Immune mechanisms of regulating the growth of uterine leiomyoma
Objective. To identify the characteristics of the expression of natural cytotoxicity receptors (NCRs), a family of NCRs by natural killer (NK) cells that invade the endometrium that is located in the projection of the myomatous node, as well as the features of collagen synthesis in various types of uterine leiomyoma.Malyshkina A.I., Voskresenskaya D.L., Voronin D.N., Antsiferova Yu.S., Sotnikova N.Yu., Malyshkina D.A.
Subjects and methods. The expression of NCRs by NK cells was determined in 51 women with symptomatic uterine leiomyoma in the endometrium. Ki67 and COL1A1 mRNA levels were measured in myomatous node tissue.
Results. The endometrium located in the projection of the myomatous node shows changes in the expression of NCRs depending on the intensity of proliferative processes. The increased collagen synthesis in the small myomatous nodes is associated with minimal proliferative activity.
Conclusion. NK cells affect tumor growth processes, inhibiting the proliferation of leiomyocytes, by indirectly enhancing tissue collagen synthesis. The shift in the activation of endometrial NK cells may be one of the mechanisms of reproductive dysfunction in uterine fibroids.
Keywords
Uterine leiomyoma (UL) is the most common benign tumor of the reproductive system in women of reproductive age [1]. Recently, ULs have been increasingly affecting younger women, who planned their pregnancies [2] and there has been an increase in the number of pregnant women of late reproductive age with UL. Having all the signs of a benign tumor, UL consists of hyperplastic smooth muscle cells and excess deposition of the extracellular matrix, specifically collagen, fibronectin, and proteoglycans [3]. It is assumed that the combined effect of hormones, growth factors, and cytokines on uterine fibroids’ cells promote cell proliferation and the accumulation of collagen in the tissue. The evidence on the pathogenesis of this type of tumor supports a special role in immune disorders. Cytokines and growth factors in fibroids and surrounding tissues are produced and controlled by immunocompetent cells [4, 5]. However, studies investigating the role of natural killer (NK) cells in this disease are lacking. NKs mediate antitumor immunity. Their cytotoxicity depends on the activation of membrane receptors, including lectin-like receptors, killer immunoglobulin-like receptors (KIR), and natural cytotoxicity receptors (NCR) [6]. It was previously shown that with UL, regardless of the clinical variant, the activity of NKs invading the endometrium increases, especially rapid tumor growth was observed against the background of a decrease in the KIR expression by endometrial NKs [7]. It is believed that the NCR ligands are present on tumor cell membranes; activation of NKs through these receptors triggers a cascade of cytotoxic reactions [8]. However, the mechanisms of their functioning in UL are not fully understood.
This study aimed to identify the features of the expression of NCRs by endometrial natural killers located in the projection of fibroids and collagen synthesis in various uterine fibroid types.
Materials and methods
The study comprised 51 women aged 27–42 with symptomatic UL, who were examined at the Department of Endoscopy of the Gynecology Clinic, V.N. Gorodkov Research Institute of Maternity and Childhood. All patients underwent general clinical and special examination, including pelvic ultrasound, and subsequently, laparoscopic myomectomy. The control group included 10 patients who sought contraceptive counseling and had no proliferative uterine diseases at the time of the study. The study did not include patients with adenomyosis and hyperplastic endometrial process.
Patients were stratified into 2 groups based on the size of uterine fibroids. Groups 1 (n = 25) and 2 (n = 26) included women with fibroids >6 cm and <6 cm, respectively. Also, the study group was divided depending on the activity of proliferative processes occurring in the tissue of fibroids. According to the assessment of the proliferation marker (Ki67), 12 and 19 patients showed high and low levels of proliferation activity in the tissue of fibroids, respectively.
The material for molecular genetic research was surgical specimens of fibroid tissue and endometrial tissue taken in the projection of fibroids. Patients in the control group underwent pipelle endometrial biopsy to select a rational method of contraception, or to remove an intrauterine device. The study was approved by the local Research Ethics Committee (protocol No. 3 of 11/27/2017).
Endometrial mononuclear cells were isolated by a standard non-enzymatic mechanical technique. The expression of surface molecules was analyzed by multicolor flow cytometry using monoclonal anti-CD3, anti-CD56, anti-NKp44, and anti-NKp46 antibodies (eBioscience, USA). Total RNA was extracted from tissue samples by the phenol method and converted into cDNA (complementary DNA) using a commercial reverse transcription kit manufactured by Fractal Bio LLC (St. Petersburg, Russia). In the obtained samples, levels of mRNA expression of the cell proliferation marker (Ki67) and the collagen type I, alpha 1 chain (COL1A1) was determined by real-time quantitative polymerase chain reaction (PCR). We used sets of enzymes, primers, and probes produced by Fractal Bio LLC (St. Petersburg, Russia). The amplification reaction and data processing were performed using the iCycler iQ (BioRad, USA). The results of gene expression analysis are presented as the number of copies of a specific gene in a sample × 103/μl for ki67 and COL1A1 mRNA normalized to mRNA levels of housekeeping genes (β-actin).
Statistical analysis was performed using Microsoft Office 2010 and Statistica for Windows 6.0. Categorical variables were compared by the Chi-square test with the Yates correction if the expected count for a cell table was from 5 to 9; the Fisher exact test was used if the expected count for a cell table was less than 5. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov and Lilliefors tests. Most samples did not show normal distribution; therefore, comparing numerical data between groups was performed with a nonparametric Mann-Whitney test. All quantitative variables were expressed as means (M) and standard deviation (SD) and presented as M (SD). Differences between the groups were considered statistically significant at p < 0.05.
Results and discussion
All patients with UL were within reproductive age; their mean age was 37 (32; 40) years. The main clinical manifestations of UL uterus were chronic pelvic pain, abnormal uterine bleeding, such as profuse uterine bleeding, dysuria, and infertility. In 9.8% (n = 5) of women, heavy menstruation was associated with mild anemia. In all cases, infertility was a reason for seeking medical attention, and in 8% (n = 4) and 9.8% (n = 5) of cases, infertility was primary and secondary, respectively. 18% (n = 9) of women had a history of spontaneous abortion in the early gestational age. All women had ultrasound-detected subserous or intramural ULs. ULs sized >6 and <6 cm were found in 25 and 26 patients, respectively. Patients with large ULs were more likely to have dysuria (n = 7; 28%) than women with smaller ULs (n = 1; 4%) (p = 0.02). Sixty-three percent (n = 32) of women had solitary uterine fibroids. The mean ultrasound-measured volume of the fibroid or dominant fibroid in patients with multiple fibroids was 137.3 (47.0; 187.0) cm3.
The expression levels of the NKp44 (NCR2) and NKp46 (NCR1) NK receptors relative to all CD3-CD56 + endometrial lymphocytes in women with different types of ULs are presented in Table. 1. Compared with women in the control group, patients with ULs had a high expression level of the NKp46 NK receptor regardless of the fibroid size. The proportion of NKp44 + NKp46 + NK in the endometrium located in the projection of small fibroids was statistically significantly higher than in the control group. In fibroids with minimal proliferative activity, the level of NKp44 + and NKp44 + NKp46 + NK in the endometrial tissue was increased. Compared with the control group, the endometrium of small fibroids with high proliferative activity had a statistically significantly higher activity of NKp46 + receptors, while fibroids with low proliferative had increased activity of double-positive receptors NKp44 + NKp46 +. Large fibroids with a high intensity of proliferative processes were found to have a decrease in the expression of endometrial NKp44 + and NKp44 + NKp46 + NK receptors.
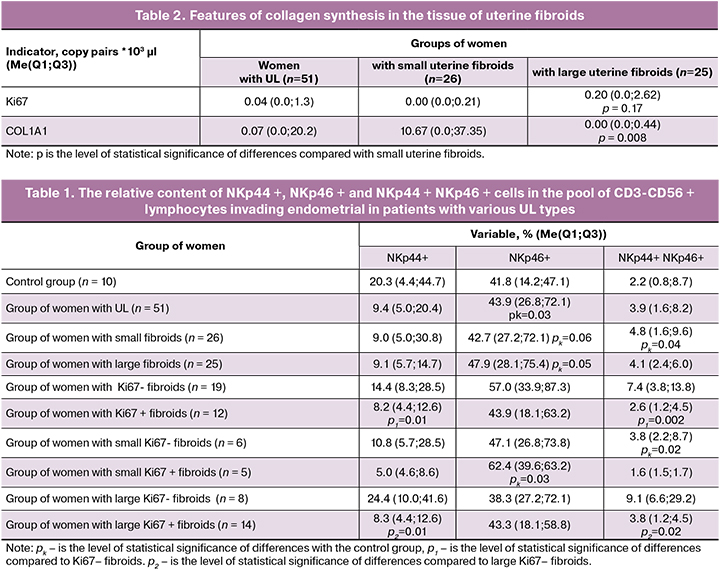
Data on the features of collagen synthesis in fibroids are presented in table. 2. Our findings showed that large fibroids had increased proliferative activity against a background of decreased collagen synthesis.
NCR receptors are known to be activated by tumor and virus-infected cells [9, 10]. Low expression of these receptors is associated with the progression of tumors of various locations, including the brain, stomach, liver, and skin [11].
Given the pronounced ability of NKp44 and NKp46 receptors to induce cytotoxicity reactions in NK, it can be assumed that a reduced level of endometrial NK expressing both receptors simultaneously leads to a breakdown of local cytotoxic reactions and a decrease in the antitumor response in uterine tissues. Insufficient cytotoxic activity of endometrial NKs results in a decreased limiting effect of immune responses on the tumor, which forms a favorable background for increasing the proliferative activity of tumor cells and the subsequent growth of the fibroid. Based on our findings, we have developed a new diagnostic method for testing the UL cells proliferative activity; a patent for invention No. 2700003 dated September 12, 2019, was granted. It has been hypothesized that the different ability of fibroids to grow is associated with the different cellular composition of small and large fibroids [12]. Increased expression of cytotoxicity receptors by endometrial NK inhibits the proliferation of leiomyocytes, while indirectly enhancing collagen synthesis, mainly in small fibroids. It has been previously shown that NK activation is accompanied by an increase in the calcium ion concentration, which is involved in intercellular adhesion and formation of connective tissue, synthesis of ECM components, in particular collagen, due to the stimulation of fibroblast division in the kidneys and heart [13, 14].
Inflammatory reactions and maintenance of precise cellular homeostasis are critical for normal defense mechanisms and tissue repair processes to proceed; any failure in regulatory mechanisms leading to chronic inflammation could result in the establishment of a conductive microenvironment favoring the initiation and progression of fibrinogenesis and tumorigenesis [15].
It is known that pregnancy occurs against the background of moderate activation of endometrial NK [16, 17]. Both an increase and a suppression of endometrial NKs caused by a growing fibroid can result in an imbalance in the production of cytokines that regulate implantation and formation of the placenta, which leads to spontaneous miscarriage in the early stages of pregnancy, and primary and secondary infertility. This fact is confirmed by our study, suggesting an increased incidence of infertility and miscarriage among patients with ULs. Impaired placental development against the background of uterine fibroids adversely affects the course and outcome of pregnancy, leading to complications such as toxemia of pregnancy, fetoplacental insufficiency, and fetal growth restriction [18].
Conclusion
Our findings suggest that the level of activation of endometrial natural killers varies depending on the proliferation activity of leiomyocytes. Marked activation of NCR expression in the NK population was found in fibroids with minimal proliferation. Changes in the activation of endometrial NKs may be one of the mechanisms underlying the pathogenesis of reproductive dysfunction in patients with UL.
References
- Sparic R., Mirkovic L., Malvasi A., Tinelli A. Epidemiology of uterine myomas: a review . Int J Fertil Steril. 2016; 9(4): 424-435.
- Тоноян Н.М., Козаченко И.Ф., Франкевич В.Е., Чаговец В.В., Адамян Л.В. Рецидивы миомы матки. Современный взгляд на проблемы диагностики, лечения и прогнозирования. Акушерство и гинекология. 2019; 3: 32–38. [Tonoyan N.M., Kozachenko I.F., Frankevich V.E., Chagovec V.V., Adamyan L.V. Recidivy miomy matki. Sovremennyj vzglyad na problemy diagnostiki, lecheniya i prognozirovaniya. Akusherstvo i ginekologiya. 2019; 3: 32–8. https://dx.doi.org/10.18565/aig.2019.3.32-38. (in Russian)]
- Fujisawa C., Castellot J. J. Jr. Matrix production and remodeling as therapeutic targets for uterine leiomyomaJ. Cell Commun. Signal. 2014; 8: 179–194. doi 10.1007/s12079-014-0234-x
- Малышкина А.И., Сотникова Н.Ю., Анциферова Ю.С., Красильникова А.К. Иммунные механизмы быстрого роста миомы матки. Иваново: ОАО Изд-во «Иваново», 2010. 272 с. [Malyshkina A.I., Sotnikova N.YU., Anciferova YU.S., Krasil’nikova A.K. Immunnye mekhanizmy bystrogo rosta miomy matki. Ivanovo: OAO Izd-vo «Ivanovo», 2010. 272s. (in Russian)]
- Chegini N. Proinflammatory and profibrotic mediators: Principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010; 28(3): 180–203. doi: 10.1055/s-0030-1251476.
- Mikhailova V, Belyakova K, Selkov S, Sokolov D. Peculiarities of NK cells differentiation: CD56dim and CD-56bright NK cells at pregnancy and in non-pregnant state. Med Immunol (Russia). 2017; 19(1): 19–26. doi: 10.15789/1563-0625-2017-1-19-26.
- Воронин Д.Н., Сотникова Н.Ю., Малышкина А.И., Лицова А.О., Анциферова Ю.С. Взаимосвязь особенностей активации эндометриальных CD56+ естественных киллеров с характером роста миоматозных узлов у пациенток с лейомиомой матки. Клиническая лабораторная диагностика. 2018; 63(2): 119–23. [Voronin D.N., Sotnikova N.YU., Malyshkina A.I., Licova A.O., Anciferova YU.S. Vzaimosvyaz’ osobennostej aktivacii endometrial’nyh CD56+ estestvennyh killerov s harakterom rosta miomatoznyh uzlov u pacientok s lejomiomoj matki. Klinicheskaya laboratornaya diagnostika. 2018; 63(2): 119–23.(in Russian)]
- Pazina T, Shemesh A, Brusilovsky M, Porgador A, Campbell K.S. Regulation of the Functions of Natural Cytotoxicity Receptors by Interactions with Diverse Ligands and Alterations in Splice Variant Expression. Front Immunol. 2017; 30(8): 369. doi: 10.3389/fimmu.2017.00369
- Vivier E., Ugolini S., Blaise D., Chabannon C., Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nature Reviews Immunology. 2012; 12(4): 239–52. doi: 10.1038/nri3174.
- Seidel E., Glasner A., Mandelboim O. Virus-mediated inhibition of natural cytotoxicity receptor recognition. Cellular and Molecular Life Sciences. 2012; 69(23): 3911–20. doi: 10.1007/s00018-012-1001-x.
- Koch J., Steinle A., Watzl C., Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013; 34(4): 182–91. doi: 10.1016/j.it.2013.01.003.
- Holdsworth-Carson S. J., Zhao D., Cann L., Bittinger S., Nowell C. J., Rogers P. A. Differences in the cellular composition of small versus large uterine fibroids. Reproduction. 2016; 152(5): 467–480. doi: 10.1530/REP-16-0216
- Toka H.R. New functional aspects of the extracellular calcium-sensing receptor. Curr Opin Nephrol Hypertens. 2014; 23(4): 352–360. doi: 10.1097/01.mnh.0000447016.21228.e0
- Zhang X., Zhang T., Wu J., Yu X., Zheng D., Yang F., et al. Calcium sensing receptor promotes cardiac fibroblast proliferation and extracellular matrix secretion. Cell Physiol Biochem. 2014; 33(3): 557–568. doi: 10.1159/000358634
- Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010; 28(3): 180–203. doi: 10.1055/s-0030-1251476.
- Fukui A., Kamoi M., Funamizu A., Fuchinoue K., Chiba H., Yokota M., et al. NK cell abnormality and its treatment in women with reproductive failures such as recurrent pregnancy loss, implantation failures, preeclampsia, and pelvic endometriosis. Reprod Med Biol. 2015; 14: 151–7. doi 10.1007/s12522-015-0207-7
- Sotnikova N., Voronin D., Antsiferova Y., Bukina E. Interaction of decidual CD56+ nk with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation. Scan J Immunol. 2014; 80(3): 198–208. doi: 10.1111/sji.12196.
- Петров Ю.А., Оздоева И.М-Б., Султыгова Л.А., Прокопцова А.А. Беременность и роды при фибромиоме матки. Международный журнал прикладных и фундаментальных исследований. 2019; 3: 76–80. [Petrov YU.A., Ozdoeva I.M-B., Sultygova L.A., Prokopcova A.A. Beremennost’ i rody pri fibromiome matki. Mezhdunarodnyj zhurnal prikladnyh i fundamental’nyh issledovanij. 2019; 3: 76-80. (in Russian)]
Received 18.11.2019
Accepted 29.11.2019
About the Authors
Anna I. Malyshkina, doctor of medical sciences, Professor, Director, Federal State Institution Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova the Ministry of Health of the Russian Federation. Tel.: +7 (4932) 33-83-20. E-mail: ivniimid@inbox.ru153045, Russia, Ivanovo, Pobedy str. 20.
Daria L. Voskresenskaya, graduate student of the laboratory of clinical immunology, Federal State Institution Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova the Ministry of Health of the Russian Federation. Tel.: +7(4932)33-69-28. E-mail: kasyanikdariakis@mail.ru
153045, Russia, Ivanovo, Pobedy str. 20.
Daria A. Malyshkina, MD, student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of the Russian Federation. Tel.: +79158156790. E-mail: nidsumi@mail.ru
117997, Russia, Moscow, Ac. Oparina str. 4.
Dmitry N. Voronin, candidate of biological sciences, senior researcher of the laboratory of clinical immunology, Federal State Institution Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova the Ministry of Health of the Russian Federation. Tel.: +7 (4932)33-69-28. E-mail: niimid.immune@mail.ru
153045, Russia, Ivanovo, Pobedy str. 20.
Yulia S. Antsiferova, doctor of biological sciences, leading scientific researcher of the laboratory of clinical immunology, Federal State Institution Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova the Ministry of Health of the Russian Federation. Tel.: +7 (4932)33-69-28. E-mail: niimid.immune@mail.ru
153045, Russia, Ivanovo, Pobedy str. 20.
Natalia Yu. Sotnikova, doctor of medical sciences, Professor, head of the laboratory of clinical immunology Ivanovo Research Institute of Maternity and Childhood named V.N. Gorodkova the Ministry of Health of the Russian Federation, Tel.: +7(4932)33-69-28. E-mail: niimid.immune@mail.ru
153045, Russia, Ivanovo, Pobedy str. 20.
For citation: Malyshkina A.I., Voskresenskaya D.L., Voronin D.N., Antsiferova Yu.S., Sotnikova N.Yu., Malyshkina D.A. Immune mechanisms of regulating the growth of uterine leiomyoma.Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 2: 111-5.(In Russian).
https://dx.doi.org/10.18565/aig.2020.2.111-115



