Hormonal sensitivity of endometrial polyps and the efficiency of LNG-IUD as a method of their secondary prevention
Background. The Endometrial polyp’s (EP) recurrence rate reaches 20%, that indicates the necessity of new pathogenically oriented prevention method’s development.Chernukha G.E., Asaturova A.V., Ivanov I.A., Korshunov A.A.
The aim of the study was to analyze the expression of sex steroids’ isoforms in EP and the efficiency of LNG-IUD as a method of their secondary prevention.
Materials and methods. 130 patients with EP were recruited for US-monitoring during a year after polypectomy, 27 of them were (treated) with LNG-IUD. 20 samples of EP, 20 – normal endometrium and 15 biopsies after LNG-IUD exposure were enrolled for assessing the expression of estrogen (ER-α, ER-β) and progesterone (PR-A,
PR-B) receptor’s isoforms.
Results. Expression of ER-β and PR-A in EP, which inhibit endometrial growth, was decreased, while PR-B’s expression, inducing proliferation – increased. The EP’s recurrence rate reached 17.1%, while in the LNG-IUD group no relapses were observed due to suppression of all ER and PR isoforms.
Conclusions. The imbalance of ER and PR isoforms can lead to EP formation, indicate their hormone sensitivity and the validity of LNG-IUD as a method of their secondary prevention.
Keywords
Endometrial polyps (EP) are the most common endometrial pathology associated with abnormal uterine bleeding (AUB), infertility, and the risk of malignancy [1–4]. One of the most challenging problems associated with EP is a high postoperative polyp recurrence rate, which, according to various sources, varies from 13 to 59.9% [4–6]. Repeated surgical interventions increase the risk of intrauterine synechia, infertility, and impair the quality of life of those affected. The risk of postoperative polyp recurrence depends primarily on the method of their removal and the surgeon’s qualification. Traditional hysteroscopy and endometrial curettage do not entirely remove the vascular pedicle of the EP, which often leads to the polyp recurrence [1, 2, 7]. Currently, hysteroscopic polypectomy remains the gold standard treatment for polyp, as it allows the ablation of the base of the EP [1, 2, 7]. However, this technique also does not guarantee a stable therapeutic effect, and the recurrence rate can reach 13.3–20.9% [6, 7]. It remains unclear whether EPs recur at the same site or form de novo, as is observed after the removal of fibroids [8]. In postmenopausal women with EP, treatment outcome may be improved by combining polypectomy with endometrial ablation. However, this method cannot be recommended for younger women who wish to preserve reproductive potential. It is also clear that surgical treatment neither eliminates the etiological factor nor affects the mechanisms of EP formation [7]. From this perspective, there is the necessity for developing pathogenetically substantiated methods of secondary prevention of EP.
To date, the issue of sensitivity of EP to hormonal stimulation remains a subject of debate. There have been many studies investigating the expression of estrogen receptor (ER) and progesterone receptor (PR) in EP, but their results were conflicting and inconclusive. Previous studies have reported both increased [9–12] and decreased [13, 14] expression of ER in polyps. The same inconsistency also applies to PR as their level in the EP can be either elevated [10–12] or reduced [13, 15]. These contradictions may be because the expression of hormonal receptors was evaluated without taking into account their isoforms that exert multidirectional effects on the endometrium. ER-α mediates proliferative action, while ER-β has an antiproliferative effect [16– 18]. Induction of PR-A is associated with endometrial secretory alterations and decidualization of endometrial stroma, while the induction of PR-B is associated with proliferation [17, 19, 20].
However, only a very small number of studies have been conducted on the expression of isoforms of steroid receptors in EP. Data about their 5–7 times more frequent occurrence during adjuvant therapy of breast cancer with tamoxifen, which has an estrogenic effect on the endometrium, may be seen as indirect evidence of the role of sex steroids in the genesis of EP [21, 22].
According to several meta-analyses, the use of a levonorgestrel-releasing intrauterine system (LNGIUS) reduces the likelihood of developing both endometrial hyperplasia and EP in patients receiving tamoxifen therapy [21, 22]. However, there are still many unresolved questions about the prevention of recurrent EP not associated with tamoxifen. Therefore, further research in this direction seems promising, both scientifically and from a practical point of view.
This study aimed to investigate the expression of isoforms of sex steroid receptors in EP and the effectiveness of LNG-IUS as a preventive therapy for polyp recurrence.
Materials and methods
The study comprised 150 women aged 20 to 50 [mean 35.6 (7.0)] who underwent hysteroscopy with an endometrial biopsy for AUB, infertility, or suspected endometrial pathology based on ultrasound examination. The study group included 130 patients with a histologically confirmed diagnosis of EP [mean age 35.5 (6.7)]. Histological findings showed that EPs were characterized as glandular-fibrous, glandular, and fibrous in 108, 15, and 7 cases, respectively.
Most often, EPs had normal adjacent endometrium in the proliferative phase (PP) (n = 91), and in 32 and 7 cases, chronic endometritis and endometrial hyperplasia were identified, respectively. The control group consisted of 20 women [mean age 36.4 (6.1)] with endometrium in PP without morphological signs of pathology and clinical symptoms. The study did not include patients who received hormone therapy for the three months preceding surgery, and those with oncological diseases and severe extragenital comorbidities. Twentyseven patients with EP and endometrial hyperplasia and those who needed contraception [mean age 34.9 (7.0)] were administered LNG-IUS (Mirena) as a secondary prevention therapy. No therapy was given to the remaining 103 patients [mean age 34.8 (7.2)].
To determine the EP recurrence rate, serial ultrasound monitoring of the endometrium was performed once every six months for 12 months. After 12 months of treatment, the patients underwent endometrial aspiration biopsy using a Pipelle sampler (Pipelle de Cornier) without preliminary extraction of LNG-IUS. In 2 cases, due to difficulties, LNG-IUS was removed and inserted again after no endometrial pathology was confirmed by a pathomorphological report.
Clinical and anamnestic data of patients in the groups receiving and not receiving therapy did not differ significantly in age, body mass index, parity, presence of AUB, gynecological comorbidities, diameter, number, and histological characteristic of EP.
The role of isoforms of sex steroid receptors in the formation of EP was estimated by using the immunohistochemical (IHC) analysis to measure the expression of ER-α, ER-β, PR-A, PR-B in 20 specimens of glandular-fibrous EP in the proliferative phase, in 20 specimens of proliferative phase endometrium, and in 15 endometrial specimens after 12 months of exposure to LNG-IUS. The IHC study was performed using a Tissue-Tek Quick-Ray kit (Unitma, Korea). IHC reactions were carried out on four μm thick paraffin sections, placed on L-polysine coated glass slides according to the standard method using the Ventana Ultra immunostainer. The primary antibodies were murine monoclonal antibodies to ER-α (clone ab75635, RTU, Abcam), ER-β (clone ab3576, RTU, Abcam), PR-A (clone 1E2, RTU, Ventana), and PR-B (clone C1A2, 1: 100, Cell Signaling). Steroid receptor expression was evaluated using an H-Score. Positive IHC staining of ER-α, ER-β, and PR-A, PR-B in EP, and endometrium specimens were presented as brown-stained nuclei of glandular and epithelial cells. Expression levels were evaluated in both the glands and the stroma.
Statistical analysis was performed using Statistica 10.0 software. The statistical significance of between-group differences for continuous variables was tested with the Mann–Whitney test. Quantitative variables are presented as the median (Me) and the quartiles Q1 and Q3. When assessing the statistical significance of differences in the expression of receptor isoforms in EP, PP, and LNG-IUS, pair-wise comparison of these groups by the Mann-Whitney test was performed. The age of the study participants was expressed as means (M) and standard deviation (SD). Qualitative variables were summarized as counts and percentages (n (%)). Categorical variables were compared by the Chi-square test. Differences were considered statistically significant at p < 0.05.
Results
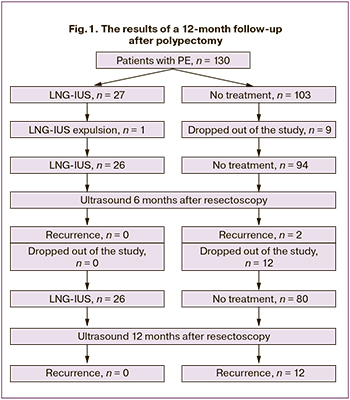 The results of a 12-month follow-up of 130 patients after a polypectomy are illustrated schematically in Fig. 1. As seen from the figure, nine women not receiving hormone therapy were excluded from the study during the first six months. Of them, 3, 1, 5 dropped out due to the onset of pregnancy, due to IVF, and for unknown reasons, respectively. One patient spontaneously expelled LNG-IUS 2 months after treatment. Based on the above data, a follow-up ultrasound examination was performed six months after polypectomy in 26 and 94 patients with and without LNG-IUS, respectively. Echographic signs of EP were found in 2 (2.1%) patients in the control group; one of them complained of heavy menstrual bleeding, which amounted to 1.1%. Among the remaining patients in this group, no AUB was reported. In the LNG-IUS group, 8 (30.76%), patients complained of minor acyclic vaginal spotting during the first six months after LNG-IUS insertion. No postoperative polyp recurrence was observed in this group; the thickness of the M-echo varied from 0.2 to 0.5 cm.
The results of a 12-month follow-up of 130 patients after a polypectomy are illustrated schematically in Fig. 1. As seen from the figure, nine women not receiving hormone therapy were excluded from the study during the first six months. Of them, 3, 1, 5 dropped out due to the onset of pregnancy, due to IVF, and for unknown reasons, respectively. One patient spontaneously expelled LNG-IUS 2 months after treatment. Based on the above data, a follow-up ultrasound examination was performed six months after polypectomy in 26 and 94 patients with and without LNG-IUS, respectively. Echographic signs of EP were found in 2 (2.1%) patients in the control group; one of them complained of heavy menstrual bleeding, which amounted to 1.1%. Among the remaining patients in this group, no AUB was reported. In the LNG-IUS group, 8 (30.76%), patients complained of minor acyclic vaginal spotting during the first six months after LNG-IUS insertion. No postoperative polyp recurrence was observed in this group; the thickness of the M-echo varied from 0.2 to 0.5 cm.
Over the next six months, among patients not receiving hormone therapy, nine women reported a pregnancy, one entered the IVF program, and two dropped out of the study for unknown reasons. In this context, at 12 months after treatment, 26 patients who received and 80 who did not receive LNG-IUS underwent ultrasound examination. In the non-treatment group, 12 (15%) patients had EP recurrence, of whom in 5 (6.1%) complained of heavy menstrual bleeding, and 3 (3.7%) reported acyclic vaginal spotting. In the LNG-IUS group, 3 (11.5%), patients experienced trace vaginal spotting with no signs of intrauterine pathology by ultrasound; the thickness of the M-echo was 0.2-0.4 cm. A total of 14 (17.1%) recurrences were observed during 12 months of follow-up among women not receiving hormone therapy; there were no recurrences in the LNG-IUS group. After 12 months of treatment, histological findings from all endometrial specimens showed atrophic changes, a decidual-like reaction of the stroma and glands lined with epithelium without signs of functional activity.
The results of IHC analysis of steroid receptors in EP, proliferative phase endometrium specimens, and endometrial biopsy specimens after 12 months of exposure to LNG-IUS are summarized in the table. The levels of ER-α expression in both the glands and the stroma were almost equal in EP and proliferative phase endometrium specimens. The expression of the ER-β receptor mediating the antiproliferative effect in EP was lower than in the unchanged endometrium in the glandular and stromal components (p = 0.01; p = 0.04).
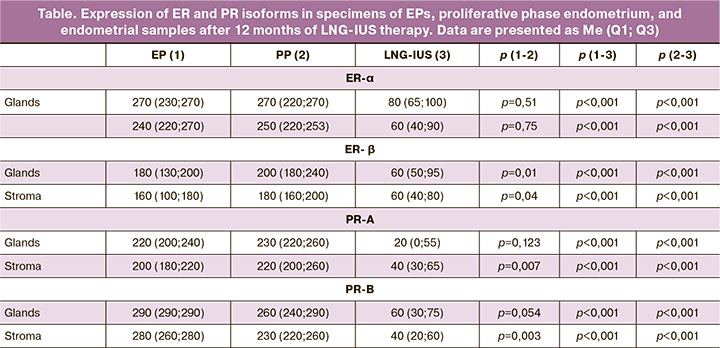
The mean expression level of PR-A, which carries out secretory transformation and decidualization, was similar in the glands of EP and proliferative phase endometrium. At the same time, in the stromal component, it was significantly lower (p = 0.007). The expression of PR-B mediating the induction of proliferation in the stromal component of EP was significantly higher compared to the proliferative phase endometrium (p = 0.003). In the glands of EP, an increase in the expression of PR-B, enhancing proliferation, was also noted, but these differences were not statistically significant (p = 0.054).
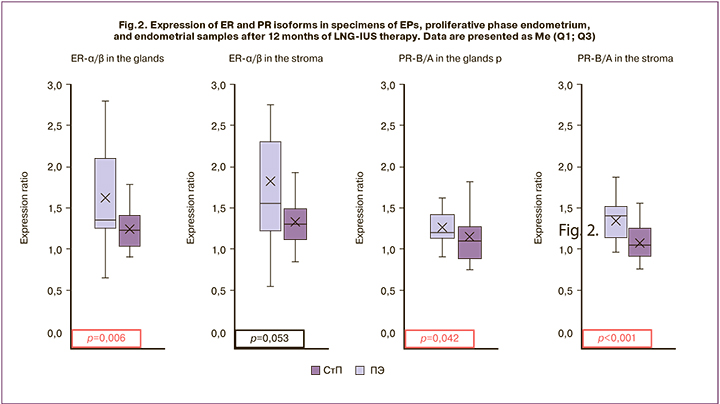
The imbalance of sex steroid receptors was also reflected in the ratio of isoforms that mediate a multidirectional effect on the endometrium, including ER-α/ ER-β and PR-B/PR-A (Fig. 2). The ratio of ER-α/β expression in the glands of EP averaged 1.4, which was significantly higher than in the glands of proliferative phase endometrium of 1.2 (p = 0.006). In the stromal component of EP, this indicator was also higher compared with unchanged endometrium (1.6 and 1.3); however, there were no statistically significant differences (p = 0.053). The ratio of PR-B/A expression was higher in EP, compared with proliferative phase endometrium, both in the glandular and stromal components of the studied specimens. In glands, this indicator was 1.2 and 1.1 (p = 0.04), and in the stroma, it was 1.4 and 1.1 (p 0.001), respectively.
After 12 months of exposure to LNG-IUS, the expression of steroid receptors was significantly suppressed both in the glandular and stromal components of the endometrium (table). The expression of ER-α showed a 3.4 and 4-fold reduction in glands and stroma, respectively, as compared with baseline levels; the expression of ER-β decreased by a factor of 2.6 and 3 in glands and the stroma, respectively. Even more, pronounced changes were identified in the expression of PR. The A-isoform was 11 times lower in the glandular and five times lower in the stromal components. PR-B showed a 4.8 and 7-fold decrease in glands and the stroma, respectively.
Discussion
Even though EPs are the most common intrauterine pathology, the underlying mechanisms of their formation and recurrence are yet unclear. One of the most debatable is the issue of sensitivity of EP to hormonal stimulation.
This study attempted to address the contradictions by a more detailed analysis of phenotypes of endometrial receptors, taking into account the expression of isoforms of ER and PR.
Traditionally, estrogens are perceived mainly as ovarian sex hormones responsible for cellular proliferation, while progestogens have a suppressive effect on the endometrium. This conception was formed back in the 1960s and was substantially revised at the end of the 20th century due to the discovery of the isoforms ER (α and β) and PR (A and B), which mediate the multidirectional effect of hormones on the uterine mucosa [16, 17]. It is now known that stimulation of ER-α induces several transcription factors (AP-1, SP-1, STAT5), cell cycle regulators (CEBPβ, cyclin D1), vascular endothelial growth factor (VEGF, CD105), signaling pathways (NFκB, Wnt pathway), leading to increased proliferation, angiogenesis, and malignancy. By contrast, the interaction of estrogens with ER-β can inhibit these factors and signaling cascades [16, 17]. This is confirmed by a study by Winuthayanon W et al., who used ER-α knockout mouse model to demonstrate that the prolonged exogenous exposure of the remaining ER-β to estradiol did not lead to proliferation, but instead increased apoptosis and endometrial atrophy [23].
Progesterone also has a multidirectional effect. Binding to PR-A, it causes decidualization, secretory transformation, and induces apoptosis. PR-B enhances endometrial growth due to the activation of transcription factors, cell cycle regulators, proliferation, and angiogenesis (CEBPβ, Ki-67, HER2, NFκB, CYR61, VEGF) [17, 20, 24–26].
The ability of PR-B to induce proliferation was demonstrated in a study by Mulac-Jericevic B et al. on PR-A knockout mice. It was noted that the administration of exogenous estrogen results in moderate proliferation of the endometrium. In contrast, a combination of estrogen and progestogen led to a significantly more pronounced endometrial growth. The authors attribute this effect to additional stimulation of PR-B in the absence of PR-Adependent decidualization and suppression of other receptors [19]. At present, it is believed that the impact of sex steroids on the endometrium depends on the ratio of the receptor isoforms in tissues, even when exposed to the same ligand [16, 17].
The study findings showed an increase in stromal expression of PR-B in EP, which induces proliferation of the endometrial mucosa. Conversely, a decrease in the levels of receptors results in a suppressive effect on the endometrium, including ER-β in the glands and the stroma, as well as PR-A in the stroma. Similarly, some authors note that the loss of the protective effect of ER-β is one of the mechanisms leading to the formation of hyperplasia, endometrial cancer, and breast and ovarian cancer [16, 27]. The imbalance of steroid receptors is most clearly demonstrated by a comparison of the ratio of proliferative and antiproliferative receptor isoforms. In EP, the expression of ER-α is predominant due to a decrease in ER-β, which can lead to local relative hyperestrogenism, inducing endometrial cell proliferation. The results also indicate the prevalence of PR-B over PR-A, which can also increase proliferative activity. An imbalance of ER and PR, reflecting an excessive proliferative and insufficient suppressive effect on the endometrium, can lead to the activation of several growth and angiogenic factors and underlie the formation of EP [2, 12, 17, 28]. These findings may indicate the sensitivity of EP to hormonal stimulation, and therefore, patients who were not interested in pregnancy were given LNG-IUS after polypectomy. Against the background of 12 months of therapy, none of the patients experienced EP recurrence. The expression of ER and PR was significantly reduced compared with baseline values. Among patients who did not receive therapy, despite the previous hysteroresectoscopy, the recurrence rate after 12 months was 17.1%, in the first six months 2.1%, in the subsequent months 15.0%. This observation is similar to published data on the recurrence rate reaching 13–20% [6,7].
Our data on the prophylactic effect of LNG-IUS are consistent with the results of a series of meta-analyses reporting the evidence that the LNG-IUS prevents the development of EPs in women taking tamoxifen as adjuvant therapy for breast cancer [21, 22]. The protective effect of LNG-IUS can be explained by the inhibition of all ER and PR isoforms and decreasing sensitivity of the endometrium to hormonal stimulation. There is no evidence in the current literature to support the inhibitory effect of LNGIUS on the expression of steroid receptors in EP. However, a similar mechanism underlies the therapeutic impact of LNG in some other gynecological conditions, such as endometrial hyperplasia [29, 30]. LNG-IUS prevents ER and PR-induced reactions that increase proliferation and angiogenesis and contribute to the formation of EP.
Conclusion
Summing up the results of the study, we can conclude that the imbalance of ER and PR isoforms represents one of the possible mechanisms of EP formation. This imbalance is associated with a decrease in the suppressive effect and an increase in the proliferative activity of the endometrium, which indicates the sensitivity of EP to hormonal stimulation. During the first year after resectoscopic polypectomy, ER recurrence can be expected in every fifth to the sixth patient, while due to the effect of LNG-IUS resulting in suppression of all isoforms of ER and PR, as well as atrophy of the glandular epithelium, no recurrences were observed. In this regard, the use of the LNG-IUS can be considered as a promising modality of secondary prevention of EP in women who are not interested in pregnancy. It is essential to conduct other, more extensive controlled clinical trials to validate these findings and integrate them into everyday clinical practice.
References
- Clark T.J., Stevenson H. Endometrial polyps and abnormal uterine bleeding (AUB-P) – What is the relationship; how are they diagnosed and how are they treated? Best Pract. Res. Clin. Obstet. Gynaecol. 2017; 40: 89-104. https://dx.doi.org/10.1016/j.bpobgyn.2016.09.005.
- Tanos V., Berry K.E., Seikkula J., Abi Raad E., Stavroulis A., Sleiman Z. et al. The management of polyps in female reproductive organs. Int. J. Surg. 2017; 43: 7-16. https://dx.doi.org/10.1016/j.ijsu.2017.05.012.
- Чернуха Г.Е., Асатурова А.В., Иванов И.А., Думановская М.Р. Структура патологии эндометрия в различные возрастные периоды. Акушерство и гинекология. 2018; 8: 129-34. https://dx.doi.org/10.18565/aig.2018.8.129-134. [Chernukha G.E., Asaturova A.V., Ivanov I.A., Dumanovskaya M.R. Endometrial lesion’s pattern in different age groups. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; 8: 129-34. (in Russian)].
- Саттаров Ш.Н., Коган Е.А., Саркисов С.Э., Мамиконян И.О., Бойко М.А., Гюрджян С.А. Молекулярные механизмы патогенеза полипов эндометрия в постменопаузе. Акушерство и гинекология. 2013; 6: 17-22. [Sattarov S.N., Kogan E.A., Sarkisov S.E. et al. Molecular mechanisms of the pathogenesis of postmenopausal endometrial polyps. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2013; 6: 17-22. (in Russian)].
- Henriquez D.D., van Dongen H., Wolterbeek R., Jansen F.W. Polypectomy in premenopausal women with abnormal uterine bleeding: effectiveness of hysteroscopic removal. J. Minim. Invasive Gynecol. 2007; 14(1): 59-63. https://dx.doi.org/10.1016/j.jmig.2006.07.008.
- AlHilli M.M., Nixon K.E., Hopkins M.R., Weaver A.L., Laughlin-Tommaso S.K., Famuyide A.O. Long-term outcomes after intrauterine morcellation vs hysteroscopic resection of endometrial polyps. J. Minim. Invasive Gynecol. 2013; 20(2): 215-21. https://dx.doi.org/10.1016/j.jmig.2012.10.013.
- Kanthi J., Mangala J., Remadevi C., Sumathy S. Clinical study of endometrial polyp and role of diagnostic hysteroscopy and blind avulsion of polyp. J. Clin. Diagn. Res. 2016; 10(6): QC01-4. https://dx.doi.org/10.7860/JCDR/2016/18173.7983.
- Paradisi R., Rossi S., Scifo M.C., Dall’O F., Battaglia C., Venturoli S. Recurrence of endometrial polyps. Gynecol. Obstet. Invest. 2014; 78(1): 26-32. https://dx.doi.org/10.1159/000362646.
- Dibi R.P., Zettler C.G., Pessini S.A., Ayub A.V., de Almeida S.B., da Silveira G.P. Tamoxifen use and endometrial lesions: hysteroscopic, histological, and immunohistochemical findings in postmenopausal women with breast cancer. Menopause. 2009; 16(2): 293-300. https://dx.doi.org/10.1097/gme.0b013e31818af10a.
- Leao R.B., Andrade L., Vassalo J., Antunes A., Aarão Pinto-Neto A., Costa-Paiva L. Differences in estrogen and progesterone receptor expression in endometrial polyps and atrophic endometrium of postmenopausal women with and without exposure to tamoxifen. Mol. Clin. Oncol. 2013; 1(6): 1055-60. https://dx.doi.org/10.3892/mco.2013.180.
- Schwartz L.B., Krey L., Demopoulos R., Goldstein S.R., Nachtigall L.E., Mittal K. Alterations in steroid hormone receptors in the tamoxifen-treated endometrium. Am. J. Obstet. Gynecol. 1997; 176(1, Pt. 1): 129-37. https://dx.doi.org/10.1016/s0002-9378(97)80025-7.
- Коган Е.А., Саттаров Ш.Н., Саркисов С.Э., Бойко М.А., Мамиконян И.О. Рецепторный статус полипов эндометрия у женщин в постменопаузе. Акушерство и гинекология. 2014; 2: 60-6. [Kogan E.A., Sattarov Sh.N., Sarkisov S.E., Boiko M.A. et al The receptor status of endometrial polyps in postmenopausal women. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 2: 60-6. (in Russian)].
- Mittal K., Schwartz L., Goswami S., Demopoulos R. Estrogen and progesterone receptor expression in endometrial polyps. Int. J. Gynecol. Pathol. 1996; 15(4): 345-8. https://dx.doi.org/10.1097/00004347-199610000-00007.
- Lopes R., Baracat E., de Albuquerque N., Ramos J., Yatabe S., Depesr D.B., Lippi U.G. Analysis of estrogen- and progesterone- receptor expression in endometrial polyps. J. Minim. Invasive Gynecol. 2007; 14(3): 300-3. https://dx.doi.org/10.1016/j.jmig.2006.10.022.
- Peng X., Li T., Xia E., Xia C., Liu Y., Yu D. A comparison of oestrogen receptor and progesterone receptor expression in endometrial polyps and endometrium of premenopausal women. J. Obstet. Gynaecol. 2009; 29(4): 340-6. https://dx.doi.org/10.1080/01443610902878775.
- Vrtačnik P., Ostanek B., Mencej-Bedrač S., Marc J. The many faces of estrogen signaling. Biochem. Med. (Zagreb). 2014; 24(3): 329-42. https://dx.doi.org/10.11613/BM.2014.035.
- Straus J.F., Barnieri R.L. Yen & jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical management, 7th ed. Philadelphia: Elsevier? 2019: 115-21.
- Hapangama D.K., Kamal A.M., Bulmer J.N. Estrogen receptor β: the guardian of the endometrium. Hum. Reprod. Update. 2015; 21(2): 174-93. https://dx.doi.org/10.1093/humupd/dmu053.
- Mulac-Jericevic B., Mullinax R.A., DeMayo F.J., Lydon J.P., Conneely O.M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000; 289(5485): 1751-4. https://dx.doi.org/10.1126/science.289.5485.1751.
- Grimm S.L., Hartig S.M., Edwards D.P. Progesterone receptor signaling mechanisms. J. Mol. Biol. 2016; 428(19): 3831-49. https://dx.doi.org/10.1016/j.jmb.2016.06.020.
- Wan Y.L., Holland C. The efficacy of levonorgestrel intrauterine systems for endometrial protection: A systematic review. Climacteric. 2011; 14(6): 622-32. https://dx.doi.org/10.3109/13697137.2011.579650.
- Dominick S., Hickey M., Chin J., Su H.I. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst. Rev. 2015; (12): CD007245. https://dx.doi.org/10.1002/14651858.CD007245.pub3.
- Winuthayanon W., Hewitt S.C., Orvis G.D., Behringer R.R., Korach K.S. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc. Natl. Acad. Sci. USA. 2010; 107(45): 19272-7. https://dx.doi.org/10.1073/pnas.1013226107.
- Rojas P.A., May M., Sequeira G.R., Elia A., Alvarez M., Martínez P. et al. Progesterone receptor isoform ratio: a breast cancer prognostic and predictive factor for antiprogestin responsiveness. J. Natl. Cancer Inst. 2017; 109(7): djw317. https://dx.doi.org/10.1093/jnci/djw317.
- Han A.R., Lee T.H., Kim S., Lee H.Y. Risk factors and biomarkers for the recurrence of ovarian endometrioma: about the immunoreactivity of progesterone receptor isoform B and nuclear factor kappa B. Gynecol. Endocrinol. 2017; 33(1): 70-4. https://dx.doi.org/10.1080/09513590.2016.1205580.
- Kaya H.S., Hantak A.M., Stubbs L.J., Taylor R.N., Bagchi I.C., Bagchi M.K. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol. Endocrinol. 2015; 29(6): 882-95. https://dx.doi.org/10.1210/me.2014-1363.
- Bottner M., Thelen P., Jarry H. Estrogen receptor beta: tissue distribution and the still largely enigmatic physiological function. J. Steroid Biochem. Mol. Biol. 2014. 139: 245-51. https://dx.doi.org/10.1016/j.jsbmb.2013.03.003.
- Indraccolo U., Di Iorio R., Matteo M., Corona G., Greco P., Indraccolo S.R. The pathogenesis of endometrial polyps: a systematic semi-quantitative review. Eur. J. Gynecol. Oncol. 2013; 34(1): 5-22.
- Weng M., Li L., Feng S., Xie M., Hong S. Effects of levonorgestrel-releasing intrauterine system on endometrial estrogen and аprogesterone receptors in patients with endometrial hyperplasia. Nan Fang Yi Ke Da Xue Xue Bao. 2012. 32(9): 1350-4.
- Gallos I.D., Devey J., Ganesan R., Gupta J.K. Predictive ability of estrogen receptor (ER), progesterone receptor (PR), COX-2, Mlh1, and Bcl-2 expressions for regression and relapse of endometrial hyperplasia treated with LNG-IUS: a prospective cohort study. Gynecol. Oncol. 2013; 130(1): 58-63. https://dx.doi.org/10.1016/j.ygyno.2013.04.016.
Received 27.03.2020
Accepted 25.05.2020
About the Authors
Galina E. Chernukha, M.D., professor, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Department of endocrinological gynecology. Tel.: +7(916)311-05-21. E-mail: g_chernukha@oparina4.ru. https://orcid.org/0000-0002-9065-5689.4 Oparina str., Moscow, 117997, Russian Federation.
Alexandra V. Asaturova, Ph.D., senior scientific researcher, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, Pathology department. Tel.: +7(926)994-43-14. E-mail: a_asaturova@oparina4.ru. https://orcid.org/0000-0001-8739-5209.
4 Oparina str., Moscow, 117997, Russian Federation.
Ilya A. Ivanov, postgraduate student, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, Department of Endocrinological gynecology. Tel.: +7(962)980-00-18. E-mail: doctor.i.ivanov@yandex.ru. https://orcid.org/0000-0003-0751-7566.
4 Oparina str., Moscow, 117997, Russian Federation.
Alexey A. Korshunov, gynecologist, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, Department of Innovative oncogynecology. Tel.: +7(916)924-09-39. E- a_korshunov@oparina4.ru.
4 Oparina str., Moscow, 117997, Russian Federation.
For reference: Chernukha G.E., Asaturova A.V., Ivanov I.A., Korshunov A.A. Hormonal sensitivity of endometrial polyps and the efficiency of LNG-IUD as a method of their secondary prevention.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 6: 65-71 (in Russian).
https://dx.doi.org/10.18565/aig.2020.6.65-71



