Study of protein oxidative modifications in patients with obesity and hyperandrogenism
Objective. To study protein oxidative modification (POM) processes in patients with hyperandrogenism and obesity.Tazina T.V., Korotkova N.V., Fomina M.A., Politova A.A., Kolobaeva A.A.
Material and methods. The intensity of tissue POM was assessed using the R.L. Levine method modified by E.E. Dubinina: the method is based on the interaction of carbonyl groups and aminogroups of oxidized amino acid residues with 2,4-dinitrophenylhydrazines (2,4-DNPH) to give rise to 2,4-dinitrophenylhydrazones having a specific absorption spectrum in the ultraviolet and visible regions.
Results. There was a statistically significant increase in spontaneous POM products in obesity; POM levels did not change in hyperandrogenism.
Secondary markers for POM were observed to increase in hyperandrogenism; the ratio of primary and secondary markers of oxidative stress was unchanged in obesity. There was a reduction in the reserve-adaption potential in obese patients.
Conclusion. Obesity is accompanied by an increase in POM and a decrease in the reserve-adaptive potential of the body. Hyperandrogenism occurs with an increase in secondary oxidative stress markers.
Keywords
According to the definition of the World Health Organization (WHO), obesity is “an abnormal or excessive fat accumulation that may impair health” [1].
Obesity is one of the most widespread chronic diseases in the world affecting not only adults but also children and teenagers. In 1997, the WHO announced this pathology as global epidemic which also remains one of the most significant problems of medicine at the present time.
According to the experts of this international organization, in 2016 more than 1.9 billion adults older than 18 years had excess weight. More than 650 million people among them had obesity.
In 2016, excess weight was revealed in 39% of adults older than 18 years (39% of men and 40% of women) and about 13% of the adult population of the planet (11% of men and 15% of women) had obesity.
During the period from 1975 to 2016 the number of people having obesity around the world became more than three times higher [1, 2]. The leading position among the countries with high incidence was taken by the USA where 34% of the population had excess body weight and 27% of people had obesity [3].
In Europe the number of people experiencing obesity reaches 10–25% of men and 10–30% of women. For the last 10 years in the majority of the European countries the prevalence of obesity increased by 10–40%. In Europe more than 50% of the population have excess weight or have obesity [1, 4].
In 2016, in the Russian Federation the number of people suffering from excess body weight was 62% and obesity 26.2% [2]. According to projections, 31% of men and 26% of women are expected to have obesity in 2020. By 2030 the model foretells that 33% of men and 26% of women will have obesity [5].
Obesity has a negative effect on the quality of life and all people’s activities often leading to the development of severe diseases, incapacity and disability. Most of people with excess body weight and obesity experience objective difficulties because of serious deviations in the state of health, physical restrictions and psychological problems.
Excess weight and obesity are risks for the development of serious cardiovascular diseases (first of all, hypertension and coronary heart disease), endocrine impairments (type II diabetes mellitus, impairment of reproductive function), diseases of the musculoskeletal system (protrusion and intervertebral disc herniation, injury to the lower extremities joints) and also oncological diseases and psychological impairments [6–9].
Chronic diseases of the cardiovascular system, oncological diseases, obesity and diabetes mellitus refer to noninfectious diseases with high mortality [10, 11].
In the world, excess weight and obesity are associated with higher mortality than being underweight. The world population amounting to 65% of people lives in the countries where people die of excess weight and obesity more often than of being underweight. The statistics include the information about all countries with high and average levels of income. Every year at least 2.8 million of adults die due to the conditions and diseases connected with excess weight or obesity [6].
According to some authors, there is a tendency for further increase in mortality caused by diseases associated with obesity, namely from 59–60% at the present time up to 69% by 2030. The main causes of this negative phenomenon besides smoking and alcohol abuse are an inactive lifestyle and an unbalanced diet especially among children and teenagers that predictably will lead to further increase in the rate of adult population with excess body weight and obesity [12].
Another common medical problem that is connected with metabolic impairments, type II diabetes mellitus, cardiovascular diseases and impairment of reproductive function is the syndrome of hyperandrogenism [13].
The syndrome of hyperandrogenism is the pathological condition caused by excessive products of androgens in the ovaries and/or adrenal glands or increase in local tissue sensitivity to the circulating androgens.
It is the most frequent endocrinopathy in women. The prevalence of a hyperandrogenism among women is 10–20%.
Timely diagnosis of hyperandrogenic states is very important as they may be followed by adverse conditions: menstrual cycle disorders, endocrine infertility, increased risk for miscarriages, premature birth, gestational diabetes [14].
According to the classification, the following forms of hyperandrogenic states can be revealed [15]:
- Nonneoplastic (functional) forms of true hyperandrogenism;
- Tumoral forms of true hyperandrogenism;
- Transport forms of hyperandrogenism resulting from decrease in globulin production in the liver, globulin in its turn connects gamic steroids;
- Receptor form of hyperandrogenism caused by an increase in activity of a 5a-reductase in target cells.
Due to the common occurrence of obesity and hyperandrogenism and their high social significance, it is relevant to study pathogenetic mechanisms of development of the presented nosologies more profoundly for the purpose of their further correction.
Any adaptive or pathological process is accompanied by the intensification of free radical oxidation of biosubstrates [16]. These processes play a key role in the metabolic processes in cells. The oxidative stress (OS), an imbalance between the oxidative and antioxidative systems of cells and tissues result from the production of oxidative free radicals and active forms of oxygen. One of the results of the increased level of active forms of oxygen is the change of structure and functions of cellular proteins [17]. There is a great number of proteins in the biomaterial, they are highly sensitive to the action of free radicals and as a result they prove to be the main targets for free radicals [18]. They are also responsible for the majority of cellular functions, therefore, studying of protein oxidative modification (POM) is one of the relevant directions in the modern experimental biochemistry [19–23].
Nowadays the role of free radical processes in pathogenesis of many various diseases has been proved by some studies and POM assessment in patients with gynecological diseases, for example, ovarian cancer [24, 25].
The objective of the research was to study POM processes in patients with hyperandrogenism and obesity.
Materials and Methods
Ten people with obesity and ten people with hyperandrogenism presented to the clinic «Medline Ryazan» for gynecological examination. Their blood plasma served as a material for the research work. The control group included ten healthy donors of the same age and sex as the patients with obesity and hyperandrogenism; people of control group did not show any pathologies accompanying increased POM. In patients, 15-ml blood samples were taken from medial cubital vein on an empty stomach once before treatment in the morning at the same time. The ADTA solution was used as anticoagulant which was prepared at the rate of 1.5 mg per 1 ml of blood.
The intensity of POM tissue was assessed using the R.L. Levine [26] method modified by E.E. Dubinina [27]: the method is based on the interaction of carbonyl groups and aminogroups of oxidized amino acid residues with 2,4-dinitrophenylhydrazines (2,4-DNPH) with the formation of 2,4-dinitrophenylhydrazones. They have a specific absorption spectrum in the ultraviolet and visible regions.
The complex assessment of POM maintenance was carried out by the patent for invention No. 2524667 (Fomina M.A., Abalenikhina Y.V., Fomin N.V., Terentyev A.A.) developed at the Department of Biological Chemistry, Ryazan State Medical University [28].
Statistical processing of the obtained data was performed using methods of nonparametric statistics with Statistica 6.0 software package. Mann Whitney U-test criterion was used for determining statistically significant distinctions of continuous sizes depending on distribution parameters. The continuous variables are presented in the form of M±s (average ± a standard deviation). Deviations were considered statistically significant with value р≤0.05 for all analyses.
Results and Discussion
The study of free radical processes in case of pathology leading to the development of metabolic impairments (obesity, diabetes mellitus, metabolic syndrome) is of critical importance because they are considered as major factors of development of cardiovascular diseases and can be the cause of infertility in women of reproductive age.
Consecutive electronic restitution (O2) leads to elaboration of reactive forms of oxygen (reactive oxygen species – ROS). One electron of molecular oxygen (e-) leads to elaboration of superoxide anion (O2-), two electrons cause elaboration of hydrogen dioxide (H2O2) and three electrons lead to elaboration of radical oxyhydroxide (HO˙).
Free radicals cause carbonylation of proteins, i.e. the formation of stable products which are formed with the participation of amino acid residues of proline, arginine, lysine, threonine. Also, carbonyl derivatives of proteins can be formed with the participation of amino acid residues of lysine, cysteic acid and histidine with products of lipids peroxide oxidation. It leads to the impairment of proteins functions and development of different pathologies requiring further correction.
It should be noted that the definition of POM has frequently been applied in clinical practice [29].
Nowadays POM in obesity is studied due to the fact that Apo-A protein oxidation as a part of lipoproteins of high density can lead to the impairment of its functions, impairment of functions of lecithin-cholesterol acyltransferase enzyme which participates in an esterification of cholesterol and its return transfer to the liver that leads to impairment of the latter and accumulation of cholesterol in blood.
 Primary and secondary markers of oxidative stress are defined as aldehyde dinitrophenylhydrazone (ADNPHH) and cetonedinitrophenylhydrazone (CDNPHH), relatively. The dominance of primary markers of proteins impairments is considered to point at the initial stage of OS and a possible reversibility of this process [30].
Primary and secondary markers of oxidative stress are defined as aldehyde dinitrophenylhydrazone (ADNPHH) and cetonedinitrophenylhydrazone (CDNPHH), relatively. The dominance of primary markers of proteins impairments is considered to point at the initial stage of OS and a possible reversibility of this process [30].
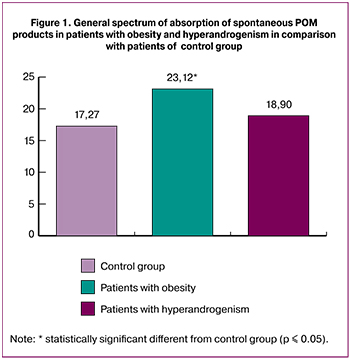
The results of the investigation showed that in patients with hyperandrogenism only slight increase of POM level was noted in comparison with values of control group, the increase was not statistically significant (Fig. 1).
The patients with obesity demonstrated statistically significant increase in total maintenance of spontaneous POM products in comparison with control group.
We analyzed primary and secondary markers of carbonyl stress and we noted that their rate in patients of control group and in patients with obesity almost did not differ despite higher total values of total number of carbonylated derivatives of proteins in obese patients (Fig. 2).
The rate of ADNPHH was 83.8% and 83.5%, respectively. These values can suggest that POM processes are reversible and they have higher adaptation opportunities of the body in this pathology.
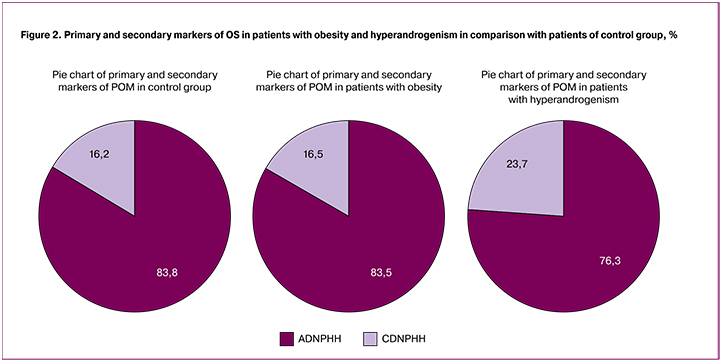
The rate of ADNPHH in patients with hyperandrogenism was lower in comparison with control group. The rate of CDNPHH increased and corresponded to value 23.7 in comparison with 16.2% in patients of control group that was statistically significant. These data can confirm aggravation of OS and its transition to later stage and also possible non-reversibility of the process.
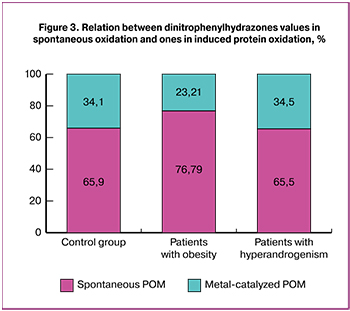 Nowadays, some methods are used to determine the level of spontaneous protein oxidation characterizing the general physiological condition of the body and metal-catalyzed POM which is an index of reserve and adaptation opportunities of the body [31]. In this investigation metal-catalyzed protein oxidation was carried out based on the Fenton reaction: it was induced with iron ions and hydrogen dioxide. The comparative analysis of the reserve and adaptation potential (RAP) was performed in patients with obesity and hyperandrogenism and in patients of control group (Fig. 3).
Nowadays, some methods are used to determine the level of spontaneous protein oxidation characterizing the general physiological condition of the body and metal-catalyzed POM which is an index of reserve and adaptation opportunities of the body [31]. In this investigation metal-catalyzed protein oxidation was carried out based on the Fenton reaction: it was induced with iron ions and hydrogen dioxide. The comparative analysis of the reserve and adaptation potential (RAP) was performed in patients with obesity and hyperandrogenism and in patients of control group (Fig. 3).
The decrease in RAP level was noted in patients with obesity in comparison with the patients of control group but it was not statistically significant. The obtained results can suggest higher sensitivity to protein oxidation in patients with this pathology.
The common RAP level in patients with hyperandrogenism was not different from that in control group.
Conclusions
- In women with gynecological pathology, obesity is accompanied by statistically significant increase in the general spectrum of absorption of spontaneous POM products. At the same time the proportion of primary and secondary markers does not change in comparison with one in control group but RAP values decrease.
- The general level of carbonylated proteins and RAP does not change in women with gynecological pathology experiencing hyperandrogenism. However, there is an increase in secondary markers of POM that can suggest OS aggravation.
References
1. Obesity: preventing and managing the global epidemic: report of a WHO consultation. WHO technical report series N°894. Geneva: World Health Organization; 2000. Available at: http://www.who.int/iris/handle/10665/42330
2. http://www.euro.who.int/ diabetes/country- profiles/rus_ru.pdf?ua=1
3. James W. The epidemiology of obesity: the size of the problem. J, Intern, Med. 2008; 263(4): 336-52. doi: 10.1111/j.1365-2796.2008.01922.x.
4. Schoeller D. The challenge of obesity in the WHO European region and the strategies for response. Med. Sci. Sports Exerc. 2008; 40(3): 590. doi: 10.1249/mss.0b013e318164f33c.
5. Euro.who.int. Russian Federation. 2015. Available at: http://www.euro.who.int/ en/health-topics/disease-prevention/nutrition/country-work/russian-federation2/ Accessed October 2, 2015.
6. ВОЗ центр СМИ. Ожирение и избыточный вес. Информационный бюллетень №311. 2015. [WHO Media centre. Obesity and overweight [internet]. Fact sheet N°311. January 2015.] Available at: http://www.who.int/mediacentre/ factsheets/fs311/en/
7. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013. Expert panel report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity (Silver Spring). 2014; 22(Suppl. 2): S41-410. doi: 10.1002/oby.20660.
8. Wong E., Tanamas S.K., Wolfe R., Backholer K., Stevenson C., Abdullah A., Peeters A. The role of obesity duration on the association between obesity and risk of physical disability. Obesity (Silver Spring). 2015; 23(2): 443-7.doi: 10.1002/oby.20936.
9. Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384(9945): 766-81.
10. Guénard F., Houde A., Bouchard L., Tchernof A., Deshaies Y., Biron S. et al. Association of LIPA gene polymorphisms with obesity-related metabolic complications among severely obese patients. Obesity (Silver Spring). 2012; 20(10): 2075-82. doi: 10.1038/oby.2012.52.
11. Beaglehole R., Bonita R., Alleyne G., Horton R., Li L., Lincoln P. et al. UN High-Level Meeting on Non-Communicable Diseases: addressing four questions. Lancet. 2011; 378(9789): 449-55. doi: 10.1016/s0140-6736(11)60879-9.
12. Пермякова Е.Ю., Година Е.З., Гилярова О.А. Влияние физической активности и суточного потребления калорий на особенности жироотложения у современных детей и подростков Архангельского региона и г. Москвы. Вестник Московского университета. Серия 23: Антропология. 2012; 4: 112-9. [Permyakova E.Yu., Godina E.Z., Gilyarova O.A. The impact of physical activity and daily calorie consumption on the features of fat deposition in modern children and adolescents of the Arkhangelsk region and the city of Moscow. Bulletin of Moscow University. Series 23: Anthropology. 2012; 4: 112-9. (in Russian)]
13. Дедов И.И., Андреева Е.Н., Карпова Е.А. Синдром поликистозных яичников. Практические рекомендации для врачей. М.: ИТМ; 2009. 52c. [Dedov I.I., Andreeva E.N., Karpova E.A. Polycystic ovary syndrome. Practical recommendations for doctors. M .: ITM; 2009. 52p. (in Russian)]
14. Луценко Л.А. Надпочечниковые гиперандрогении: мультидисциплинарный подход к решению проблемы. Міжнародний ендокринологічний журнал. 2016; 8: 29-34. [Lutsenko L.A. Adrenal hyperandrogenism: a multidisciplinary approach to problem solving. International Endocrinology Journal. 2016; 8: 29-34. (in Russian)]
15. Дедов И.И., ред. Синдром гиперандрогении у женщин. Патогенез, клинические формы, дифференциальная диагностика и лечение. Методическое пособие для врачей. М.; 2003. 32с. [Dedov I.I, ed. Hyperandrogenism syndrome in women. Pathogenesis, clinical forms, differential diagnosis and treatment. Methodical manual for doctors. M .; 2003. 32c. (in Russian)]
16. Губский Ю.И., Беленичев И.Ф., Левицкий Е.Л., Коваленко С.И., Павлов С.В., Ганчева О.В., Марченко А.Н. Токсикологические последствия окислительной модификации белков при различных патологических состояниях (обзор литературы). Журнал АМН Украiни. 2008; 814(7): 49-54. [Gubsky Yu.I., Belenichev I.F., Levitsky E.L., Kovalenko S.I., Pavlov S.V., Gancheva O.V., Marchenko A.N. Toxicological effects of oxidative modification of proteins under various pathological conditions (review of literature). Journal of the Academy of Medical Sciences of Ukraine. 2008; 814 (7): 49-54.(in Russian)]
17. Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., de Bittencourt P.I. Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016; 473(24): 4527-50.
18. Colak E. New markers of oxidative damage to macromolecules. J. Med. Biochem. 2008; 27: 1-16.
19. Balogh A., Santer D., Pásztor E.T., Tóth A., Czuriga D., Podesser B.K. et al. Myofilament protein carbonylation contributes to the contractile dysfunction in the infracted. LV region of mouse hearts. Cardiovasc. Res. 2014; 101(1): 108-19.
20. Безручко Н.В., Рубцов Г.К. Методология и метод оценки окислительной модификации белков в комплексе с молекулами средней массы, перспективы их применения. Вестник Томского государственного педагогического университета. 2014; 8: 185-9. [Bezruchko N.V., Rubtsov G.K. Methodology and method for assessing the oxidative modification of proteins in combination with medium-weight molecules, the prospects for their use. Bulletin of Tomsk State Pedagogical University. 2014; 8: 185-9. (in Russian)]
21. Кулемина М.В., Гаврилова Н.В., Короткова Н.В. Изучение окислительной модификации белков под действием сангвинарина в неседиментируемой фракции гомогенатов печени крыс in-vitro. В кн.: Кошель В.И., ред. «Неделя науки – 2016»: материалы Всероссийского молодежного форума с международным участием. Ставрополь: Изд-во СтГМУ; 2016: 412. [Kulemina M.V., Gavrilova N.V., Korotkova N.V. Studying the oxidative modification of proteins under the action of sanguinarine in the non-depreciated fraction of rat liver homogenates in vitro. In the book: Koshel VI, ed. “Science Week - 2016”: materials of the All-Russian Youth Forum with international participation. Stavropol: Publishing House of the State Medical University; 2016: 412. (in Russian)]
22. Калинин Р.Е., Абаленихина Ю.В., Пшенников А.С., Сучков И.А., Исаков С.А. Взаимосвязь окислительного карбонилирования белков и лизосомального протеолиза плазмы в условиях экспериментального моделирования ишемии и ишемии-реперфузии. Наука молодых. 2017; 3: 338-51. [Kalinin R.Ye., Abalenikhina Yu.V., Pshennikov A.S., Suchkov I.A., Isakov S.A. The relationship of oxidative carbonylation of proteins and lysosomal plasma proteolysis under conditions of experimental modeling of ischemia and ischemia-reperfusion. Science is young. 2017; 3: 338-51. (in Russian)]
23. Арапова А.И., Фомина М.А. Изучение влияния L-карнитина на изменение активности катепсинов B, L, H и окислительной модификации белков в мышечных органах крыс. Российский медико-биологический вестник им. академика И.П. Павлова. 2016; 2: 13-20. [Arapova A.I., Fomina M.A. The study of the effect of L-carnitine on the change in the activity of cathepsins B, L, H and the oxidative modification of proteins in the muscle organs of rats. Russian Medical and Biological Bulletin them. Academician I.P. Pavlova. 2016; 2: 13-20. (in Russian)]
24. Долгова Д.Р., Генинг С.О., Антонеева И.И., Пирмамедова С.С., Михеенко А.А., Мясникова Д.Ф., Наковкина Е.С., Насырова Е.Ю. Окислительная модификация белков в эритроцитах больных раком яичников после полихимиотерапии по схеме САР. Фундаментальные исследования. 2014; 7(ч. 4): 689-92. [Dolgova D.R., Gening S.O., Antoneeva I.I., Pirmamedova S.S., Mikheenko A.A., Myasnikova D.F., Nakovkina E.S., Nasyrova E.Yu. Oxidative modification of proteins in erythrocytes of patients with ovarian cancer after polychemotherapy according to the SAR scheme. Basic research. 2014; 7 (Part 4): 689-92. (in Russian)]
25. Горошинская И.А., Неродо Г.А., Сурикова Е.И., Качесова П.С., Внуков В.В., Шалашная Е.В., Нескубина И.В., Немашкалова Л.А., Максимова Н.А., Сергеева М.М. Интенсивность хемилюминисценции, состояние антиоксидантной системы и окислительная модификация белков плазмы крови при развитии рецидива рака яичников. Сибирский онкологический журнал. 2013; 4: 45-9. [Goroshinskaya I.A., Nerodo G.A., Surikova E.I., Kachesova P.S., Vnukov V.V., Shalashnaya E.V., Neskubina I.V., Nemashkalova L.A., Maksimova N.A., Sergeeva M.M. The intensity of chemiluminescence, the state of the antioxidant system and the oxidative modification of plasma proteins during the development of ovarian cancer recurrence. Siberian Oncology Journal. 2013; 4: 45-9. (in Russian)]
26. Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G. et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990; 186: 464-78.
27. Дубинина Е.Е., Бурмистров С.О., Ходов Д.А., Поротов И.Г. Окислительная модификация белков сыворотки крови человека, метод ее определения. Вопросы медицинской химии. 1995; 41(1): 24-6. [Dubinina E.E., Burmistrov S.O, Khodov D.A, Porotov I.G. Oxidative modification of human serum proteins, method of its determination. Questions of medical chemistry. 1995; 41 (1): 24-6. (in Russian)]
28. Пат. 2524667 РФ. МПК G01N 33/52. Способ комплексной оценки содержания продуктов ОМБ в тканях и биологических жидкостях / Фомина М.А., Абаленихина Ю.В., Фомина Н.В., Терентьев А.А. Ряз гос. мед. ун-т им. акад. И.П. Павлова. Номер заявки 2013102618/15; заявлено 21.01.2013; опубл.27.07.2014, Бюл. № 21.
29. Вавилов Н.В., Шилов Ю.И., Годовалов А.П. Методические аспекты определения окислительной модификации белка. Медицинский альманах. 2018; 2: 19-22. [Vavilov N.V., Shilov Yu.I., Godovalov A.P. Methodical aspects of determining the oxidative modification of a protein. Medical Almanac. 2018; 2: 19-22. (in Russian)]
30. Муравлева Л.Е., Молотов-Лучанский В.Б., Клюев Д.А., Бакенова Р.А., Култанов Б.Ж., Танкибаева Н.А., Койков В.В., Омарова Г.А. Окислительная модификация белков: проблемы и перспективы исследования. Фундаментальные исследования. 2010; 1: 74-8. [Muravleva L.E., Molotov-Luchansky V.B., Klyuev D.A., Bakenova R.A., Kultanov B.Zh., Tankibaeva N.A., Koykov V.V., Omarova G.A. Oxidative modification of proteins: problems and prospects for research. Basic research. 2010; 1: 74-8. (in Russian)]
31. Никитина Ю.В., Мухина И.В. Изменение окислительных процессов в ткани головного мозга и крови крыс в раннем онтогенезе. Вестник Нижегородского университета им. Н.И. Лобачевского. 2009; 6(1): 124-31. [Nikitina Yu.V., Mukhina I.V. Changes in oxidative processes in brain tissue and blood of rats in early ontogenesis. Bulletin of Nizhny Novgorod University. N.I. Lobachevsky. 2009; 6 (1): 124-31. (in Russian)]
Received 06.04.2018
Accepted 20.04.2018
About the Authors
Tazina, Tatiana V., assistant of the Department of Surgery, Obstetrics and Gynecology, Federal State Medical University of Ryazan State Medical University.390026, Russia, Ryazan, Vysokovol’tnaya str. 9. Tel.: +74912367970. E-mail: tazina@inbox.ru
Korotkova, Natalya V., PhD, senior lecturer of the Department of Biological Chemistry, Ryazan State Medical University.
390026, Russia, Ryazan, Vysokovol’tnaya str. 9. Tel.: +74912460898. E-mail: fnv8@yandex.ru
Fomina, Maria A., PhD, associate professor of the Department of Biological Chemistry, Ryazan State Medical University.
390026, Russia, Ryazan, Vysokovol’tnaya street, 9, Tel.: +74912460898. E-mail: marya.fom@yandex.ru
Politova, Anastasia A., resident of the Clinical and Research Laboratory of Immunophenotyping Blood Cells and Bone Marrow National Medical Research Center of Hematology, Ministry of Health of the Russian Federation.
125167, Russia, Moscow, Novy Zykovsky Proezd str. 4. Tel.: +74954992016.
E-mail: naka.politova@gmail.com
Kolobaeva, Anna A., 6-th year student of the medical and preventive faculty of the Ryazan State Medical University.
390026, Russia, Ryazan, Vysokovol’tnaya str. 9.
For citations: Tazina T.V., Korotkova N.V., Fomina M.A., Politova A.A., Kolobaeva A.A. Study of protein oxidative modifications in patients with obesity and hyperandrogenism. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (12): 82-7. (in Russian)
http://dx.doi.org/10.18565/aig.2018.12.82-87



