Body surface cultures in preterm neonates on the first day of life: clinical usefulness
Background: Early-onset neonatal sepsis and pneumonia remain one of the leading causes of neonatal loss. The role of microbiological examination of body surface cultures on the first day of life (DOL) in preterm neonates with high risk for early-onset infections currently remains insufficiently studied. Objective: To estimate the clinical value of microbiological examination of body surface cultures in preterm neonates on the first day of life. Materials and methods: The study included 173 preterm neonates at 24–36 weeks’ gestation admitted directly from the delivery room to the Neonatal Intensive Care Unit (NICU) from January 2020 to April 2021. Microbiological examination was carried out using classical (microscopic, cultural) and innovative methods (proteometric MALDI-TOF-MS analysis). Biological material was taken from three loci (blood culture and two surface cultures, namely oral and rectal swabs). All neonates were divided into two main groups: group I included 43 neonates with positive body surface cultures, group II included 130 neonates with a negative surface culture result. According to their gestational age (GA), the patients were compared in the following subgroups: a) GA<33 weeks (Ia n=10 vs IIa n=47); b) GA=33–36 weeks (Ib n=33 vs IIb n=83). The relationship between surface culture results and the incidence of early neonatal infections (pneumonia, sepsis), complications, as well as severity scores (nSOFA, NEOMOD), inflammation markers, and complications was studied. Results: The subgroups did not differ in anthropometric data, antenatal steroids rates, Apgar, NEOMOD and nSOFA scores. There were no differences in the incidence of early-onset neonatal infections (sepsis and/or pneumonia) (8/10 vs 36/47, in subgroups Ia and IIa, respectively, p>0.05; 11/33 vs 27/83 in subgroups Ib vs IIb, p>0.05). However, additional analysis without regard to commensal bacteria revealed statistically significant increase in early-onset neonatal clinical sepsis (EONS) in the neonates <33 weeks GA with positive surface cultures (E. coli and/or Candida sp., and/or Klebsiella sp. and/or Acinetobacter sp. and/or Enterococcus sp.): 4/7 vs 7/47 in Ia and IIa, respectively (p=0.02); this subgroup also had a higher level of C-reactive protein on DOL3 (Me=2.7 mg/L, Q1–Q3 1.6–23.4 vs Me=0.95 mg/L, Q1–Q3 0.33–5.0 in subgroups Ia vs IIa, respectively, p=0.08). This pattern was absent in neonates of GA 33–36 weeks. Necrotizing enterocolitis (NEC) incidence was found to be higher in all preterm neonates colonized with E. coli and Candida sp. on DOL1 (E. сoli: RR=4.8 (95% CI 1.6–14.7); Candida sp.: RR=9.6 (95% CI 3.5–26.7)). Conclusion: Microbiological examination of body surface cultures on DOL1 may be considered as a valuable clinical tool for EONS in preterm neonates born before 33 weeks GA. It can also be regarded as a prognostic method in all premature babies: the absence of E. coli and/or Candida colonization significantly decreases the risk of subsequent development of NEC.Krogh-Jensen O.A., Nikitina I.V., Bragina O.N., Isaeva E.L., Priputnevich T.V., Zubkov V.V., Degtyarev D.N., Lenyushkina A.A.
Keywords
Early neonatal losses are mainly caused by infectious and inflammatory diseases. The diagnosis of such diseases in preterm neonates presents considerable difficulties all over the world. The clinical picture of neonatal infections is nonspecific and it is similar to a number of other conditions characteristic of premature patients. Besides, the laboratory markers of the systemic inflammatory response (C-reactive protein (CRP), the level of leukocytes, neutrophils and platelets) are not accurate enough in infections of the early neonatal period (early-onset neonatal sepsis (EONS), congenital pneumonia (CP)) [1–3]. The diagnosis of sepsis is traditionally made using microbiological methods which detect the causative agent in the blood, but the frequency of positive results of hemocultures in newborns is extremely low. The incidence of EONS in preterm infants which is confirmed microbiologically ranges from 3.7 to 9.7 per 1000 live births in the developed countries of the world (0.4–1%) [4–6]. At the same time, the incidence of clinical sepsis (which is not confirmed microbiologically) occurs 6-16 times higher than confirmed one and reaches 24.4% of neonates born earlier than 33 weeks gestation [4, 7–9]. The role of microbiological examination of the body surface cultures collected from mucosal sites in the upper respiratory tract (URT) and gastrointestinal tract (GIT) on the first day of life remains underestimated in the complex diagnosis of infectious diseases of the early neonatal period. Thus, the clinical significance and necessity of routine microbiological screening of non-sterile loci in newborns admitted to the neonatal intensive care units (NICU) become questionable. Given the extremely low frequency of detection of positive hemocultures, the study of the material obtained from non-sterile loci along with the study of the microflora of the cervical and vaginal discharge of women shortly before childbirth, as well as the study of amniotic fluid and membranes are the only sources of information about the spectrum of possible perinatal pathogens. Moreover, this information is of great epidemiological importance.
The aim of the study was to determine the clinical significance of microbiological examination of the cultures obtained from mucosal sites in the URT and GIT on the first day of life in preterm neonates admitted to the NICU.
Materials and Methods
This was a prospective observational study which included 205 preterm neonates born before 37 weeks gestation. All of them were born at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia, from January 2020 to April 2021. The neonates were admitted directly from the delivery room to the NICU on the first day of life for the examination and treatment. The newborns with congenital malformations, hereditary metabolic disorders and chromosomal abnormalities were not included in the study.
Biological material was taken from all the neonates from three loci (blood culture and two surface cultures, namely oral and rectal swabs). Microbiological examination was carried out using microscopic and cultural methods aimed at the specific identification of microorganisms with MALDI-TOF-MS analysis (Matrix-Assisted Lazer Desorption / Ionization Time-of-Flight Mass Spectrometry). The specific media for the detection of intracellular microorganisms (Ureaplasma sp., Micoplasma sp.) were not used in this study of newborns.
During the preliminary analysis of the collected biomaterial, 32 patients were excluded from the study as they did not have a full range of microbiological examinations on the first day of life (the biomaterial from one of the loci was missing). The newborns enrolled in the final analysis (n=173) were divided into two main groups depending on the growth of opportunistic microorganisms in the cultures collected from mucosal sites in the URT and GIT: group I consisted of 43 newborns who demonstrated the growth of opportunistic microorganisms, group II included 130 newborns who had a negative result after the microbiological examination of biomaterial from the above–mentioned loci. According to their gestational age (GA), the patients were compared in the following subgroups: a) GA<33 weeks, b) GA=33–36 weeks. The flow diagram of children included in the study is presented in Figure 1.
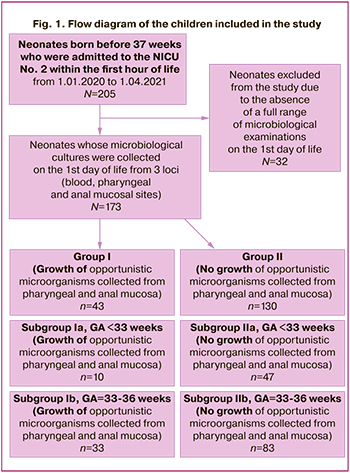
In order to detect/exclude congenital infection, all newborns underwent a standard clinical and laboratory examination which included a full blood count with the estimation of the absolute number of leukocytes, platelets, neutrophils and calculation of the neutrophil index (NI), concentration of CRP; radiography of the thoracic cavity was performed, and hemoculture was analyzed. After obtaining the results of the clinical, laboratory and instrumental examination and evaluation of the dynamics of the newborn’s clinical condition within 72 hours of life, a conclusion was made about the presence or absence of a congenital infection. The differential diagnosis of CP, respiratory distress syndrome (RDS) and transient tachypnea of the newborn (TTN) was made according to the clinical guidelines [10, 11]. The diagnosis of “early neonatal confirmed sepsis” was made on the basis of the detection of positive hemoculture in the neonate within the first 72 hours of life and the presence of one or more clinical signs of an infectious process (Appendix) [11–13]. The diagnosis of “clinical early neonatal sepsis” was made within the first 72 hours of life if microorganisms were not detected in a blood sample, but there were at least two clinical and at least two laboratory signs of an infectious process according to the criteria adopted at “The Expert Meeting on Neonatal and Paediatric Sepsis, London 2010” [14].
Antibacterial therapy was administered in accordance with clinical guidelines, the treatment started with a combination of the following drugs: ampicillin+gentamicin [11]. Due to the suspected EONS and CP, antibacterial therapy was prescribed to the following categories of children with respiratory disorders on the first day of life: 1) patients with very low body weight, 2) newborns undergoing invasive mechanical ventilation. If there were any indications based on the results of the primary clinical and laboratory examination, antibacterial therapy was prescribed to patients with a birth weight of over 1500 g, patients with respiratory disorders but who did not require a mechanical ventilation, as well as those who received non-invasive respiratory therapy (continuous positive airway pressure (CPAP), non-invasive mechanical ventilation) or patients who did not receive respiratory therapy. Antibacterial therapy that was started on the first day of life due to the suspected EONS and CP was canceled in case of absence of clinical, laboratory and instrumental data confirming CP within 72 hours of life. Antifungal drugs were prescribed to newborns strictly according to indications; prophylactic administration of antifungals in newborns included in this study was not used [15].
In the study subgroups, the following parameters were evaluated: the incidence of EONS and CP, complications (disseminated intravascular coagulation (DIC), intraventricular hemorrhages (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis, bronchopulmonary dysplasia (BPD), retinopathy of prematurity) and death rate; the rate of mechanical ventilation was assessed, the frequency and duration of antibacterial and cardiotonic therapy were studied as well. The diagnosis of necrotizing enterocolitis (NEC) was made according to the criteria proposed by Bell et al. (1978) which were modified by Walsh and Kliegman (1987) [16–18]. The patients were monitored until they reached the postconceptional age (PCA) of 37 weeks or before the discharge from the hospital, if it occurred before PCA 37 weeks.
We analyzed the relationship between the results of the microbiological study and the values of the severity of the condition based on the NEOMOD scale, assessment of the sequential organ failure (nSOFA scale) on the first and third day of life [19, 20].
On receiving the positive results of hemocultures and cultures collected from mucosal sites in the URT and GIT, we compared the frequency of detecting identical strains of opportunistic microorganisms, and we also analyzed the species composition of opportunistic pathogens isolated from newborns in the main and control groups. The frequency of increase and absolute values of laboratory markers of systemic inflammatory reaction on the first and third days of life were analyzed, depending on the results of microbiological examination of the mucosa in the URT and GIT.
Statistical analysis
Statistical analysis of the data was performed using software package IBM SPSS Statistics version 23 (USA). Normality of distribution was assessed using the Kolmogorov-Smirnov test and the Lilliefors test. Abnormal distribution was detected in all the groups. In order to find out statistical significance of differences among groups, the Mann–Whitney U-test was used. The results are shown in a median line (Ме) and interquartile range of the 25–75 percentile, minimum and maximum values (Min–Max). The differences were considered as verifiable at the significance level of p<0.05. In the analysis of nominal variables, Pearson’s chi-squared test together with Yates’ correction as well as Fisher’s exact test were used for a small number of observations, relative risk (RR) was calculated. To assess the informative value of the diagnostic methods, the following operational characteristics were used: sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV).
Results
The newborns in the subgroups did not differ in anthropometric data, in Apgar, NEOMOD and nSOFA scores. Preterm neonates who were born before 33 weeks gestation were more likely to be delivered by Caesarean section than neonates who were 33–36 weeks gestation (82 and 67%, respectively). The proportion of infants delivered by Caesarean section did not differ in the subgroups of extremely preterm newborns. The proportion of neonates delivered by operative techniques in the group of children aged 33–36 weeks was statistically significantly lower among newborns who had negative results of cultures on the first day of life compared with newborns who had an increase in opportunistic microorganisms detected in the mucosa of the URT and GIT.
The data on antenatal steroid preventive measures were comparable in subgroups of newborns. In the subgroup IIb (GA<33 weeks, without growth of opportunistic microorganisms), there were four deaths of extremely preterm newborns (two of them had feto-fetal transfusion syndrome, one had a severe hemolytic disease of newborns, the condition after intrauterine treatment) caused by multiple organ failure accompanied by EONS. There were no deaths in the subgroup of neonates who demonstrated the growth of opportunistic microorganisms detected in the mucosa of the URT and GIT; however, the differences between the subgroups were not statistically significant. The frequency of neonatal complications, such as NEC, IVH, PVL, retinopathy of prematurity, BPD did not differ in the compared subgroups. The analysis of the length of hospital stay, respiratory therapy and cardiotonic therapy was carried out without taking into account the data of neonates who died. The clinical characteristics of the patients included in the study are shown in Table 1.
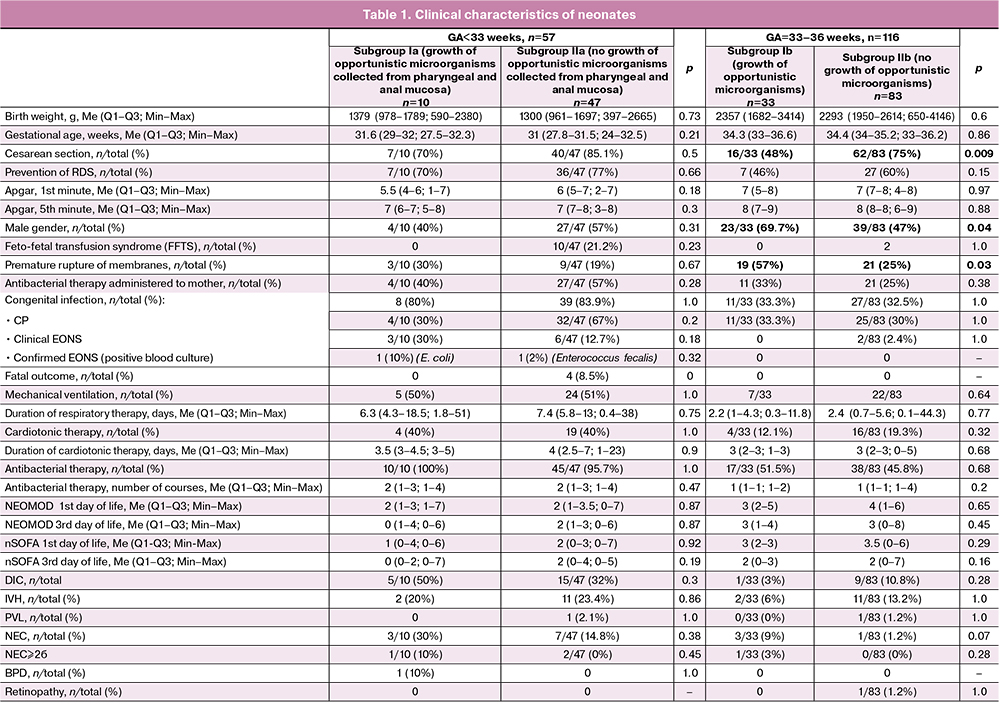
The analysis of data on microbiological examination of neonates born before 33 weeks gestation
Positive hemoculture was detected in one child from subgroup Ia (GA<33 weeks) (10%); the species of opportunistic microorganism (Escherichia coli) detected in the hemoculture was the same as in the culture of the mucosa obtained from the URT and GIT. In subgroup IIa, positive hemoculture was also revealed in one newborn (2.1%), it was Enterococcus fecalis; however, there was no growth of opportunistic microorganisms in non-sterile loci. Thus, identical stain of opportunistic microorganism was isolated only in one case of obtaining a positive culture result in blood sample and non-sterile loci.
The frequency of detecting positive cultures in the mucosa of the URT and GIT was 17.5% (10/57). The examination of the mucosa of the URT and GIT in the neonates born before 33 weeks gestation revealed that the most common opportunistic microorganisms were fungi from the genus Candida, Escherichia coli, and Staphylococcus epidermidis which could be isolated or in association with other microorganisms (Fig. 2).
The microbiological examination of the mucosa of the URT and GIT revealed the growth of Candida species in three patients and in all cases it was accompanied by the development of congenital infections, such as clinical EONS, CP, invasive candidiasis of the intestine and skin. Blood culture for sterility was negative in all cases. The isolation of Escherichia coli from non-sterile loci was observed in two neonates out of ten newborns of subgroup Ia and it was also accompanied by the development of congenital infections in both cases: CP and early neonatal E. coli-sepsis which was confirmed microbiologically. The detection of the growth of Staphylococcus epidermidis (coagulase negative staphylococci, CONS) in two out of three cases was associated with the development of congenital pneumonia.
However, it should be noted that the development of EONS and pneumonia was observed in newborns with negative results of primary isolation of culture collected from the URT and GIT with the same frequency. The regression analysis did not reveal a statistically significant relationship between the development of early neonatal infections and positive results of microbiological examination of the mucosa of the URT and GIT on the first day of life.
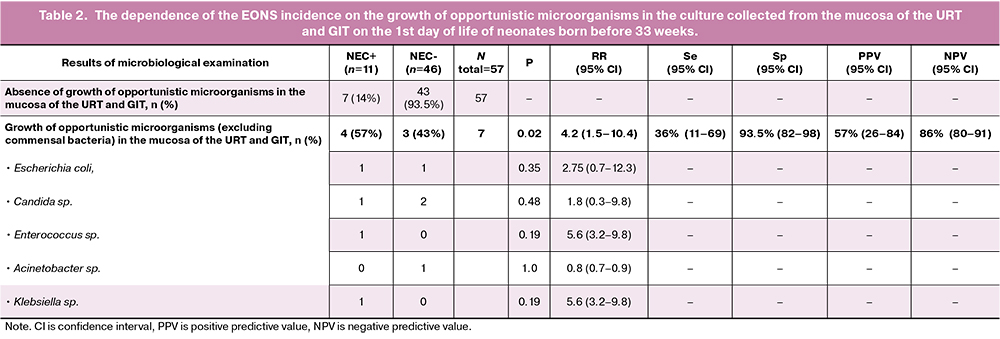
Next, we analyzed the results excluding newborns whose microbiological examination identified only commensal bacteria (CONS, alpha-hemolytic streptococci, Bacillus spp., non-hemolytic streptococci, etc.) [21, 22]. Thus, after correction, there were seven patients in the subgroup of neonates born before 33 weeks with positive results of microbiological examination of the mucosa of the URT and GIT. Therefore, the proportion of neonates colonized with opportunistic microorganisms (E. coli, Candida sp., K. pneumoniae, Acinetobacter sp., Enterococcus faecium) in the subgroup of children born before 33 weeks was 12.2% (7/57), and microorganisms in the culture collected from the mucosal sites of the URT and GIT were distributed as follows: Candida sp. – 3/7 (43%), E. coli – 2/7 (29%), E. faecium – 1/7 (14%), Acinetobacter sp. – 1/7 (14%), K. pneumoniae – 1/7 (14%). A microbial association, namely E. coli +Candida sp., was noted in one out of seven newborns who had positive cultures. The analysis of the dependence of early neonatal infections on the presence of opportunistic microorganisms in the cultures collected from mucosa of the URT and GIT excluding commensal microorganisms revealed that the frequency of EONS (confirmed clinically and microbiologically) was statistically significantly higher in the subgroup of neonates with positive results of microbiological examination compared with neonates without increased growth of cultures from pharyngeal mucosa and one from GIT: 4/7 vs. 7/50 (p=0.02). The results are presented in Table 2. It should also be noted that there was a high level of specificity (Sp 93.5%) and negative predictive value (NPV 86%) in terms of EONS.
There was no difference in either frequency of neonatal complications or the assessment of the severity of the condition by nSOFA and NEOMOD scores in newborns of the study subgroups on the first and third days of life. The comparison of the levels of the markers of systemic inflammatory response in the subgroups revealed a higher level of CRP on the third day of life: newborns of subgroup Ia with the growth of opportunistic microorganisms had Me=2.7 mg/L, Q1–Q3 1.6–23.4 in comparison with Me=0.95 mg/L, Q1–Q3 0.33–5.0 in newborns of subgroup IIa without growth of opportunistic microorganisms in the pharyngeal and rectal mucosa, p=0.008. The levels of leukocytes, neutrophils, and platelets on the first and third days of life did not differ among the subgroups.
The analysis of data on microbiological examination of neonates born at 33–36 weeks of gestation
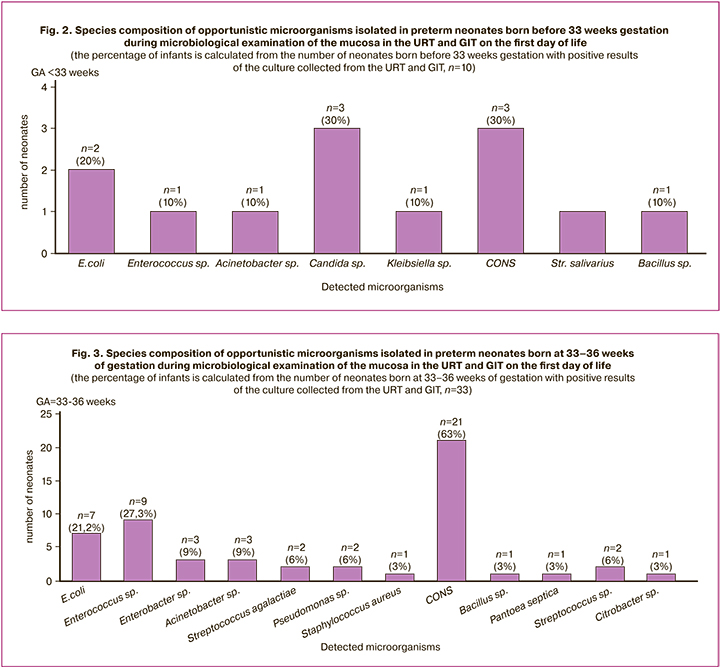
The spectrum of opportunistic microorganisms isolated during microbiological examination of the mucosa from the URT and GIT in neonates born at 33–36 weeks of gestation is presented in Figure 3.
There were no positive hemocultures in this subgroup of newborns. The frequency of detection of microbial growth in the pharyngeal and rectal mucosa was 28.5% (33/116) and it was significantly higher than in neonates born before 33 weeks gestation (17.5%). There was a significantly greater species diversity of opportunistic microorganisms in newborns born after 33 weeks of gestation which could be associated with a larger number of neonates born by spontaneous vaginal delivery among the newborns of the same GA. The microbiological examination revealed the growth of opportunistic microorganisms in the mucosa in 16/78 (20%) of neonates who were born by cesarean section compared to 17/38 (45%) of neonates born by vaginal delivery (p=0.009). Also, the growth of E. coli (5/38 (13%) vs 2/78, p=0.04) and microbial associations (11/38 (64%) vs 5/78 (32%), p=0.003) was significantly more frequent in newborns in case of spontaneous vaginal delivery. The growth of group B Streptococcus (Streptococcus agalactiae) was detected only in neonates born by spontaneous vaginal delivery.
The analysis of the data on microbiological examination excluding commensal bacteria [21, 22] revealed the frequency of the growth of opportunistic microorganisms in the mucosa of the URT and GIT was 17.2%. There were no differences in the frequency of EONS, CP, and other neonatal complications depending on the presence or absence of the growth of opportunistic microorganisms in the mucosa of the URT and GIT. There were no significant differences in the severity of the clinical condition of newborns in the early neonatal period; it was confirmed by the absence of a statistically significant difference in the assessment by the NEOMOD and nSOFA scores. There were no significant differences in the severity of systemic inflammatory response (absolute number of leukocytes, neutrophils, platelets, neutrophil index and CRP values) on the first and third days of life between the subgroups Ib and IIb.
In order to identify the dependence of the frequency of early neonatal infections, as well as the development of their complications, on the detection of certain opportunistic microorganisms in the cultures collected from the mucosa of the URT and GIT on the first day of life, the relative risk analysis was carried out. Opportunistic microorganisms were assessed as predictors/risk factors for the development of diseases separately and in associations. The statistically significant prognostic significance was obtained exclusively for E. coli and NEC in a subgroup of neonates at 33–36 weeks of gestation. The proportion of children who subsequently had NEC was 3/33 (9%) in subgroup Ib (with the growth of opportunistic microorganisms) vs 1/83 (1.2%) in subgroup IIb (without the growth of opportunistic microorganisms). The growth of E. coli in the culture collected from the mucosa of the URT and GIT was noted in 2/4 of newborns with NEC on the first day of life and was an independent factor associated with the development of this complication (p=0.01). At the same time, the risk of developing NEC in neonates with negative culture results was 15.6 times lower compared to neonates colonized with E. coli at birth (RR=15.6 (95% CI: 2.6–91), NPV is 98%, PPV is 50%, p=0.018.
The analysis of all NEC cases in preterm neonates at 24–36 weeks gestation
Taking into account the increased risk of NEC in preterm neonates, as well as the data of a number of studies on the relationship of uncontrolled growth of opportunistic microflora with the development of NEC, we conducted an additional stage of analysis in order to identify opportunistic microorganisms which could be potentially associated with the development of this neonatal complication from the first day of life.
In this study, the development of NEC stages I–III was noted in 15/173 (8.6%) newborns, 11 of them were born before 33 weeks gestation, 4 of them were born at 33–36 weeks gestation. The incidence of NEC was 11/57 (19%) in neonates born before 33 weeks gestation and 4/116 (3.4%) in neonates born at 33–36 weeks gestation. Depending on the severity of NEC, the neonates were distributed the following way: NEC Ia-b – 5/15, NEC IIa – 7/15, NEC IIb – 1/15, NEC IIIb – 2/15. Thus, the incidence of NEC ≥2b stage was 1.7% among all preterm neonates (3/173). Surgical intervention for intestinal perforation was necessary in two cases of NEC (13%, 2/15). The development of this complication was observed in three newborns who had a growth of E. coli at high titers (107 CFU/ml) in the culture collected from the mucosa of the URT and GIT. The relationship between the detection of E. coli and the development of NEC was statistically significant (RR=4.8 (95% CI: 1.6–14.7), p=0.03) (Table 3). The analysis of the mode of delivery revealed that two of these three children were born by vaginal delivery which affects the frequency of early colonization of the mucosa in newborns on the first day of life, according to the above data. Another group of opportunistic microorganisms which was statistically significantly associated with the development of NEC was yeast-like fungi of the Candida species (RR=9.6 (95% CI: 3.5-26.7), p=0.02) (Table 3). The results of the study showed high values of specificity (Sp) and negative predictive value (NPV) for E. coli and Candida sp. (99.2 and 93%; 95.3 and 93%, respectively) (Table 3).
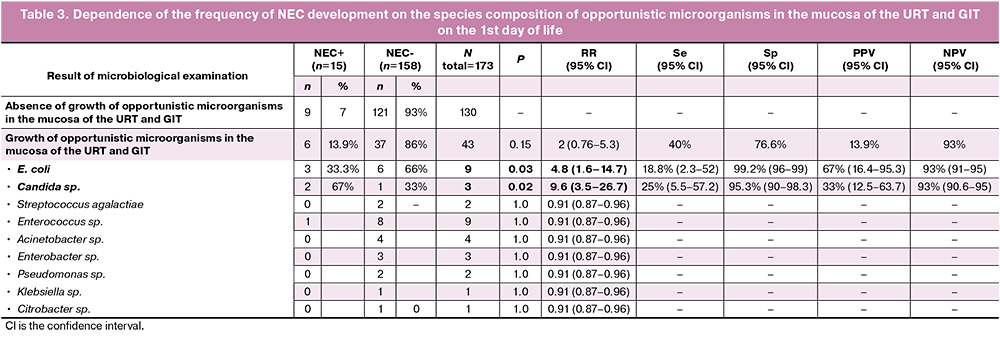
The positive result of the culture collected from the mucosa of the URT and GIT on the first day of life did not affect the frequency of NEC, as well as the detection of other opportunistic microorganisms, except E. coli and Candida sp.
The analysis of sensitivity of opportunistic microorganisms which were detected during microbiological examination of the mucosa of the URT and GIT on the 1st day of life to antibacterial and antifungal drugs
In this study, microbiological examination was carried out on the first day of life of newborns, and, therefore, the data on sensitivity of opportunistic microorganisms to initial antibacterial therapy (ampicillin and gentamicin) were of particular clinical interest. The combination of these medications was used as an empirical treatment for neonates with suspected congenital infection. This analysis includes the results of a microbiological study but it does not take into account the cases of isolation of opportunistic microorganisms associated with commensal bacteria (CONS, alpha-hemolytic streptococci, Bacillus sp., non-hemolytic streptococci).
E. coli was detected in nine patients, and there was sensitivity to both ampicillin and gentamicin in six cases. It was resistant to ampicillin in three cases, but it was sensitive to gentamicin in 100% of cases.
Candida sp. The growth of Candida species was detected in three neonates (Candida albicans in two cases, Candida parapsilosis in one case) during the initial microbiological examination. Candida albicans was resistant to fluconazole in one newborn out of three and it was sensitive to micafungin and amphotericin B. In the above case, the results of a microbiological examination of an extremely preterm neonate in combination with clinical and laboratory signs of the course of the infectious disease were objective sources of information. The target antifungal therapy was prescribed timely on the basis of these data.
The growth of Enterococcus sp. was detected in ten preterm neonates, sensitivity to ampicillin and gentamicin was revealed in 80% of cases. However, there was an increase in Enterococcus faecium in 2/10 patients; it was sensitive exclusively to vancomycin and linezolid, therefore, after obtaining clinical data, initial scheme of antibacterial therapy was changed.
Strains of Acinetobacter sp., Enterobacter sp., Klebsiella pneumoniae, Citrobacter freundii resistant to gentamicin were not revealed in this study.
Streptococcus agalactiae was detected in two children who had sensitivity to penicillin antibiotics. The sensitivity data are shown in Figure 4.
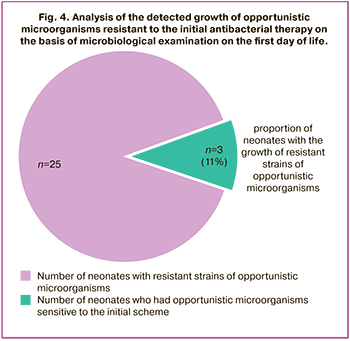
The number of newborns who had a growth of opportunistic microorganisms resistant to initial antibacterial therapy was 11% (three neonates had Enterococcus fecalis+Candida albicans; one neonate had Enterococcus faecalis; one neonate had Pseudomonas oryzihabitans). It was necessary for two neonates born at 32 weeks with congenital pneumonia to change antibacterial therapy within the first 72 hours of life due to clinical and laboratory data on the ineffectiveness of the initial scheme of antibacterial therapy. After obtaining the results of their primary microbiological examination, therapy was changed.
Discussion
The main aim of this study was to determine the prognostic and diagnostic significance of microbiological examination of the mucosa in the URT and GIT on the first day of life in preterm neonates admitted to the NICU due to the risk of the development of early neonatal infections.
The course of the study showed the following:
- The detected growth of opportunistic microorganisms (E. coli, Candida sp., Klebsiella sp., Enterococcus sp.) is associated with a higher risk of developing EONS and systemic congenital candidiasis in neonates born before 33 weeks and is accompanied by a higher level of CRP on the third day of life.
- The neonates of 33–36 weeks gestation had a growth of opportunistic microorganisms in the culture collected from the mucosa of the URT and GIT, but this growth was not a statistically significant prognostic factor for the development of early neonatal infections on the first day of life and did not affect the severity of the condition of newborns and mortality rate.
- There was a higher incidence of NEC in preterm newborns who had a growth of E. coli and/or Candida sp. collected from the mucosa of the URT and GIT on the first day of life.
In order to compare the obtained results, we conducted a search for relevant national and foreign studies.
One of the most extensive studies in the field of epidemiology and etiology of EONS is the research conducted by B. Stoll et al. [5]; a total of 217,480 newborns were enrolled in the study, including 30,879 preterm neonates from 18 maternity hospitals of the USA. This study provides the details of opportunistic microorganisms detected in blood samples depending on GA and body weight of neonates at birth; it also presents the results of the analysis of hemocultures separately for full-term and preterm neonates. According to B. Stoll et al., the most frequently detected pathogens of EONS in preterm neonates are E. coli (51%) and group B Streptococcus (GBS, 13%), Haemophylus sp. (5,3%), Klebsiella sp. (5.3%), Candida albicans (3.1%); these data are consistent with the results of our study. Despite the fact that the isolation of positive hemoculture is considered to be the standard for the diagnosis of EONS, the frequency of positive hemocultures is extremely low in all studies and in our study as well. It was 0.42% in preterm neonates in the study conducted by B. Stoll and it was inversely proportional to GA: 1.84% at 22–28 weeks gestation, 0.62% at 29–33 weeks gestation, 0.07% at 34–36 weeks gestation, respectively.
In our study, the frequency of positive hemocultures on the first day of life was 1.16% (2/173) among all preterm neonates and 3.5% (2/57) in the subgroup of neonates born before 33 weeks. Such positive hemocultures as E. coli (one newborn) and Enterococcus faecalis (one newborn) were noted only among neonates born before 33 weeks. The frequency of positive hemocultures revealed by us in the study is slightly higher than the frequency detected by other scientists (0.37% or 3.71 per 1000 live births among all preterm neonates at 22–36 weeks gestation and 1.1% among neonates with very low body weight); these results are probably due to the fact that only patients admitted to NICU were included in this study [23]. The low number of pathogens detected in blood cultures for sterility may be explained by the low level of bacteremia in newborns which preceded antibacterial therapy administered to mother, the sensitivity of microorganisms to cultivation conditions, impossible repeated blood sampling and collection of large-volume blood samples due to the risk of phlebotomy losses.
The analysis of the slightly higher frequency of detection of opportunistic microorganisms in blood cultures in preterm neonates showed that this indicator is most likely due to the peculiarities of the patients admitted to the Centre (medical organization of IIIb level) which provides healthcare services to the patients with extremely severe somatic, obstetric and gynecological medical history; as a rule, such patients come from different regions of the Russian Federation and need a prolonged hospital stay. Moreover, the relatively high frequency of positive hemocultures may be due to a number of factors depending on the microbiological examination technology [24, 25], namely:
1) The volume of the analyzed material. Sensitivity of blood culture to sterility has been proven to decrease by 10–40% when 0.5 ml of blood is used for analysis compared to 1.0 ml [7, 26]. In most cases, 0.5 ml is enough to detect bacteremia with a high concentration of opportunistic microorganisms. However, if the concentration of the pathogen is low (less than 4 CFU/ml), a blood volume of at least 1.0 ml is required for its identification.
2) Standards of cultivation and sample preparation, characteristics of the selective media used in the study.
3) The use of additional identification methods, such as: real–time polymerase chain reaction (PCR), innovative proteometric methods, namely Matrix-Assisted Lazer Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS), as well as methods based on the targeted and genome-wide sequencing [27].
It should be noted that the use of the optimal volume of the analyzed biomaterial as well as the use of MALDI-TOF-MS analysis for identification of opportunistic microorganisms in positive hemoculture in our study increased the sensitivity of the method and the speed of microbiological examination.
It is also worth mentioning the fact of detecting identical strains of opportunistic microorganisms in hemoculture and cultures collected from the pharyngeal and rectal mucosa. These data are consistent with the results of the study conducted by A. Berger et al. [4]: the strains of opportunistic microorganisms isolated from the blood (E. coli and Listeria monocytogenes) in neonates with positive hemocultures were 100% the same as the strains found in the cultures obtained from non-sterile loci. This fact may have clinical significance for choosing a combination of antimicrobial drugs.
Most of the studies conducted earlier in the 80–90s of the previous century demonstrated the lack of prognostic value of microbiological examinations of non-sterile loci in terms of the development of early neonatal sepsis [4, 28–30]. However, one should take into account their methodological heterogeneity, as well as the fact that most of the studies were carried out using exclusively standard microbiological methods. In this regard, it is difficult to compare the data of our research with the results of these studies. It should also be noted that the vast majority of the studies do not analyze the results of microbiological examinations depending on the neonate GA, although it is extremely important as there are proven differences in the colonization resistance of the mucosa and the characteristics of the immune response when interacting with opportunistic microorganisms, depending on the degree of maturity of the fetus and newborn [4, 23, 29, 31–35].
The most up-to-date research, which is most similar in design and methods to our work, is a study conducted by A. Berger in 2004 [4], which included preterm neonates born before 34 weeks (n=221) by caesarean section. Among the microbiological methods of the study there was the cultivation of microflora on special nutrient media, including those which could be used for the identification of Ureaplasma urealyticum and Mycoplasma hominis, but the identification of opportunistic microorganisms was carried out without the use of proteometric methods. The biological material for the culture study contained samples of amniotic fluid, placental membrane, as well as two smears/cultures of the discharge from the external auditory canal and/or oropharynx, and/or skin. The frequency of sepsis which was confirmed microbiologically in this study was 1% (2/198) among all examined neonates born before 34 weeks. The GA median of neonates affected by sepsis was 29.3 weeks in comparison with 31 weeks of GA in subgroup a of our study. The frequency of clinical sepsis which was not confirmed microbiologically was 24.4% (48/198) compared to 15.7% (9/57) in our study in a subgroup of neonates born before 33 weeks. However, it should be noted that A. Berger et al. used the criteria of clinical sepsis which were adopted before the publication of the definitions established by the expert meeting (London, 2010) [4, 14]. The total frequency of sepsis mentioned in the article by A. Berger et al. was 50/198 (25.2%), which is also slightly higher than in our work, 11/57 (19.2%). The growth of opportunistic microorganisms in the cultures collected from the skin and/or oropharyngeal mucosa was detected in 17.2% of cases (34/198). The most commonly identified opportunistic microorganisms were U. urealyticum (68%, 23/34), group B Streptococcus (Streptococcus agalactiae) (8.8%, 3/34) and E. coli (5.9%, 2/34). Body surface cultures were analyzed without taking into account commensal bacteria (CONS, alpha-hemolytic streptococci, Bacillus spp., etc.) [21]. The identification of Ureaplasma was not carried out in our study; when comparing the frequency of positive cultures without taking into account Ureaplasma and commensal bacteria, the number of positive results in our study was almost twice as high as in the A. Berger study: 12.2% (7/57) vs 5.5% (11/198).
In the study conducted by A. Berger, the detection of the growth of opportunistic microorganisms in body surface cultures of the infants was not associated with the development of EONS and was found to be economically impractical. In comparison with the cultures collected from the skin surface, the growth of opportunistic microorganisms was detected more frequently during the examination of the amniotic cavity: it was 38% in total, 85/221 (separately amniotic membranes – 34%, amniotic fluid – 19.2%, placental membranes – 26%); it was statistically significantly associated with the development of EONS in newborns (OR=6.1, high NPV=84.1%, relatively low PPV=57.1%). Despite the fact that the most frequent opportunistic microorganism revealed in the amniotic cavity as well as in the cultures collected from the skin surface was U. urealyticum (65%, 56/85), the most remarkable correlation with the development of EONS was observed in the detection of other opportunistic microorganisms (E. coli, group B Streptococcus, Enterococcus sp., Candida sp., etc.). The newborns were prescribed ampicillin+gentamicin and antibacterial therapy did not include macrolides.
The species composition of opportunistic microorganisms, the distribution and frequency of positive results of detecting opportunistic microorganisms in the body surface cultures in our study differed from those revealed by A. Berger et al.; this can partly be explained by methodological differences (only infants after caesarean section were included in the study by A. Berger, cultures from the anus were not examined, and only cultures from the surface of the skin and auricle were studied; there were also differences in the microbiological methods. The objectives of our study did not include the analysis of antibacterial therapy administered to mothers as well as intranatal antibiotic prophylaxis of infections caused by group B Streptococcus. According to major epidemiological studies, the distribution of opportunistic microorganisms detected in hemocultures of preterm infants in the early neonatal period has changed over the past 10–15 years due to the preventive measures of infection caused by group B Streptococcus. Despite the fact that the incidence of sepsis associated with group B Streptococcus has significantly decreased, there is an increase in the number of cases of sepsis caused by E. coli (8.68 per 1000 live births in 2017 compared to 5.07 in 2009, p=0.008). The most unpleasant fact is an increase in the proportion of resistant strains of E. coli. Lack of sensitivity to both ampicillin and gentamicin was noted in 7.8% of cases. Resistance to gentamicin has increased in recent years from 3 to 11% [5, 36].
The detection of Candida species mainly in preterm neonates born before 33 weeks is most likely associated with prolonged hospitalization of mothers in an obstetric hospital and multiple courses of antibacterial therapy aimed at the treatment of obstetric infections; however, this fact requires further detailed study.
Our study revealed a statistically significant association between the detection of E. coli and Candida sp. during the microbiological examination of cultures collected from the mucosa of the URT and GIT on the first day of life in all preterm neonates at 24–36 weeks gestation with a higher risk for the subsequent development of NEC; this can be explained by the remarkable immaturity of the immune system, excessive activation of TLR4, as well as low colonization resistance of the mucous membranes of preterm newborns.
The correlation of early (from the first day of life) E. coli colonization of the mucosa of the URT and GIT with the subsequent development of NEC in newborns is currently not sufficiently studied. However, the association of detecting E. coli and NEC in the late neonatal period is well known [37, 38]. In addition, numerous modern histological studies of resected intestinal sites in newborns and experimental animals with NEC prove that excessive bacterial (E. coli) stimulation of TLR4 receptors causes the release of a cascade of proinflammatory cytokines, activation of nuclear factor-kB (NF-kB) and triggers the processes of apoptosis and necroptosis in NEC; bacterial translocation through the intestinal mucosa activates TLR4 on the endothelium of the intestinal vascular wall which can lead to a decrease in blood flow and the development of intestinal wall ischemia and necrosis [39–46].
Conclusion
This study shows the clinical significance of the microbiological examination of the mucosa of the URT and GIT in extremely preterm neonates; the revealed growth of opportunistic microorganisms is a statistically significant factor associated with the development of the EONS and accompanied by a higher level of CRP. However, this relationship is absent in neonates born after 33 weeks. Moreover, the risk for developing NEC is significantly lower in preterm neonates with negative results of microbiological examination compared to newborns whose mucous membranes were colonized by E. coli and/or Candida species from birth.
In other cases, the growth of opportunistic microorganisms in the culture collected from the mucosa of the URT and GIT on the first day of life is not a statistically significant factor associated with the development of the infectious process in newborns, the severity of the condition of newborns and outcomes.
Understanding of the species composition of opportunistic microorganisms in the culture collected from the mucosa of the URT and GIT, as well as in the hemoculture of preterm neonates, information about their sensitivity to antibacterial and antifungal drugs are a valuable source of information when choosing antimicrobial therapy and determining its duration.
Thus, the study of the spectrum of opportunistic microorganisms colonizing the mucous membranes of preterm infants of various GA at birth with the help of modern microbiological methods is a valuable tool for assessing the risk of early neonatal infections and requires further study.
Economic efficiency of microbiological examination of the mucosa from the URT and GIT in all preterm neonates also requires additional study.
Strengths and limitations of the study
The limitation of this study is a relatively small sample size of neonates born before 33 weeks.
There are the following strengths of the study: 1) the use of modern high-tech microbiological methods; 2) a detailed analysis of the results of microbiological examination of preterm infants depending on gestational age; 3) the comparison of the obtained data with clinical and laboratory signs of the infectious process, as well as the severity of the condition of patients and the development of complications; 4) the detection of a statistically significant correlation between certain opportunistic microorganisms revealed on the mucosa of the URT and GIT and the development of EONS and NEC; 5) updating epidemiological data on the frequency of detection and species composition of opportunistic microorganisms in preterm patients admitted to the NICU in the early neonatal period.
Directions for further research: identification of the diagnostic value and economic efficiency of an extended microbiological examination using additional biomaterial (amniotic fluid, amniotic membranes, amniotic membrane of the placenta); further analysis of opportunistic microorganisms in terms of the prediction of NEC.
References
- Hornik C.P., Benjamin D.K., Becker K.C., Benjamin D.K. Jr., Li J., Clark R.H. et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr. Infect. Dis. J. 2012; 31(8): 799-802. https://dx.doi.org/10.1097/INF.0b013e318256905c.
- Shane A.L., Sánchez P.J., Stoll B.J. Neonatal sepsis. Lancet. 2017; 390(10104): 1770-80. https://dx.doi.org/10.1016/S0140-6736(17)31002-4.
- Pettinger K.J., Mayers K., McKechnie L., Phillips B. Sensitivity of the Kaiser Permanente early-onset sepsis calculator: a systematic review and meta-analysis. EClinicalMedicine. 2019; 19: 100227. https://dx.doi.org/10.1016/j.eclinm.2019.11.020.
- Berger A., Witt A., Haiden N., Kretzer V., Heinze G., Pollak A. Amniotic cavity cultures, blood cultures, and surface swabs in preterm infants - useful tools for the management of early-onset sepsis? J. Perinat. Med. 2004; 32(5): 446-52. https://dx.doi.org/10.1515/JPM.2004.145.
- Stoll B.J., Puopolo K.M., Hansen N.I., Sánchez P.J., Bell E.F., Carlo W.A. et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020; 174(7): e200593. https://dx.doi.org/10.1001/jamapediatrics.2020.0593.
- Jiang S., Hong L., Gai J., Shi J., Yang Y., Lee S.K. et al. Early-onset sepsis among preterm neonates in China, 2015 to 2018. Pediatr. Infect. Dis. J. 2019; 38(12): 1236-41. https://dx.doi.org/10.1097/inf.0000000000002492.
- Klingenberg C., Kornelisse R.F., Buonocore G., Maier R.F., Stocker M. Culture-negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 2018; 6: 285. https://dx.doi.org/10.3389/fped.2018.00285.
- Drageset M., Fjalstad J.W., Mortensen S., Klingenberg C. Management of early-onset neonatal sepsis differs in the north and south of Scandinavia. Acta Paediatr. 2017; 106(3): 375-81. https://dx.doi.org/10.1111/apa.13698.
- Cantey J.B., Wozniak P.S., Pruszynski J.E., Sánchez P.J. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect. Dis. 2016; 16(10): 1178-84. https://dx.doi.org/10.1016/S1473-3099(16)30205-5.
- Ионов О.В., Никитина И.В., Зубков В.В., Митрохин С.Д., Крохина К.Н., Киртбая А.Р., Балашова Е.Н., Левадная А.В., Любасовская Л.А., Рюмина И.И., Дегтярев Д.Н., Крючко Д.С. Порядок обследования новорожденных с подозрением на инфекционную патологию и правила назначения антибактериальной терапии, принятые в отделении реанимации и интенсивной терапии новорожденных ФГБУ «Научный центр акушерства, гинекологии и перинатологии им. акад. В.И. Кулакова» Минздрава России. Неонатология: новости, мнения, обучение. 2014; 1: 95-106. [Ionov O.V., Nikitina I.V., Zubkov V.V., Mitrokhin S.D., Krokhina K.N., Kirtbaya A.R. et al. The procedure for examination of newborns suspected of infectious pathology and rules of antibacterial therapy, adopted at Department of resuscitation and intensive therapy of newborn at Kulakov Research Center for Obstetrics, Gynecology and Perinatology. Neonatology: news, opinions, training. 2014; 1: 95-106. (in Russian)].
- Антонов А.Г., Байбарина Е.Н., Балашова Е.Н., Дегтярев Д.Н., Зубков В.В., Иванов Д.О., Ионов О.В., Карпова А.Л., Киртбая К.Н., Крохина К.Н., Крючко Д.С., Ленюшкина А.А., Ли А.Г., Малютина Л.В. Врожденная пневмония (клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4: 133-48. [Antonov A.G., Baibarina E.N., Balashova E.N., Degtyarev D.N., Zubkov V.V., Ivanov D.O., et al. Congenital pneumonia (clinical practice guidelines). Neonatology: news, opinions, training. 2017; 4: 133-48. (in Russian)].
- Гельфанд Б.Р., ред. Сепсис: классификация, клинико-диагностическая концепция и лечение. М.: Медицинское информационное агентство; 2017. 408с. [Gelfand B.R., ed. Sepsis: classification, clinical and diagnostic concept and treatment. Moscow: Medical Information Agency; 2017.(in Russian)].
- McGovern M., Giannoni E., Kuester H., Turner M.A., van den Hoogen A., Bliss J.M. et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr. Res. 2020; 88(1): 14-26. https://dx.doi.org/10.1038/s41390-020-0785-x.
- European Medicines Agency. Science Medicines Health. Report on the expert meeting on neonatal and paediatric sepsis. UK: London; 2010. Available at: https://www.ema.europa.eu/en/documents/report/report-expert-meeting-neonatal-paediatric-sepsis_en.pdf
- Антонов А.Г., Байбарина Е.Н., Балашова Е.Н., Володин Н.Н., Дегтярев Д.Н., Зубков В.В., Иванов Д.О., Ионов О.В., Карпова А.Л., Киртбая К.Н., Климко Н.Н., Крючко Д.С., Ленюшкина А.А. Инвазивный кандидоз у новорожденных (клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4: 120-32. [Antonov A.G., Baibarina E.N., Balashova E.N., Volodin N.N. Invasive candidiasis in newborns (clinical guidelines). Neonatology: news, opinions, training. 2017; 4: 120-32. (in Russian)].https://dx.doi.org/10.24411/2308-2402-2017-00048.
- Дорофеева Е.И., Подуровская Ю.Л., Буров А.А., Рюмина И.И., Нароган М.В., Грошева Е.В., Ионов О.В., Балашова Е.Н., Киртбая А.Р., Дегтярев Д.Н., Хаматханова Е.Н. Диагностика и консервативное лечение новорожденных с некротизирующим энтероколитом (проект клинических рекомендаций). Неонатология: новости, мнения, обучение. 2014; 2: 84-92. [Dorofeeva E.I., Podurovskaya Y.L., Burov A.A., Ryumina I.I., Narogan M.V., Grosheva E.V. et al. Diagnosis and conservative treatment of newborns with necrotizing enterocolitis (draft clinical recommendations). Neonatology: news, opinions, training. 2014; 2: 84-92. (in Russian)].
- Bell M.J., Ternberg J.L., Feigin R.D., Keating J.P., Marshall R., Barton L. et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978; 187(1): 1-7. https://dx.doi.org/:10.1097/00000658-197801000-00001.
- Walsh M.C., Kliegman R.M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. North Am. 1986; 33(1): 179-201.https://dx.doi.org/10.1016/S0031-3955(16)34975-6.
- Wynn J.L., Polin R.A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 2020; 88(1): 85-90. https://dx.doi.org/10.1038/s41390-019-0517-2.
- Janota J., Simak J., Stranak Z., Matthews T., Clarke T., Corcoran D. Critically ill newborns with multiple organ dysfunction: assessment by NEOMOD score in a tertiary NICU. Ir. J. Med. Sci. 2008; 177(1): 11-7. https://dx.doi.org/10.1007/s11845-008-0115-5.
- National Healthcare Safety Network (NHSN). Patient Safety Component Manual. 2022; 432p.
- 2020 NHSN Laboratory Confirmed Bloodstream Infection (LCBI) Checklist. Available at: https://www.cdc.gov/nhsn/pdfs/checklists/2020/lcbi-checklist-508.pdf
- Mukhopadhyay S., Puopolo K.M. Neonatal early-onset sepsis: epidemiology and risk assessment. NeoReviews. 2015; 16(4): e221-e230. https://dx.doi.org/10.1542/neo.16-4-e221.
- Припутневич Т.В., Зубков В.В., Трофимов Д.Ю., Шевырева М.П., Марьин Г.Г., Тутельян А.В., Акимкин В.Г., Брико Н.И., Костенко Н.А., Байбарина Е.Н., Сухих Г.Т. Эволюция технологий в микробиологии - ключ к формированию новых возможностей надзора и профилактики инфекций в родовспоможении. Вестник Российской академии медицинских наук. 2019; 74(6): 364-70. [Priputnevich T.V., Zubkov V.V., Trofimov D.Yu., Shevyreva M.P., Maryin G.G., Tutelyan A.V. et al. The evolution of technologies in microbiology is the key to creating new opportunities for surveillance and prevention of infections in obstetrics. Annals of the Russian Academy of Medical Sciences. 2019; 74(6): 364-70. (in Russian)]. https://dx.doi.org/10.15690/vramn1198.
- Припутневич Т.В., Мелкумян А.Р., Любасовская А.А., Муравьева В.В., Ильина Е.Н., Сухих Г.Т. Масс-спектрометрия в микробиологической практике научного центра акушерства, гинекологии и перинатологии. Журнал микробиологии, эпидемиологии и иммунобиологии. 2016; 1: 52-8. [Priputnevich T.V., Melkumyan A.R., Lyubasovskaya L.A., Muravieva V.V., Ilina E.N., Sukhikh G.T. Мass-spectrometry in microbiological practice of scientific centre of obstetrics, gynecology and perinatology. Journal of Microbiology, Epidemiology and Immunobiology. 2016; 1: 52-8. (in Russian)]. https://dx.doi.org/10.36233/0372-9311-2016-1-52-58.
- Schelonka R.L., Chai M.K., Yoder B.A., Hensley D., Brockett R.M., Ascher D.P. Volume of blood required to detect common neonatal pathogens. J. Pediatr. 1996; 129(2): 275-8. https://dx.doi.org/10.1016/S0022-3476(96)70254-8.
- Samaranayake W.A.M.P., Dempsey S., Howard-Jones A.R., Outhred A.C., Kesson A.M. Rapid direct identification of positive paediatric blood cultures by MALDI-TOF MS technology and its clinical impact in the paediatric hospital setting. BMC Res. Notes. 2020; 13(1): 12. https://dx.doi.org/10.1186/s13104-019-4861-4.
- Dobson S.R., Isaacs D., Wilkinson A.R., Hope P.L. Reduced use of surface cultures for suspected neonatal sepsis and surveillance. Arch. Dis. Child. 1992; 67(1 Spec No): 44-7. https://dx.doi.org/10.1136/adc.67.1_Spec_No.44.
- Goldmann D.A., Leclair J., Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J. Pediatr. 1978; 93(2): 288-93. https://dx.doi.org/10.1016/S0022-3476(78)80523-X.
- Choi Y., Saha S.K., Ahmed A.S., Law P.A., Chowdhury M.A., Islam M. et al. Routine skin cultures in predicting sepsis pathogens among hospitalized preterm neonates in Bangladesh. Neonatology. 2008; 94(2): 123-31. https://dx.doi.org/10.1159/000119722.
- Sgro M., Kobylianskii A., Yudin M.H., Tran D., Diamandakos J., Sgro J. et al. Population-based study of early-onset neonatal sepsis in Canada. Paediatr. Child Health. 2019; 24(2): e66-e73. https://dx.doi.org/10.1093/pch/pxy018.
- Ebenebe C.U., Hesse F., Blohm M.E., Jung R., Kunzmann S., Singer D. Diagnostic accuracy of interleukin-6 for early-onset sepsis in preterm neonates. J. Matern. Fetal Neonatal Med. 2021; 34(2): 253-8. https://dx.doi.org/10.1080/14767058.2019.1606194.
- Romagnoli C., Frezza S., Cingolani A., De Luca A., Puopolo M., De Carolis M.P. et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur. J. Pediatr. 2001; 160(6): 345-50. https://dx.doi.org/10.1007/PL00008445.
- Melville J.M., Moss T.J.M. The immune consequences of preterm birth. Front. Neurosci. 2013; 7: 79. https://dx.doi.org/10.3389/fnins.2013.00079.
- Никитина И.В., Донников А.Е., Крог-Йенсен О.А., Ленюшкина А.А., Быстрицкий А.А., Крючко Д.С., Ионов О.В., Зубков В.В., Дегтярев Д.Н. Генетические полиморфизмы у детей, ассоциированные с развитием врожденных инфекций. Акушерство и гинекология. 2019; 11: 175-85. [Nikitina I.V., Donnikov A.E., Krogh-Jensen O.A., Lenyushkina A.A., Bystritsky A.A., Kryuchko D.S., Ionov O.V., Zubkov V.V., Degtyarev D.N. Congenital infection-associated genetic polymorphisms in children. Obstetrics and Gynegology. 2019; 11: 175-85. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.175-185.
- Stoll B.J., Hansen N.I., Sánchez P.J., Faix R.G., Poindexter B.B., Van Meurs K.P. et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011; 127(5): 817-26. https://dx.doi.org/10.1542/PEDS.2010-2217.
- Cushing A.H. Necrotizing enterocolitis with Escherichia coli heat-labile enterotoxin. Pediatrics. 1983; 71(4): 626-30. https://dx.doi.org/10.1542/peds.71.4.626.
- Nolan L.S., Wynn J.L., Good M. Exploring clinically-relevant experimental models of neonatal shock and necrotizing enterocolitis. Shock. 2020; 53(5): 596-604. https://dx.doi.org/10.1097/SHK.0000000000001507.
- Hui L., Dai Y., Guo Z., Zhang J., Zheng F., Bian X. et al. Immunoregulation effects of different γδT cells and toll-like receptor signaling pathways in neonatal necrotizing enterocolitis. Medicine (Baltimore). 2017; 96(8): e6077.https://dx.doi.org/10.1097/MD.0000000000006077.
- Egan C.E., Sodhi C.P., Good M., Lin J., Jia H., Yamaguchi Y. et al. Toll-like receptor 4–mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J. Clin. Invest. 2016; 126(2): 495-508. https://dx.doi.org/10.1172/JCI83356.
- Jia H., Sodhi C.P., Yamaguchi Y., Lu P., Martin L.Y., Good M. et al. Pulmonary epithelial TLR4 activation leads to lung injury in neonatal necrotizing enterocolitis. J. Immunol. 2016; 197(3): 859-71. https://dx.doi.org/10.4049/jimmunol.1600618.
- Liu T., Zong H., Chen X., Li S., Liu Z., Cui X. et al. Toll-like receptor 4-mediated necroptosis in the development of necrotizing enterocolitis. Pediatr. Res. 2022; 91(1): 73-82. https://dx.doi.org/10.1038/s41390-021-01457-y.
- Levy E., Xanthou G., Petrakou E., Zacharioudaki V., Tsatsanis C., Fotopoulos S. et al. Distinct roles of TLR4 and CD14 in LPS-induced inflammatory responses of neonates. Pediatr. Res. 2009; 66(2): 179-84. https://dx.doi.org/10.1203/PDR.0b013e3181a9f41b.
- Tremblay É., Thibault M.P., Ferretti E., Babakissa C., Bertelle V., Bettolli M. et al. Gene expression profiling in necrotizing enterocolitis reveals pathways common to those reported in Crohn’s disease. BMC Med. Genomics. 2016; 9: 6.https://dx.doi.org/10.1186/s12920-016-0166-9.
- Roy S.K., Meng Q., Sadowitz B.D., Kollisch-Singule M., Yepuri N., Satalin J. et al. Enteral administration of bacteria fermented formula in newborn piglets: a high fidelity model for necrotizing enterocolitis (NEC). PLoS One. 2018; 13(7): e0201172. https://dx.doi.org/10.1371/journal.pone.0201172.
- Никитина И.В., Донников А.Е., Крог-Йенсен О.А., Крашенинникова Р.В.,Непша О.С., Ленюшкина А.А., Дегтярев Д.Н. Молекулярно-генетические предикторы некротизирующего энтероколита у новорожденных. Акушерство и гинекология. 2020; 12: 150-8. [Nikitina I.V., Donnikov A. E., Krogh-Jensen O.A., Krasheninnikova R.V., Nepsha O.S.,Lenyushkina A.A. Genetic predictors of necrotizing enterocolitis in neonates. 2020; 12: 150-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.150-158.
Received 03.06.2022
Accepted 10.08.2022
About the Authors
Olga A. Krogh-Jensen, M.D., Ph.D., Neonatologist at NICU No. 2, Institute of Pediatrics and Neonatology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Associate Professor at the Neonatal Department, Pediatric Faculty, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University, 119991, Russia, Moscow, Trubetskaya str., 8/2, +7(926)014-01-35, o_krogh@oparina4.ru, olgaborisevich@gmail.com, Scopus Author ID: 57214220453, https://orcid.org/0000-0002-5178-5659Irina V. Nikitina, Dr. Med. Sci., Leading Researcher at the NICU No. 2, Institute of Neonatology and Pediatrics, Associate Professor at the Neonatology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)531-44-44 (ex. 2700, 2697), i_nikitina@oparina4.ru, https://orcid.org/0000-0002-1103-1908, Researcher ID: AAH-3465-2019, Scopus Author ID: 57189233499, 117997, Russia, Moscow, Oparina str., 4.
Olga N. Bragina, Clinical Resident at the Department of Neonatology, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(987)123-34-20, bragina_medicina@mail.ru, https://orcid.org/0000-0001-8029-4667, 117997, Russia, Moscow, Oparina str., 4.
Elena L. Isaeva, M.D., Ph.D., Senior Researcher at the Laboratory of Microbiology, Institute of Microbiology, Clinical Pharmacology and Epidemiology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-25-33 (ex. 2776), e_isaeva@oparina4.ru, https://orcid.org/0000-0001-6224-8202,
117997, Russia, Moscow, Oparina str., 4.
Tatiana V. Priputnevich, Corresponding Member of the RAMS, Dr. Med. Sci., Head of the Institute of Microbiology, Clinical Pharmacology and Epidemiology, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Oparina str., 4; Professor at the Department of Microbiology and Virology, Faculty of Pediatrics, N.I. Pirogov RNRMU, Ministry of Health of Russia, 117997, Russia, Moscow, Ostrovityanov str., 1, +7(495)438-25-10 (ex. 2770), priput1@gmail.com,
https://orcid.org/0000-0002-4126-9730
Viktor V. Zubkov, Dr. Med. Sci., Director of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Oparina str., 4; Professor at The Neonatal Department, Pediatric Faculty, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8/2, victor.zubkov@mail.ru, https://orcid.org/0000-0002-9697-9596
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Vice director on Scientific Work, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Oparina str., 4; Head of the Neonatal Department, Pediatric Faculty, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University),
119991, Russia, Moscow, Trubetskaya str., 8/2, +7(495)438-25-33, d_degtiarev@oparina4.ru, https://orcid.org/0000-0001-8975-2425
Anna A. Lenyushkina, MD, Ph.D., Head of the NICU No. 2, Institute of Neonatology and Pediatrics, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)531-44-44 (ex. 2700, 2697), a-lenushkina@yandex.ru, Scopus Author ID: 57202802436, WOS ID: AAJ-6896-2021, https://orcid.org/0000-0001-8929-2991,
117997, Russia, Moscow, Oparina str., 4.
Authors’ contributions: Nikitina I.V., Lenyushkina A.A., Krogh-Jensen O.A. – developing the concept and design of the study; Nikitina I.V., Isaeva E.L. – collection of biomaterial; Krogh-Jensen O.A., Bragina O.N. – statistical analysis and interpretation; Krogh-Jensen O.A., Nikitina I.V., Lenyushkina A.A. – writing the manuscript; Nikitina I.V., Lenyushkina A.A., Priputnevich T.V. – editing; Priputnevich T.V., Zubkov V.V., Degtyarev D.N. – coordination of research and laboratory investigations.
Conflicts of interest: Authors declare no conflict of interests.
Funding: The study was performed as part of the state assignment of Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia for 2021–2023 “Early minimally invasive diagnosis and prediction of infectious and inflammatory diseases in newborns using modern echographic, microbiological, immunological and molecular genetic research methods”, 121032500123-2.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia.
Patient Consent for Publication: Mothers of newborns provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Krogh-Jensen O.A., Nikitina I.V., Bragina O.N., Isaeva E.L., Priputnevich T.V., Zubkov V.V., Degtyarev D.N., Lenyushkina A.A. Body surface cultures in preterm neonates
on the first day of life: clinical usefulness.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 8: 108-123 (in Russian)
https://dx.doi.org/10.18565/aig.2022.8.108-123



