Clinical risk factors for infertility in reproductive-age patients with intramural uterine fibroids
Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S.
Objective: To determine the clinical risk factors for infertility and develop a relative risk score for infertility in reproductive-aged patients with intramural uterine fibroids (UF).
Materials and methods: This was a single-center retrospective case-control study with multivariate logistic regression. The study included an analysis of the medical records of 200 reproductive-age patients with intramural UF measuring 3–5 cm (FIGO 3–6 type), who were examined and treated at the University Clinic "I am healthy!" (clinical base of the Department of Obstetrics and Gynecology with a perinatology course at the People’s Friendship University) from 2017 to 2021. The patients were divided into two groups: 100 infertile women with intramural UF ("case") and 100 fertile patients with UF ("control"). Factors, such as complaints and diseases with a duration exceeding 12 months, which could potentially affect fertility at the time of pregnancy planning, were assessed. The development of a relative risk score scale involved selecting parameters and combining them into a score scale. The binary logistic regression method was used to predict single-step (prognostic) conditions such as infertility.
Results: The relative risk score scale included anamnestic parameters such as long and heavy menstruation (lasting more than 12 months), chronic pelvic pain (lasting more than 12 months), insulin resistance, obesity (lasting more than 12 months), long-term iron deficiency anemia, history of endometrial hyperplastic processes, and phenotypic signs of undifferentiated connective tissue dysplasia (two or more signs). Mathematical calculations allowed us to determine the significance of each risk factor. The sum of these factors provides an estimate of the relative risk of infertility based on the obtained odds ratio value expressed in points. A score of >6 indicates an increased relative risk of infertility (p<0.001), while a score of >13 indicates a high relative risk of infertility with statistical significance (p<0.001). During the ROC analysis with recalculated point coefficients for each patient, we obtained a "cut off" value of 0.5035, above which the probability of infertility increased, reaching the maximum sensitivity and specificity.
Conclusion: The developed relative risk point scale, which includes clinical and phenotypic parameters, can be a valuable tool for assessing the risk of infertility (as well as the potential causes of failure in in vitro fertilization programs) in patients with intramural UF, including during the stages of pregnancy planning.
Authors' contributions: Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S. – conception and design of the study; Kolesnikova S.N., Alyoshkina E.V. – material collection and processing, drafting of the manuscript; Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Patrice Lumumba Peoples' Friendship University of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S. Clinical risk factors for infertility in reproductive-age patients with intramural uterine fibroids.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (8): 96-105 (in Russian)
https://dx.doi.org/10.18565/aig.2024.128
Keywords
Uterine fibroids (UF) are the most common benign tumors of the female reproductive system, with an incidence rate of up to 70% among women of reproductive age [1]. The average age at UF detection is reported to be between 32 and 34 years [1]. The causes of UF development are not well understood, as the disease is multifactorial and involves genetic, epigenetic, inflammatory, hormonal, and immunological factors [2–4]. Emerging theories on the influence of the "microenvironment" and sterile inflammation on the transformation of undifferentiated myometrial cells into tumor cells have gained importance [5]. Inflammation is triggered by the increased secretion of DAMPs in response to cellular damage. Chronic inflammation is sustained by cytokines secreted by the immune system and undifferentiated cells [6–9], which engage in complex crosstalk with tumor cells. These cytokines affect proliferation, fibrosis, and angiogenesis, thereby supporting the formation, growth, and clinical manifestations of myomas [10].
UF exhibits significant heterogeneity in terms of pathophysiology, size, location, number of nodules, and symptoms [11], which poses challenges for research on the disease.
Infertility is a major social and economic problem, particularly in recent times, when individuals delay their reproductive function. Moreover, human reproduction is generally inefficient, with only approximately 30% of conceptions resulting in birth (approximately 30% end in the preimplantation stage, and another 30% end in the early stages after implantation, confirmed only by an increase in serum concentrations of chorionic gonadotropin) [12]. Approximately 10% of pregnancies terminate after implantation.
The effect of UF on fertility largely depends on its location. Subserous UFs generally do not significantly affect conception [13]. Submucosal and intramural UF, which distort the uterine cavity, are recognized as contributing factors to infertility. However, the effect of intramural UF, which do not disrupt the anatomy of the uterine cavity, on fertility remains controversial, and the literature contains conflicting data [13, 14]. The mechanisms through which intramural UF affects implantation and gestation in the absence of other obvious causes of infertility are also unclear. Based on our assumption, as well as previous studies [15–18], patients with intramural UF and infertility exhibit various characteristics (somatic, epigenetic, genetic, and metabolic), which can be collectively described as chronic systemic aseptic inflammation. Homeostatic disturbances in some patients with intramural UF appear to be one of the pathophysiological causes of impaired fertility. Currently, there is a need to identify practical tools that provide objective information and assess risks, which can greatly assist both doctors and patients in formulating an appropriate treatment strategy.
This study aimed to identify the clinical risk factors for infertility and develop a relative risk score for infertility in reproductive-aged patients with intramural UF.
Materials and methods
This single-center clinical retrospective case-control study included 200 patients of reproductive age with intramural UF (FIGO type 3–6) who were evaluated and treated at the University Clinic "I am healthy!" from 2017 to 2021. Among them, 100 patients with intramural UF and infertility (UF+I) were included in the study group, and 100 fertile patients with intramural UF (UF-I) were included in the control group. This study involved a retrospective analysis of the medical records, including ultrasound findings.
The inclusion criteria for the study group were age 20–35 years, UF 3–5 cm (FIGO type 3–6), no pregnancy for more than 1 year of regular sexual intercourse, regular menstrual cycle, no use of hormonal drugs in the last 6 months, history of unsuccessful IVF attempts, normal ovarian reserve (AMH>1.2 ng/ml), no history of UF surgery, and informed consent to participate in the study.
Inclusion criteria for the control group were age 20–35 years; UF 3–5 cm (FIGO type 3–6); regular menstrual cycle, women seeking routine and outpatient medical care and choice of contraception within 12 months after delivery, diagnosed with UF during pregnancy and/or before pregnancy, and informed consent to participate in the study.
Exclusion criteria from the study group were submucosal UF (FIGO type 0–2) and subserous UF (FIGO type 7); uterus enlarged by fibroids to more than 12 weeks of pregnancy [1]; acute pelvic organ inflammatory diseases; female infertility of tubal origin, verified at the time of the study; female infertility of cervical origin; female infertility associated with male factors; female infertility associated with the absence of ovulation; low ovarian reserve (AMH<1.2 ng/ml); ovarian tumors; history of surgical treatment for UF; the presence of common forms of endometriosis verified by laparoscopy; oncological diseases, hyperprolactinemia associated with macro- or microadenoma of the pituitary gland (according to magnetic resonance imaging), body mass deficiency (body mass index <19.9 kg/m2). The exclusion criteria for the control group were submucosal UF (FIGO type 0–2) and subserous UF (FIGO type 7) and uterine enlargement by fibroids to more than 12 weeks of pregnancy [1].
The location of fibroids was determined using the FIGO classification [12] and the effects of large and small sizes on fertility (according to the clinical recommendations of the Ministry of Health of Russia on UF from 2020, fibroids >12 weeks of pregnancy should be considered large [1]). Ultrasound examinations were performed using a transvaginal volumetric sensor on the Voluson E 10 Expert device; the examination was performed by one researcher who was not aware of the clinical data of the patients.
The study included an analysis of the sociodemographic and clinical-anamnestic data of patients in the study groups. The clinical and anamnestic study included assessment of patients’ complaints (including dyspareunia and chronic pelvic pain), medical history (time of first menstruation, type of menstrual function, regularity and duration of menstrual cycles, reproductive function, and presence of gynecological, extragenital, and endocrine diseases), and general and gynecological examinations. Particular attention was paid to the clinical characteristics of patients at the time of pregnancy planning. Complaints and diseases that lasted for >12 months were assessed as risk factors and factors with a possible impact. That is, these factors were potentially present at the time of pregnancy planning. In accordance with the clinical guidelines for connective tissue dysplasia, the phenotypic signs and manifestations of undifferentiated connective tissue dysplasia (uCTD) were assessed [20]. The diagnosis of uCTD was established based on the presence of signs from at least two body systems.
Statistical analysis
Statistical analysis was performed using SPSS (version 10.0.7) and Statistica (version 6.0) for Windows, and some calculations were performed in Excel. Differences between the groups were considered statistically significant at p<0.05.
The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Continuous variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Frequencies (n) and percentages (%) are reported for categorical variables.
Differences between the independent groups for categorical variables were assessed using the chi-square test (χ2) or Fisher’s exact test for small samples. Continuous variables were compared between the two groups using Student’s t-test and Mann–Whitney test. The critical significance level when testing the statistical hypotheses was set at 0.05.
Receiver operating characteristic (ROC) analysis was performed with constructing ROC curves. If the AUC was 0.9–1.0, the value of the feature was very high; if the AUC was 0.5–0.6, it was considered unsatisfactory. Differences were considered statistically significant at p<0.05 (95% significance level) and p<0.01 (99% significance level). Each characteristic was assigned a score corresponding to the level of significance. By summing the scores, it was possible to obtain a total score that reflected the risk of infertility in patients with intramural UF and infertility.
The study used binary logistic regression analysis with forward selection, and the choice of parameters was determined by the variables with the highest values of calculated relative risk. The inclusion of each subsequent factor was accompanied by a cross-check of the resulting point scale with the determination of sensitivity and specificity. The criteria for sensitivity and specificity were assumed to be at least 80%, and the significance of the included variables was set at p<0.05.
Development of a score scale. In the first stage, a selection of variables encoding characteristics was carried out, taking into account theoretical ideas about the relationship of these characteristics with both the factor and outcome of the study. Within the framework of the task, the following characteristics were selected: age, presence of obesity, comorbidities, medical history, phenotypic signs of uCTD, and clinical symptoms (yes: 1, no: 0). The independent variables were the presence or absence of the most clinically significant characteristics, and the outcome variable was the presence of infertility in women with intramural UF.
Calculation of conditional probabilities. A logit model was used to calculate the conditional probabilities. The dependent binary variable was the fact that the patient belonged to the infertility group (coded as 1, without infertility: 0). The independent variables were the characteristics that potentially affected the presence of infertility. Binary logistic regression was used to analyze all significant risk factors for infertility and to calculate the regression coefficient for each factor.
Risk scale quality assessment. ROC curve analysis was used to assess the quality of classification of the resulting risk scale. This analysis also allows the selection of the optimal probability threshold for separating women with or without infertility to achieve an acceptable level of sensitivity and specificity of the risk scale.
The Area Under Curve (AUC) was used to analyze the classification ability of the risk scale. The AUC varies from 0.5 (no separation) to 1 (perfect separation). It is usually considered that AUC of 0.9–1 corresponds to excellent scale quality, 0.8–0.9 – very good, 0.7–0.8 – good, 0.6–0.7 – average, 0.5–0.6 – unsatisfactory. It should be noted that the AUC was intended only for comparative analysis of scales. When analyzing the quality of a risk scale using AUC, the Gini index is often calculated. This indicator converts the area under the curve into a range from 0 to 1; the higher its value, the higher the discriminatory ability of the risk scale. The Gini index was calculated using the following equation: D=2*(AUC–0.5), where AUC is the area under the ROC curve. The classification quality of the model developed based on logistic regression analysis can be assessed as good because the Gini index was 0.86.
Results
The study design is illustrated in Figure 1. The patients were divided into two groups: 100 infertile women with intramural UF ("case") and 100 fertile patients with UF ("control").
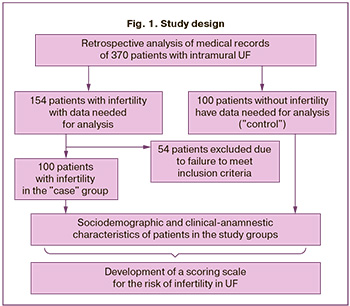
The results of the assessment of the sociodemographic, clinical-anamnestic, and phenotypic characteristics of the patients have been presented previously [15–18]. Descriptive statistics of the study participants are presented in Table 1.
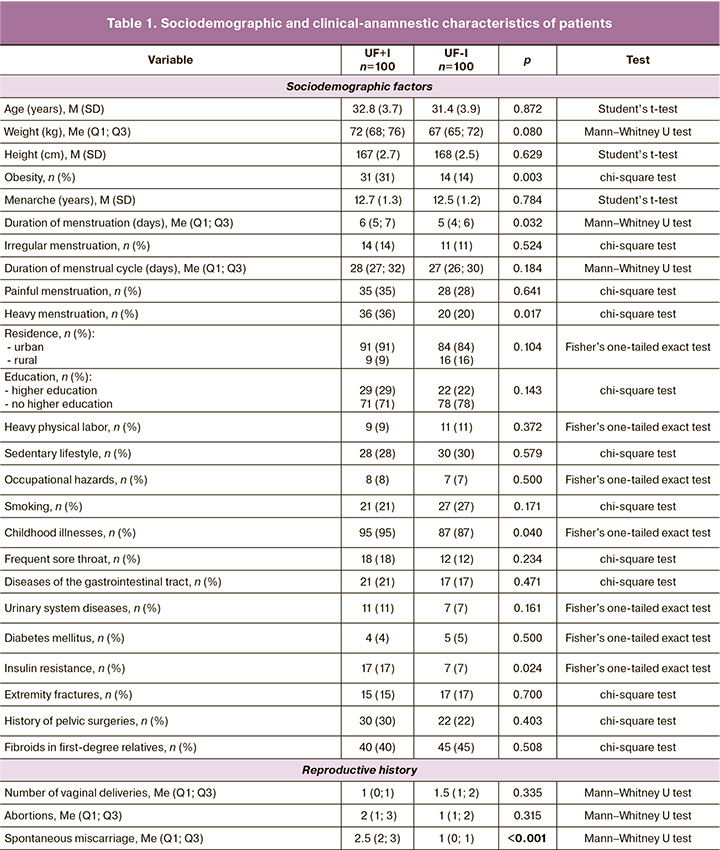
Thus, the groups were generally comparable in terms of the main sociodemographic and clinical-anamnestic characteristics, with the exception of the spontaneous miscarriage rate (2.5 (2; 3)).
Table 2 presents the analysis of the risk factors and their association with infertility in the study group.
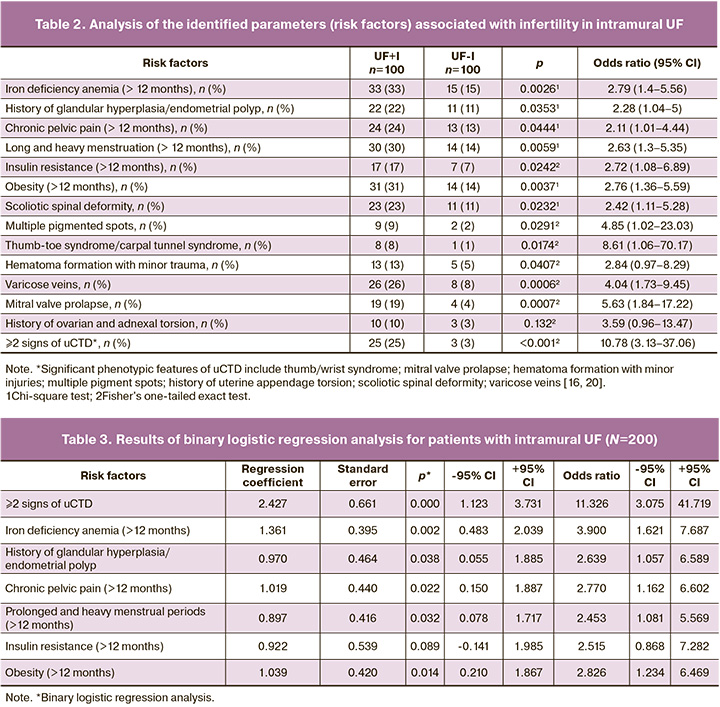
Using binary logistic regression analysis, a relative risk score was obtained (Chi2(7)=57.459, p<0.0001), with the presence of infertility coded as 1 and the absence of infertility as 0 (Table 3).
Cross-validation of the resulting model revealed the following sensitivity, specificity, and overall accuracy: sensitivity – 82% (82/100); specificity – 85% (85/100); the overall accuracy – 83.5% (167/200).
An equation was obtained in which the presence or absence of infertility was coded as 1 or 0, respectively:
Z=-1.404+2.427*[2 or more uCTD signs]+1.361*[Iron deficiency anemia]+0.970*[History of glandular hyperplasia/endometrial polyp]+1.019*[Chronic pelvic pain]+0.919*[Long and heavy menstruation]+0.922*[Insulin resistance]+1.039*[Obesity].
Considering that after rounding the obtained coefficients of the regression equation to the first decimal place (after a linear transformation of the rounding with a step of 0.5), the obtained coefficients were 1.5 and 2.5, in order to obtain integer values of the coefficients, each of the parameters was assigned a corresponding value by multiplying by 2 and rounding to whole units to the nearest whole number.
Table 4 presents the results.
During the ROC analysis for risk factors recalculated into point coefficients for each patient, we obtained a very good quality risk point scale, in which the AUC was 0.930±0.018 (95% CI 0.894–0.966, p<0.0001), the “cut off” value=0.5035 (upon reaching the total maximum sensitivity and specificity), above which the probability of infertility increased (Fig. 2).
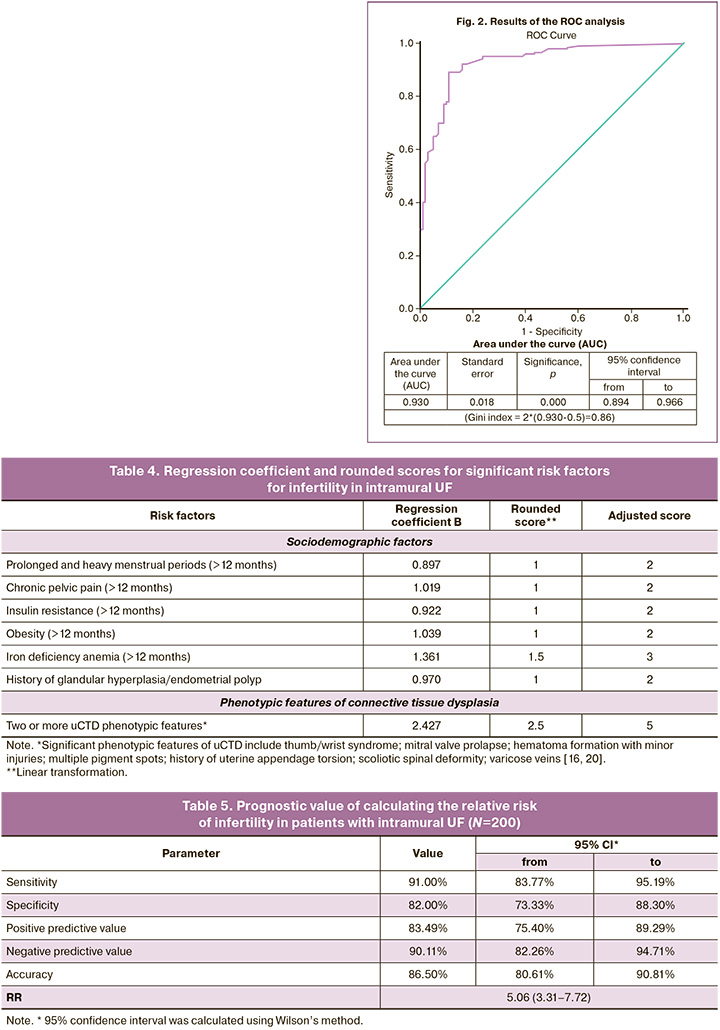
The results of the assessment of prognostic value of the proposed method are presented in Table 5.
The clinical significance of the obtained relative risk score scale proves that the risk of infertility in patients with intramural UF in terms of reproductive age and significant risk factors for infertility is increased by 5.06 times compared to that in women with UF and without these additional risk factors (RR=5.06, 95% CI 3.31–7.72, p<0.001).
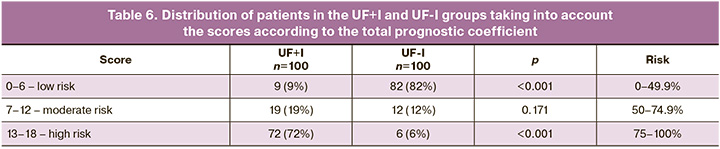
Therefore, a relative risk score scale for infertility in intramural UF was developed and proposed. The distribution of patients within each group, considering the risk assessed using the proposed model, is presented in Table 6.
Discussion
This study allowed the identification of the clinical risk factors associated with infertility in patients with intramural uterine fibroids. These risk factors include prolonged and heavy menstruation lasting for more than 12 months, chronic pelvic pain lasting for more than 12 months, insulin resistance and obesity lasting for more than 12 months, long-term iron deficiency anemia, history of polyps/endometrial hyperplasia, and presence of two or more signs of uCTD. Case-control studies are commonly conducted to identify potential causes of diseases (in this case, infertility) and to form hypotheses about their etiology.
Mathematical calculations were conducted to determine the significance of each risk factor. By summing these factors, the relative risk of infertility can be estimated based on the obtained odds ratio. This study was able to develop a "portrait" of a patient with intramural UF and infertility. These patients exhibit clinical and metabolic disorders, which indicate mild systemic chronic inflammation (confirming the hypothesis), along with signs of uCTD, which characterize the state of the extracellular matrix (likely influenced by genetics/epigenetics). These systemic changes likely affect both local changes in the UF and the function of the reproductive system, disrupting ovulation, embryo transport, and implantation.
The relationship between intramural UF and infertility remains contentious. The only known fact is the negative impact of submucosal myomas on fertility [21]. Most studies present conflicting findings [22, 23]. The challenges in assessment arise from difficulties in UF classification; determining infertility; varying number, size, and location of fibroids; and combination with other infertility factors (e.g., endometriosis or advanced reproductive age). Determining the appropriate management approach is particularly challenging when UF is present in patients with multiple unsuccessful in vitro fertilization attempts and no other apparent objective reasons.
In recent years, chronic aseptic inflammation has been considered as a potential cause of both UF and infertility. It impacts physiological inflammatory processes in infertility and disrupts the function of the reproductive system through increased production of free oxygen radicals [24, 25]. However, the specific mechanisms involved are not fully understood. Interestingly, all the clinical parameters (obesity, insulin resistance, iron deficiency anemia) found to be significantly associated with infertility in intramural UF are manifestations of a systemic chronic aseptic inflammatory process [26–28]. Heavy menstrual bleeding, which is characteristic of patients with infertility in this study, is probably associated with systemic and local chronic inflammation, as supported by modern data and the existing literature [29].
In this study, patients with infertility demonstrated phenotypic features of uCTD, indicating systemic disturbances in the composition of the extracellular matrix. The extracellular matrix plays a role in processes such as menstruation, ovulation, and implantation [30]. Additionally, systemic features of the extracellular matrix contribute to fibrotic changes in the UF [31].
Conclusion
The developed relative risk score, which incorporates clinical and phenotypic parameters, can be a valuable tool for assessing the risk of infertility and potential causes of failure in in vitro fertilization programs for patients with intramural UF, even during pregnancy planning.
However, this study has some limitations. First, the number of patients included in the study was relatively small. Second, as with any case-control study, there may be a risk of recall bias, as individuals may not accurately remember the duration of the studied exposure. Nevertheless, this study allowed us to formulate and support a hypothesis regarding the potential causes of infertility in UF, highlighting the presence of chronic systemic aseptic inflammation and systemic changes in the extracellular matrix. These findings can form the basis for further epidemiological studies.
References
- Министерство здравоохранения Российской Федерации. Миома матки. Клинические рекомендации. 2020. [Ministry of Health of the Russian Federation. Uterine fibroids. Clinical guidelines. 2020. (in Russian)].
- Дубинская Е.Д., Гаспаров А.С., Колесникова С.Н., Холбан И.В., Бабичева И.А. Эпигенетика в клинической гинекологии. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(2): 110-6. [Dubinskaya E.D., Gasparov A.S., Kolesnikova S.N., Kholban I.V., Babicheva I.A. Epigenetics in Clinical Gynecology. Gynecology, Obstetrics and Perinatology. 2021; 20(2): 110-6. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-2-110-116.
- Дубинская Е.Д., Колесникова С.Н., Гаспаров А.С., Холбан И.В., Алешкина Е.В. Роль фетального программирования при миоме матки. Вопросы гинекологии, акушерства и перинатологии. 2022; 21(5): 27-35. [Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S., Kholban I.V., Aleshkina E.V. Role of fetal programming in uterine fibroids. Gynecology, Obstetrics and Perinatology. 2022; 21(5): 27-35. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2022-5-27-35.
- Zhang L., Lu Q., Chang C. Epigenetics in health and disease. Adv. Exp. Med. Biol. 2020; 1253: 3-55. https://dx.doi.org/10.1007/978-981-15-3449-2_1.
- Protic O., Toti P., Islam M.S., Occhini R., Giannubilo S.R., Catherino W.H. et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell. Tissue Res. 2016; 364(2): 415-27. https://dx.doi.org/10.1007/s00441-015-2324-3.
- Elinav E., Nowarski R., Thaiss C., Hu B., Jin C., Flavell R. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013; 13(11): 759-71. https://dx.doi.org/10.1038/nrc3611.
- Ma S., Xie N., Li W., Yuan B., Shi Y., Wang Y. Immunobiology of mesenchymal stem cells. Cell. Death Differ. 2014; 21(2): 216-25. https://dx.doi.org/10.1038/cdd.2013.158.
- Orciani M., Sorgentoni G., Torresetti M., Di Primio R., Di Benedetto G. MSCs and inflammation: new insights into the potential association between ALCL and breast implants. Breast Cancer Res. Treat. 2016; 156(1): 65-72. https://dx.doi.org/10.1007/s10549-016-3745-8.
- Orciani M., Sorgentoni G., Olivieri F., Mattioli-Belmonte M., Benedetto G., Primio R. Inflammation by breast implants and adenocarcinoma: not always a bad company. Clin. Breast Cancer. 2017; 17(4): 286-92. https://dx.doi.org/10.1016/j.clbc.2017.01.001.
- Islam M.S., Protic O., Stortoni P., Grechi G., Lamanna P., Petraglia F. et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil. Steril. 2013; 100(1): 178-93. https://dx.doi.org/10.1016/j.fertnstert.2013.03.007.
- Jayes F., Liu B., Feng L., Aviles-Espinoza N., Leikin S., Leppert P. Evidence of biomechanical and collagen heterogeneity in uterine fibroids. PLoS One. 2019; 14(4): e0215646. https://dx.doi.org/10.1371/journal.pone.0215646.
- Goodwin N. Improving integrated care: can implementation science unlock the 'black box' of complexities? Int. J. Integr. Care. 2019; 19(3): 12. https://dx.doi.org/10.5334/ijic.4724.
- Bonanni V., Reschini M., La Vecchia I., Castiglioni M., Muzii L., Vercellini P. et al. The impact of small and asymptomatic intramural and subserosal fibroids on female fertility: a case-control study. Hum. Reprod. Open. 2022; 2023(1): hoac056. https://dx.doi.org/10.1093/hropen/hoac056.
- Freytag D., Günther V., Maass N., Alkatout I. Uterine fibroids and infertility. Diagnostics (Basel). 2021; 11(8): 1455. https://dx.doi.org/10.3390/diagnostics11081455.
- Дубинская Е.Д., Колесникова С.Н., Алёшкина Е.В., Гаспаров А.С., Башкирова Е.С., Леффад М.Л. Дополнительные факторы инфертильности при интрамуральной миоме матки. Акушерство и гинекология. 2023; 5: 75-82. [Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S., Bashkirova E.S., Leffad M.L. Supplementary infertility factors in patients with intramural uterine fibroids. Obstetrics and Gynecology. 2023; (5): 75-82 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.28.
- Дубинская Е.Д., Колесникова С.Н., Алёшкина Е.В., Гаспаров А.С. Клинические и фенотипические признаки недифференцированной дисплазии соединительной ткани при интрамуральной миоме матки как маркеры прогнозирования инфертильности. Вопросы гинекологии, акушерства и перинатологии. 2023; 22(4): 57-65. [Dubinskaya E.D., Kolesnikova S.N., Alyoshkina E.V., Gasparov A.S. Clinical and phenotypic signs of undifferentiated connective tissue dysplasia in intramural uterine leiomyoma as markers for predicting infertility. Gynecology, Obstetrics and Perinatology. 2023; 22(4): 57-65. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2023-4-57-65.
- Дубинская Е.Д., Колесникова С.Н., Гаспаров А.С., Алёшкина Е.В. Молекулярные паттерны, связанные с повреждением, при интрамуральной миоме матки и бесплодии. Акушерство и гинекология. 2024; 5: 108-17. [Dubinskaya E.D., Kolesnikova S.N., Gasparov A.S., Alyoshkina E.V. Damage-associated molecular patterns in patients with intramural uterine fibroids and infertility. Obstetrics and Gynecology. 2024; (5): 108-17. (in Russian)]. https://dx.doi.org/10.18565/aig.2024.48.
- Дубинская Е.Д., Колесникова С.Н., Алёшкина Е.В., Гаспаров А.С. Хроническое стерильное воспаление в патогенезе доброкачественных изменений миометрия. Вопросы гинекологии, акушерства и перинатологии. 2024; 23(1): 84-93. [Dubinskaya E.D., Kolesnikova S.N., Aleshkina E.V., Gasparov A.S. Chronic sterile inflammation in the pathogenesis of benign myometrial diseases. Gynecology, Obstetrics and Perinatology. 2024; 23(1): 84-93. (in Russian)]. https://dx.doi.org/10.20953/1726-1678- 2024-1-84-93.
- Российское научное медицинское общество терапевтов. Дисплазии соединительной ткани. Клинические рекомендации. 2017. [Russian Scientific Medical Society of Therapists. Connective tissue dysplasias. Clinical Guidelines. 2017. (in Russian)].
- Metwally M., Raybould G., Cheong Y.C., Horne A.W. Surgical treatment of fibroids for subfertility. Cochrane Database Syst. Rev. 2020; 1(1): CD003857. https://dx.doi.org/10.1002/14651858.CD003857.pub4.
- Karlsen K., Mogensen O., Humaidana P., Kesmodel U., Ravn P. Uterine fibroids increase time to pregnancy: a cohort study. Eur. J. Contracept. Reprod. Health Care. 2020; 25(1): 37-42. https://dx.doi.org/10.1080/13625187.2019.1699047.
- Stewart E., Cookson C., Gandolfo R., Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017; 124(10): 1501-12. https://dx.doi.org/10.1111/1471-0528.14640.
- Don E., Mijatovic V., Huirne J. Infertility in patients with uterine fibroids: a debate about the hypothetical mechanisms. Hum. Reprod. 2023; 38(11):2045-54. https://dx.doi.org/10.1093/humrep/dead194.
- Ojo O., Nwafor-Ezeh P., Rotimi D., Iyobhebhe M., Ogunlakin A., Ojo A. Apoptosis, inflammation, and oxidative stress in infertility: a mini review. Toxicol. Rep. 2023; 10: 448-62. https://dx.doi.org/10.1016/j.toxrep.2023.04.006.
- Hall J., Mouton A., da Silva A., Omoto A., Wang Z., Li X. et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc. Res. 2021; 117(8): 1859-76. https://dx.doi.org/10.1016/j.toxrep.2023.04.006.
- Bilsen J., Brink W., Hoek A., Dulos R., Caspers M., Kleemann R. et al. Mechanism-based biomarker prediction for low-grade inflammation in liver and adipose tissue. Front. Physiol. 2021; 12: 703370. https://dx.doi.org/10.3389/fphys.2021.703370.
- Lanser L., Fuchs D., Kurz K., Weiss G. Physiology and inflammation driven pathophysiology of iron homeostasis-mechanistic insights into anemia of inflammation and its treatment. Nutrients. 2021; 13(11): 3732. https://dx.doi.org/10.3390/nu13113732.
- Bahrami A., Bahrami-Taghanaki H., Khorasanchi Z., Timar A., Jaberi N., Azaryan E. et al. Menstrual problems in adolescence: relationship to serum vitamins A and E, and systemic inflammation. Arch. Gynecol. Obstet. 2020; 301(1): 189-97. https://dx.doi.org/10.1007/s00404-019-05343-1.
- O'Connor B., Pope B., Peters M., Ris-Stalpers C., Parker K. The role of extracellular matrix in normal and pathological pregnancy: Future applications of microphysiological systems in reproductive medicine. Exp. Biol. Med. (Maywood). 2020; 245(13): 1163-74. https://dx.doi.org/10.1177/1535370220938741.
- Afrin S., Islam M.S., Patzkowsky K., Malik M., Catherino W.H., Segars J.H. et al. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am. J. Obstet. Gynecol. 2020; 223(5): 733.e1-733. https://dx.doi.org/10.1016/j.ajog.2020.05.012.
Received 28.05.2024
Accepted 02.08.2024
About the Authors
Ekaterina D. Dubinskaya, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology, Patrice Lumumba Peoples' Friendship University of Russia,8 Miklukho-Maklaya str., Moscow, 117198, Russia, +7(903)117-55-58, eka-dubinskaya@yandex.ru, https://orcid.org/0000-0002-8311-0381
Svetlana N. Kolesnikova, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Pediatrics, MMU Reaviz, 2-2 Krasnobogatyrskaya str., Moscow, 107564, Russia, +7(916)500-10-99, ksnmed@mail.ru, https://orcid.org/ 0000-0001-9575-02741
Elizaveta V. Alyoshkina, Teaching Assistant at the Department of Obstetrics, Gynecology and Reproductive Medicine, Faculty of Postgraduate Education, Patrice Lumumba Peoples' Friendship University of Russia, 8 Miklukho-Maklaya str., Moscow, 117198, Russia, +7(926)768-44-27, alyoshkina.ev@yandex.ru,
https://orcid.org/0000-0001-5339-1285
Alexander S. Gasparov, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics, Gynecology and Perinatology, Peoples' Friendship University of Russia,
8 Miklukho-Maklaya str., Moscow, 117198, Russia, +7(903)117-55-58, 13513520@mail.ru, https://orcid.org/0000-0001-6301-1880
Corresponding author: Ekaterina D. Dubinskaya, eka-dubinskaya@yandex.ru



