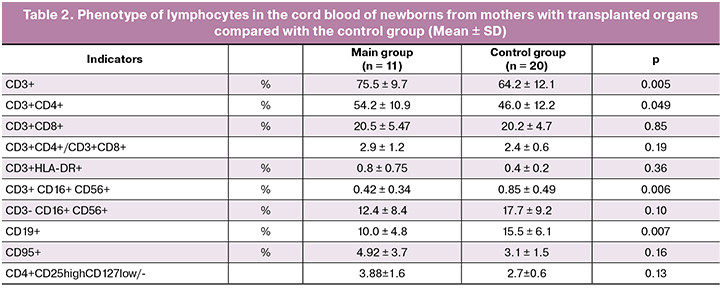
It is noteworthy that significant differences in the ratios of lymphocytes of different phenotypes were found between the peripheral blood of mothers and umbilical cord blood of children. The ratio of CD4+ and CD8+ T lymphocytes in newborns in both groups was significantly higher than that of their mothers. The proportion of activated T lymphocytes (CD3+HLA-DR+) and the proportion of lymphocytes bearing Fas antigen (CD95), a marker of cell readiness for apoptosis, were significantly lower in both groups of infants compared to their mothers.
Table 3 presents the data characterizing the immune status of children from mothers with transplanted organs in dynamics in the neonatal period.
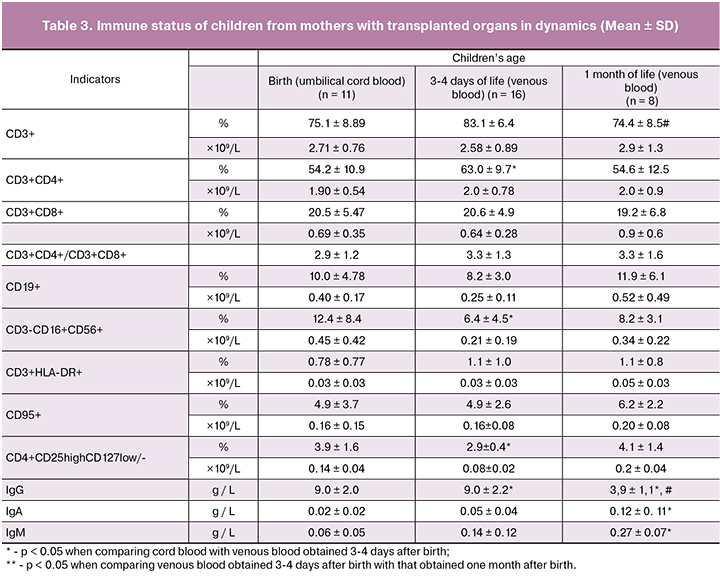
A comparative analysis of the percentage and absolute cell content of different lymphocyte subpopulations did not reveal significant differences between the cord blood and the venous blood of the child obtained in the early neonatal period (3-4 days after birth), except for a decrease in CD3-CD16+CD56+-NK-cells. Significant changes in the subpopulation composition of lymphocytes in the peripheral blood of the child in a month were not revealed. However, there was a significant decrease in the concentration of IgG (9.0 ± 2.2 g / L and 3.9 ± 1.1 g / L, p = 0.0004) and an increase in the content of IgM and IgA.
Discussion
At present, there is little data in the literature concerning the state of health of children exposed to immunosuppressive drugs in utero. According to the Food and Drug Administration (FDA), all immunosuppressive drugs that patients receive after transplantation fall into safety category B, that is, in animal studies, these drugs showed a negative effect on the fetus. Currently, there are no adequate and well-controlled studies of these drugs in humans and, given the need for their use in transplantation, they can be used in pregnant women, despite the possible risks [11]. A coordinated work of an obstetrician and a transplant specialist is also required to maintain an optimal level of immunosuppression keeping a balance between prevention of graft rejection and the adverse side effects of immunosuppression.
According to the results of five-year observations of McKay D.B., Adams P.L, almost 26% of children born to mothers with a transplanted kidney showed significant cardiac impairment [12]. In our study, there were no serious cardiovascular disorders in newborns born to mothers with a transplanted kidney.
In the study of the immune status of the newborn from main and control groups, there were no significant differences in the number of leukocytes, the level of granulocytes and lymphocytes between the groups of newborns, which is consistent with the data of other authors [6]. However, according to Ono et al., fewer platelets, leukocytes, neutrophils, and eosinophils were detected in children from mothers with a transplanted kidney at birth than in children from the control group [13].
Our data are also consistent with the results of studies in which it was shown that at birth children from mothers with a transplanted organ had a significant decrease in the number of B cells [13]. Previously it was assumed that the immune changes observed in newborns from mothers with transplanted organs may interfere with the normal response of children to vaccination. The possibility of postponing vaccination to 6 months of age was considered in order to avoid undesirable immunological reactions in adverse conditions to live vaccines [14, 15]. Evidence has been published confirming that babies born to these mothers may have an adequate serological response to the vaccine without a higher percentage of adverse outcomes [16]. It is suggested that children from mothers with a transplant should be immunized against vaccine-preventable diseases as soon as possible, since they are in constant contact with mothers who are in an immunodeficient state and have an increased risk of infectious diseases.
Conclusion
The pregnancy in women with transplanted kidney or liver who used immunosuppressive therapy during gestation was most often complicated by the threatened miscarriage, mild to moderate anemia, and arterial hypertension. Women in this group showed the signs of imbalance in the cellular component of the immune system, which were manifested by a decrease in the T lymphocyte content, an immunoregulatory index (the ratio of CD3+CD4+ and CD3+CD8+ subpopulations of T lymphocytes), the number of B lymphocytes and natural killer cells, an increase in the content of activated T lymphocytes and lymphocytes ready for apoptosis.
Children born to women with organ transplants are more likely to have lower mass growth rate, a lower gestational age and an increased incidence of complications of the early neonatal period. In the cord blood of newborns from mothers who received immunosuppressive therapy during pregnancy, a significant decrease in B lymphocytes was detected in the absence of a decrease in immunoregulatory index, despite a significant increase in the percentage of CD3+CD4+ T lymphocytes.
The study of the immune status of these children in the neonatal period in the dynamics did not reveal any significant changes in the subpopulation composition of lymphocytes, compared with the content in the umbilical cord blood. There was a highly significant decrease in the concentration of IgG and an increase in the levels of IgM and IgA by the end of the neonatal period, which may indicate a natural decrease in the content of maternal IgG and an increase in the production of its own IgM and IgA.
For a successful outcome of pregnancy in women with transplanted organs who used immunosuppressive drugs during gestation as well as the normal formation and development of the cellular basis of adaptive immunity in the fetus, further improvement of treatment with an interdisciplinary character is required, with careful selection of drugs and their dosage.
References
1. National Transplantation Pregnancy Registry (NTPR). Annual report: gift of life institute. Philadelphia, PA; 2014.
2. Zachariah M.S., Tornatore K.M., Venuto R.C. Kidney transplantation and pregnancy. Curr. Opin. Organ Transplant. 2009; 14(4): 386-91.
3. Concepcion B.P., Schaefer H.M. Caring for the pregnant kidney transplant recipient. Clin. Transplant. 2011; 25(6): 821-9.
4. Coscia L.A., Constantinescu S., Davison J.M., Moritz M.J., Armenti V.T. Immunosuppressive drugs and fetal outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2014; 28(8): 1174-87. doi: 10.1016/j.bpobgyn.2014.07.020.
5. Deshpande N.A., James N.T., Kucirka L.M., Boyarsky B.J., Garonzik-Wang J.M., Montgomery R.A., Segev D.L. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am. J. Transplant. 2011; 11(11): 2388-404.
6. Kociszewska-Najman B., Pietrzak B., Cyganek A., Szpotanska-Sikorska M., Schreiber-Zamora J., Jabiry-Zieniewicz Z., Wielgos M. Intrauterine hypotrophy and premature births in neonates deliveredby female renal and liver transplant recipients. Transplant. Proc. 2011; 43(8): 3048-51.
7. Готье С., Мойсюк Я., Хомяков С. Донорство и трансплантация органов в Российской Федерации в 2013 году. VI сообщение регистра Россйского трансплантологического общества. Вестник трансплантологии и искусственных органов. 2014; 16(2): 5-23. doi:10.15825/1995-1191-2014-2-5-23.
8. Parhar K.S., Gibson P.S., Coffin C.S. Pregnancy following liver transplantation: review of outcomes and recommendations for management. Can. J. Gastroenterol. 2012; 26(9): 621-6.
9. Leroy C., Rigot J.M., Leroy M., Decanter C., Le Mapihan K., Parent A.S. et al. Immunosuppressive drugs and fertility. Orphanet J. Rare Dis. 2015; 10: 136.
10. Casale J.P., Doligalski C.T. Pharmacologic considerations for solid organ transplant recipients who become pregnant. Pharmacotherapy. 2016; 36(9): 971-82. doi: 10.1002/phar.1800.
11. Шаталова Е.А., Зубков В.В., Подуровская Ю.Л., Кравченко Н.Ф., Ванько Л.В. Беременность после трансплантации паренхиматозных органов: осложнения, исходы, перспективы. Акушерство и гинекология. 2017; 7: 5-11.
12. McKay D.B., Adams P.L., Bumgardner G.L., Davis C.L., Fine R.N., Krams S.M. et al. Reproduction and pregnancy in the transplanted patient: current practices. Prog. Transplant. 2006; 16(2): 127-32.
13. Ono E., dos Santos A.M., Viana P.O., Dinelli M. I.S., Sass N., De Oliveira L. et al. Immunophenotypic profile and increased risk of hospital admission for infection in infants born to femalе kidney transplant recipients. Am. J. Transplant. 2015; 15(6): 1654-65.
14. Di Paolo S., Schena A., Morrone L.F., Manfredi G., Stallone G., Derosa C. et al. Immunologic evaluation during the first year of life of infants born to cyclosporine-treated female kidney transplant recipients: analysis of lymphocyte subpopulations and immunoglobulin serum levels. Transplantation. 2000; 69(10): 2049-54.
15. Schena F.P., Stallone G., Schena A., Manfredi G., Derosa C., Procino A., Di Paolo S. Pregnancy in renal transplantation: immunologic evaluation of neonates from mothers with transplanted kidney. Transpl. Immunol. 2002; 9(2-4):161-4.
16. Dinelli M.I.S., Ono E., Viana P.O., Spina F.G., Weckx L.Y., dos Santos A.M.N., de Moraes-Pinto M.I. Response to immunization in children born to renal transplant recipients using immunosuppressive drugs during gestation. Vaccine. 2016; 34(4): 404-7.
Received 17.02.2018
Accepted 02.03.2018
About the Authors
Shatalova, Ekaterina A., neonatologist of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakovof Ministry of Healthcare of Russian Federation, 4, Oparin street, Moscow, Russian Federation, 117997, +7-(495)-438-11-83. е-mail: e_shatalova@oparina4.ru
Matveeva, Natalia K., candidate of biological science, senior researcher of clinical immunology laboratory National Medical Research Center for Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russian Federation, 4, Oparin street, Moscow, Russian Federation,
117997, +7-(495)-438-11-83. е-mail: matveeva_nk@mail.ru.
Vanko, Ludmila V., doctor of medicine, professor, leading research worker of clinical immunology laboratory National Medical Research Center of Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russia Federation,
4, Oparin street, Moscow, Russian Federation, 117997, +7-(495)-438-11-83. e-mail: lvanko@mail.ru.
Kravtchenko, Natalia F., candidate of medicine sciences, obstetrician-gynecologist, senior researcher of the Department of pregnant pathology of National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russia Federation,
4, Oparin street, Moscow, Russian Federation, 117997, E-mail: n_kravchenko@oparina4.ru
Zhukova, Anastasia S., research worker of clinical immunology laboratory National Medical Research Center of Obstetrics, Gynecology and Perinatology named
after Academician V.I.Kulakov of Ministry of Healthcare of Russia Federation,
4, Oparin street, Moscow, Russian Federation, 117997, +7-(495)-438-11-83. E-mail: a_belyaeva@oparina4.ru
Makieva, Mziya I., neonatologist, pediatrician, the head of the clinical work of the department of newborns National Medical Research Center of Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russia Federation,
4, Oparin street, Moscow, Russian Federation, 117997, +7-(495)-438-11-83. е-mail: m_makieva@oparina4.ru
Krechetova, Lyubov V., candidate of medicine sciences, head of clinical immunology laboratory National Medical Research Center of Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russia Federation,
4, Oparin street, Moscow, Russian Federation, 117997, +7-(495)-438-11-83. E-mail: k_l_v_@mail.ru.
Zubkov, Victor V., Doctor of Medicine , Head of the Department of Neonatology and Pediatrics National Medical Research Center of Obstetrics,
Gynecology and Perinatology named after Academician V.I.Kulakov of Ministry of Healthcare of Russia Federation.
4, Oparin street, Moscow, Russian Federation, 117997, +7 (495) 438-22-66. E-mail: v_zubkov@oparina4.ru
For citations: Shatalova E.A., Matveeva N.K., Vanko L.V., Kravchenko N.F., Zhukova A.S., Makieva M.I., Krechetova L.V., Zubkov V.V. Clinical and immunological characteristics in the newborn infants of mothers with organ transplants. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 106-13. (in Russian)
https://dx.doi.org/10.18565/aig.2018.11.106-113



