Clinical and epidemiological aspects of corpus uteri cancer in the context of prevention of recurrent endometrial hyperplasia
Objective. To analyze trends in the incidence of corpus uteri cancer (CUC) in the Omsk Region in 2002–2018 to prioritize cancer prevention in patients with endometrial hyperplasia (EH). Material and methods. A continuous descriptive observational retrospective epidemiological study of CUC incidence rates was conducted using official statistics. Results. There was a moderate tendency for higher CUC incidence rates in the region (Growth rate (Gr) = +1.8%; p < 0.001) and in the Russian Federation (Gr = +1.7%; p <0.001). The highest proportion was 32.3% of women aged 60–69 years. There was a moderate tendency for increased CUC incidence rates among 30 to 39 year olds and a marked tendency for higher ones in women aged 35–39 years (Gr = +5.6%; p <0.05). A rationale was provided for an integrated approach to managing patients with EH in the context of prevention of the recurrent disease. Conclusion. It was established that the Omsk Region showed a rise in CUC incidence and a tendency for patients with this disease to become younger. One of the measures to reduce the risk of HE recurrence and progression is the need for a preventive therapy cycle, by taking into account endometrial morphological control.Klinyshkova T.V., Turchaninov D.V., Frolova N.B.
Keywords
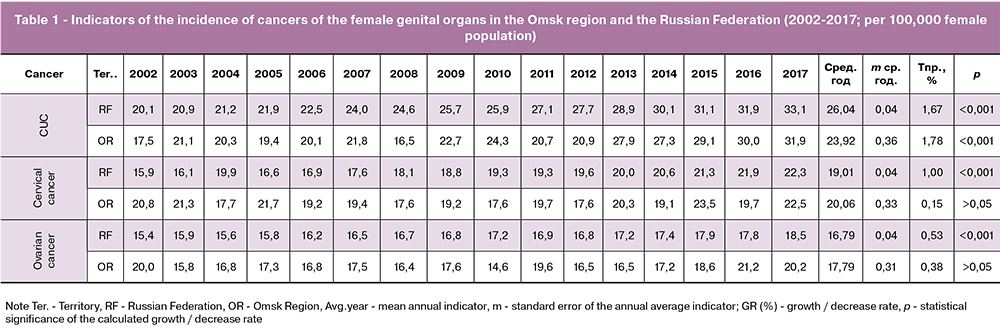
Malignant neoplasms of the female reproductive system account for the largest proportion (39.2%) of malignancy-related morbidity among women in the Russian Federation (RF), and neoplasms of female genital organs constitute 18.2% of all malignancies in women [1]. In 2017, cancer of the corpus uteri (CUC) was the third leading malignancy (7.8%) among the female population in Russia after breast cancer (21.1%) and skin neoplasms (16.6%) [1], and was most commonly occurring gynecological cancer. According to the Federal Service for State Statistics, the prevalence of CUC in the Russian Federation in 2008 and 2017 was 128.6 0 and 175.5 per 100,000 female population, respectively. An increase in the CUC incidence rate in the Russian Federation was also recorded in 2007 (24.00 per 100,000) and 2017 (33.1 per 100,000 women). The increase in the CUC incidence rate from 2007to 2017 reached 39.6%. The mean age of CUC patients in the Russian Federation in 2017 was 62.9 years [1].
According to WHO and the International Agency for Research on Cancer (IARC), in 2018, the incidence rate of CUC worldwide, in France, New Zealand, and the Russian Federation was 10.1, 31.9, 27.9, and 33.6 per 100,000 women, respectively [2].
Endometrial adenocarcinoma has been known to be closely associated with endometrial hyperplasia (EH). However, the literature is lacking data on the prevalence of EH since there is no official registration of EH incidence. Analysis of the incidence and structure of EH in the Russian Federation showed no decrease in the incidence of the disease in recent years, along with a high incidence of atypical EH and endometrial adenocarcinoma in reproductive age women. This observation confirms the need for a cancer prevention program, which includes an expert morphological assessment of the endometrium, identification of patients at high risk for cancer, and improvement in EH management [3]. Currently, insufficient attention is being paid to the prevention of EH recurrence due to the lack of regulatory documents.
This study was aimed to analyze the dynamics of CUC incidence rates in the Omsk region for the years 2002 to 2018 and set priorities for cancer prevention in patients with EH.
Material and methods
This was a descriptive observational retrospective epidemiological study of the incidence of CUC for the years 2002–2017. The study was conducted using official epidemiology and health statistics from P. Hertsen MORI - the branch of the National Medical Radiology Research Centre of Minzdrav of Russia, official statistical forms of the Clinical Oncology Center (form No. 7 Information on Malignant Tumors and form No. 35 Information on Patients with Malignant Neoplasms). Detailed data on patients’ age were analyzed for the years 2009–2018.
Statistical analysis was performed using Microsoft Excel. In accordance with the algorithm of descriptive observational epidemiological research, intensive (incidence, per 100,000 female population) and extensive (prevalence, expressed as a percentage) indicators, and their standard errors (Vlasov VV, Epidemiology, 2004) were calculated. To assess the error of relative values, the standard error of the indicator was calculated by the formula:

where n is the sample size, p is the rate of the events, and m is the standard error. To indicate a range of values that’s likely to include a population value with a certain degree of confidence, a confidence interval (CI) was calculated, in which the value for the population is found with a probability of 95%. To obtain 95% CI, m was multiplied by (t1-a/2), where a is the accepted level of alpha risk. Alpha risk corresponds to a 5% probability of error. The dynamic analysis was performed with least-squares smoothing and the calculation of increase (decrease) rates, expressed as a percentage. The critical level of significance when testing statistical hypotheses was considered at p <0.05.
Results and discussion
The changes in the incidence of CUC in the OR were analyzed in comparison with the data for the Russian Federation for the years 2002-2017. Over the study period, CUC was a leading malignancy among other genital cancers (Table 1). The incidence of CUC in the Russian Federation ranged from 20.9 in 2002 to 33.1 per 100,000 women in 2017. The incidence of CUC in the OR in 2002 was 17.5 per 100,000 women versus 31.9 per 100,000 in 2017 (p <0.001; Fig. 1). There was a moderate upward trend in the incidence of CUC (growth rate GR = + 1.8%, p <0.001), as well as in the Russian Federation (GR = + 1.7%, p <0.001) for the years 2002–2017. Over the study period, the incidence rates in the OR were slightly lower than the national average (23.2 ± 0.4 and 26.0 ± 0.040 / 0000, respectively, p <0.001), but in recent years they have become comparable. In 2018, the incidence rate in the OR increased to 35.1 per 100,000 women, reaching a maximum.

The age structures of CUC patients in the OR and RF did not differ significantly (p> 0.05). Women aged 60–69, 50–59, ≥70, 40–49, 30–39, and 20–29 constituted 32.3% 31.2%, 23.4%, 10,5%, 2.3%, and 0.34%, respectively (Fig. 2).
 It is worth noting that there was a moderate upward trend in CUC incidence rate among women aged 30–39 for the years 2009–2018, with a pronounced upward trend in the incidence rate among women aged 35–39 (GR = + 5.6%; p <0, 05). These observations correlate with the age distribution of various types of EH (2008–2012) in age-related peaks of morbidity. So, the peak of atypical EH - endometrial pre-cancer, was recorded at the age of 35–40 years, mainly in nulliparous women, while the peak of simple and complex EH was noted at the age of the menopausal transition [3]. In this study, the incidence of endometrial cancer in reproductive-aged women was 13.5% of all cases of endometrial cancer (5–9.6% in the world literature).
It is worth noting that there was a moderate upward trend in CUC incidence rate among women aged 30–39 for the years 2009–2018, with a pronounced upward trend in the incidence rate among women aged 35–39 (GR = + 5.6%; p <0, 05). These observations correlate with the age distribution of various types of EH (2008–2012) in age-related peaks of morbidity. So, the peak of atypical EH - endometrial pre-cancer, was recorded at the age of 35–40 years, mainly in nulliparous women, while the peak of simple and complex EH was noted at the age of the menopausal transition [3]. In this study, the incidence of endometrial cancer in reproductive-aged women was 13.5% of all cases of endometrial cancer (5–9.6% in the world literature).
CUC incidence rates in the female population of the OR by age groups are presented in Fig. 3. The following age risk groups were determined: 35–39 years (marked increase in indicators), 45–49 years (a significant increase compared with the previous age), and 60–69 years (maximum incidence rates). No significant differences were found between age-specific CUC incidence rates in the OR and the national average (p> 0.05).
 It is important to note that for standardized incidence rates (WHO World Standard Population was used), the CUC incidence rate in the ORwas slightly higher than the national average (but not statistically significant, 22.17 ± 0.35 and 21.8 ± 0.04, respectively; p> 0, 05).
It is important to note that for standardized incidence rates (WHO World Standard Population was used), the CUC incidence rate in the ORwas slightly higher than the national average (but not statistically significant, 22.17 ± 0.35 and 21.8 ± 0.04, respectively; p> 0, 05).
Along with obesity and insulin resistance, the malignant transformation of the endometrium is associated with hyperplastic endometrium processes, including the atypical form (EIN according to the binary classification system of endometrial hyperplasia, WHO 2014). Worthy of considerable attention is the lack of measures aimed at preventing recurrence of EH, as well as organizational problems (incomplete examination of treated patients in the absence of morphological control of the endometrium after completion of EH treatment with or without atypia, especially among nulliparous women). It seems debatable to expand the indications for pipelle endometrial biopsy because of its lack of information for the diagnosis of focal changes in the uterine mucosa. Thus, focal complex EH was established in every second case in the background of simple EH, in every fourth case in the background of unchanged endometrium and every tenth in the background of the endometrial polyp [3].
Patients with EH who had no indications for hysterectomy underwent conservative treatment taking into account their age (EIN in peri-, postmenopausal women, combined uterine hyperplasia, a progression of EH, etc.) using gestagens or gonadotropin-releasing factor agonists for combined EH [4-10]. The effectiveness of gestagens varies depending on the gestagen type, treatment regimen and duration of therapy. It has been proven that local intrauterine (levonorgestrel-releasing intrauterine system [LNG-IUS]) progestogens are most effective in achieving regression, and, therefore, should be the first-line medical treatment. Many studies reported that both continuous oral and local progestogens are more effective than a cyclic regimen. In patients with endometrial hyperplasia without atypia, the efficacy of oral progestins (medroxyprogesterone acetate (MPA), dydrogesterone, norethisterone) and LNG-IUS is 55.6–80% and 92–100%, respectively [4–11]. Moreover, the rate of hysterectomy, which is subsequently performed in these patients, is two-fold higher in those treated with oral progestins, compared with LNG-IUS (37.2–57.4% versus 20–22.1%, p <0.001). As is known, the duration of therapy for patients with EH is six months, followed by histological surveillance of the endometrium. Failure to achieve regression of complex EH without atypia and EH with atypia confers a high risk of endometrial cancer [9].
Endometrial ablation for EH has been a subject of debate in the literature. According to the NICE guideline (2016) endometrial ablation is not recommended for the treatment of EH with and without atypia, because complete and persistent endometrial destruction cannot be ensured and intrauterine adhesion formation may preclude future endometrial histological surveillance [12].
During treatment of EH, patients may experience regression of EH, persistence of EH (lack of morphological changes after the main treatment course), progression of EH, and long-term relapse. Recurrence of EH is a serious clinical problem. The results of multicenter RCTs with the participation of 153 women with EH without atypia aged 30 to 70 years, who were randomized to one of the following three treatment arms: LNG-IUS; 10 mg of oral MPA administered for 10 days per cycle for 6 months; or 10 mg of oral MPA administered daily for 6 months. Despite the higher efficacy of LNG-IUS in comparison with progestins at the end of treatment, follow-up evaluation showed different results. Within 24 months of follow-up, histological relapse was observed in 41% of patients regardless of the assigned treatment regimen and most relapses (63.6%) occurred during the first 6 months of observation period [13].
Often in clinical practice, the management of patients with EH without atypia is limited to the first stage, despite the existing possibilities of prophylactic hormone therapy. The second stage of the integrated management of patients with EH (after histological confirmation of regression) is a key priority for the prevention of disease recurrence [5, 11, 13, 14]. Depending on the patient’s age and patients’ reproductive plans, the following options for the second stage management are possible when EH regression is achieved:
- cyclic progestogens in reproductive age patients, including those planning a pregnancy;
- LNG-IUS in patients who have completed their reproductive life and are in perimenopause (including the combination of LNG-IUS with estrogens as menopausal hormone therapy), as well as in patients with concomitant adenomyosis and for the prevention of recurrence of peritoneal endometriosis;
- combined oral contraceptives (COCs) in reproductive age patients who need contraception, as well as in the early stage of the menopausal transition.
The Federal Guidelines indicate that long-term prophylaxis of EH after the end of therapy is advisable for two years or more in patients who have completed their reproductive life [15]. This approach can be considered as one of the measures to prevent recurrence of EH without atypia, progression to EIN, and possible malignant transformation.
Conclusion
CUC is the most commonly occurring gynecological cancer both in the Russian Federation and the Omsk region. Between 2002 and 2018, OR experienced an increase in the incidence of CUC per 100,000 women (17.5 in 2002 and 35.1 in 2018; GR = + 1.8%; p <0.001), with increasing incidence trends among younger women (GR = + 5, 6% among women aged 35–39, p < 0.05). The high recurrence rate of EH without atypia necessitates at least two years of prophylactic hormonal therapy after morphological confirmation of the complete treatment response and taking into account the patient’s reproductive plans.
References
- Каприн А.Д., Старинский В.В., Петрова Г.В., ред. Злокачественные новообразования в России в 2017 году (заболеваемость и смертность). М.: МНИОИ им. П.А. Герцена – филиал ФГБУ «НМИЦ радиологии» Минздрава России; 2018. 250 с.[Kaprin A.D., Starinsky V.V., Petrova G.V., ed. Malignant neoplasms in Russia in 2017 (morbidity and mortality). M.: MNIII im. P.A. Herzen - a branch of the Federal State Budgetary Institution Scientific Research Center for Radiology of the Ministry of Health of Russia; 2018.250 s.(in Russian)].
- World Health Organization: GLOBOCAN 2018. Cancer Today –IARC. https://gco.iarc.fr/today/home
- Думановская М.Р., Чернуха Г.Е., Асатурова А.В., Коган Е.А. Частота выявления и структура гиперплазии эндометрия в различные возрастные периоды. Акушерство и гинекология. 2015; 3: 40–4. [Dumanovskaya M.R., Chernukha G.Ye., Asaturova A.V., Kogan Ye.A. The frequency of detection and structure of endometrial hyperplasia in different age periods. Akusherstvo i ginekologija/Obstetrics and gynecology. 2015; 3: 40-44. (in Russian)]
- Orbo A., Vereide A., Arnes M., Pettersen I., Straume B. Levonorgestrel-impregnated intrauterine device as treatment forendometrial hyperplasia: a national multicentre randomised trial. BJOG. 2014; 21(4): 477–86. doi: 10.1111/1471-0528.12499.
- Marnach M.L., Butler K.A., Henry M.R., Hutz C.E., Langstraat C.L., Lohse C.M., Casey P.M. Oral Progestogens Versus Levonorgestrel-Releasing Intrauterine System for Treatment of Endometrial Intraepithelial Neoplasia. J Womens Health (Larchmt). 2017; 26(4): 368–73. doi: 10.1089/jwh.2016.5774.
- El Behery M.M., Saleh H.S., Ibrahiem M.A., Kamal E.M., Kassem G.A., Mohamed Mel S. Levonorgestrel-releasing intrauterine device versus dydrogesterone for management of endometrial hyperplasia without atypia. Reprod Sci. 2015; 22(3): 329–34. doi: 10.1177/1933719114542014
- Abu Hashim H., Zayed A., Ghayaty E., El Rakhawy M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: a randomized controlled trial. J Gynecol Oncol. 2013; 24(2):128–34. doi: 10.3802/jgo.2013.24.2.128.
- Gallos I.D., Shehmar M., Thangaratinam S., Papapostolou T.K., Coomarasamy A., Gupta J.K. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Аm J Obstet Gynecol. 2010; 203(6): 547.e1-10. doi:10.1016/j.ajog.2010.07.037
- Gallos I.D., Krishan P., Shehmar M., Ganesan R., Gupta J.K. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod. 2013; 28(11): 2966–71. doi: 10.1093/humrep/det320
- Клинышкова Т.В., Фролова Н.Б., Мозговой С.И. Клинико-морфологическое обоснование оптимизации лечения больных с гиперплазией эндометрия. Российский вестник акушера-гинеколога. 2010; 3: 16–20. [Klinyshkova T.V., Frolova N.B., Mozgovoy S.I. Clinical and morphological substantiation of optimization of treatment of patients with endometrial hyperplasia. Rossiyskiy vestnik akushera-ginekologa/ Russian Bulletin of Obstetrician-Gynecologist. 2010; 3: 16–20. (in Russian)]
- Чернуха Г.Е., Могиревская О.А., Шигорева Т.В., Грибанова Н.Д. Клинико-морфологические аспекты внутриматочного воздействия левоноргестрела при гиперплазии эндометрия. Акушерство и гинекология. 2011; 4: 56–62. [Chernukha G.Ye., Mogirevskaya O.A., Shigoreva T. V., Gribanova N. D. Clinical and morphological aspects of intrauterine exposure to levonorgestrel in endometrial hyperplasia. Akusherstvo i ginekologija /Obstetrics and gynecology. 2011; 4: 56–62. (in Russian)].
- Management of Endometrial Hyperplasia. Green-top Guideline No. 67 RCOG/BSGE Joint Guideline | February 2016 https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_67_endometrial_hyperplasia.pdf
- Ørbo A., Arnes M., Vereide A.B., Straume B. Relapse risk of endometrial hyperplasia after treatment with the levonorgestrel-impregnated intrauterine system or oral progestogens. BJOG. 2016;123(9):1512–9. doi: 10.1111/1471-0528.13763.
- Тихомиров А.Л. Обоснование использования комбинированных оральных контрацептивов для профилактики рецидивов типичных гиперплазий эндометрия. Гинекология. 2018; 20(4): 26–8. [Tikhomirov A.L. Rationale for the use of combined oral contraceptives for the prevention of recurrence of typical endometrial hyperplasia. 2018; 20(4): 26–28. (in Russian)]
- Гиперпластические процессы эндометрия. Гинекология. Национальное руководство. М., 2017. С. 303–308. [Hyperplastic processes of the endometrium. Gynecology. National leadership. M., 2017; 303–308. (in Russian)]
Received 27.05.2019
Accepted 21.06.2019
About the Authors
Tatiana V. Klinyshkova, MD, Full Professor, Professor of the Graduate Education Department of obstetrics and gynecology, State Budgetary Educational Institutionof Higher Professional Education “Omsk State Medical University” of the Public Health Ministry of the Russian Federation, tel.:+7620315480,
е-mail: klin_tatyana@mail.ru; https://orcid.org/0000-0002-0544-8184
644043 Russia, Omsk, Lenin Str., 12.
Denis V. Turchaninov, MD, Full Professor, Head of the Department of Hygiene and Nutrition, State Budgetary Educational Institution of Higher Professional Education
“Omsk State Medical University” of the Public Health Ministry of the Russian Federation, tel.+73812605418, е-mail: omskgsen@yandex.ru;
https://orcid.org/0000-0002-6298-4872
644043, Russia, Omsk, Lenin Str. 12.
Natalia B. Frolova, PhD, Head of gynecological Department, Non-state Healthcare Institution Division Hospital of Omsk Passazhirskii JSCo RZD.
tel. +7 9139686808, e-mail: nbfrolova@yandex.ru; https://orcid.org/0000-0002-4393-6904
644005 Russia, Omsk, Karbysheva str., 41.
For citation: Klinyshkova T.V., Turchaninov D.V., Frolova N.B. Clinical and epidemiological aspects of corpus uteri cancer in the context of prevention of recurrent endometrial hyperplasia .
Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2020; 1: 135-40. (In Russian).
https://dx.doi.org/10.18565/aig.2020.1.135-140



