Characteristic features of blood circulation and angiogenesis in patients of reproductive age with uterine myoma, adenomyosis and intrauterine septum: functional MRI-based assessment of expression levels of angiogenesis markers in the endometrium
Objective. To evaluate characteristic features of blood circulation and angiogenesis in patients of reproductive age with uterine myoma, nodular adenomyosis and intrauterine septum using functional MRI-based assessment of the level of expression of angiogenesis markers in the endometrium.Kozachenko I.F., Makiyan Z.N., Fayzullina N.M., Bychenko V.G., Shchegolev A.I., Adamyan L.V.
Materials and methods. 862 patients with benign uterine diseases and infertility were examined and surgically treated before the IVF program. Among them 200 patients were with nodular adenomyosis, 510 patients – with uterine fibroids, 152 patients – with intrauterine septum. Infertility was treated in accordance with the IVF protocol. Histological and immunohistochemical (IHC) analysis of endometrial biopsy samples during the "implantation window" in cycles before surgical treatment and before IVF was performed in 30 patients in the main groups and in 10 patients with tubal-peritoneal infertility factor without endometrial and myometrial pathology. Magnetic resonance imaging (MRI) was performed using magnetic induction field of 3T and gadolinium contrast agent.
Results. IHC analysis of endometrial biopsy samples showed statistically significant differences in the expression of angiogenesis markers in the endometrium (vascular endothelial growth factor A (VEGF-A), atrix metalloproteinases (MMP-2.9), αvβ3-integrin) in patients before and after surgical treatment and in the control group. In patients with intrauterine septum, blood flow in intrauterine septum decreased by more than 30%, which was comparable to a high level of pregnancy losses in this group (84%) and expression level of receptivity markers in the endometrium, in particular, – angiogenesis. Functional MRI was used to assess blood flow in the myometrium, myoma nodes and adenomyosis, and to determine the structure and mapping of myoma nodes and adenomyosis.
Conclusion. In patients with adenomyosis, uterine fibroids, and intrauterine septum, there was a lower expression of angiogenesis markers (VEGF, MMP 2,9, and avß3-integrin) in the endometrium before surgery compared to the expression of these markers after surgery and to the control group (without endometrial and myometrial pathology). The method of functional contrast-enhanced MRI allows to assess the blood flow in different myometrial loci and intrauterine septum, reliably to assess the blood flow in different myometrial pathologies, including uterine myoma, adenomyosis, to determine the structure and mapping of myoma nodes and adenomyosis.
Keywords
Vascular bed is the most important component of the stroma of any organ, it ensures functional support to all metabolic processes. The occurrence of pathological conditions leads to structural and functional changes, which necessitate adaptive restructuring of vascular bed.
Formation of blood cells, which carry nutrients and oxygen, underlies the most physiological and pathological processes. On the one part, the active growth of blood vessels accompanies the normal growth and development of an organism in the prenatal and postnatal periods, wound healing, development of the placenta and yellow body. On the other part, it gives rise to oncological diseases [1, 2].
Neoangiogenesis, including vasculogenesis and angiogenesis is the major process in restructuring the vascular bed [3, 4]. Svetozarsky N.L. et al. (2015) [4] defined angiogenesis as a complex of morphogenetic process, which involves proteolytic "rupture" of blood vessel basement membranes and the extracellular matrix around the capillaries mainly due to increased activity of matrix metalloproteinases (MMPs) leading to migration, attachment and proliferation of endothelial cells to the extravascular space; then the tubular structures and anastomoses with the nearest blood vessels are formed; and blood flow through the newly formed capillaries is initiated. Vacculogenesis is a process of blood vessel formation from mesenchymal cells at the early stage of embryogenesis or endothelial precursor cells migrating from the red bone marrow in the postnatal period.
Physiological angiogenesis in female reproductive organs is manifested in the cyclical (development of follicles) or continuous growth of blood vessels (transformation of the endometrium during entire menstrual cycle, as well as during pregnancy [4]. New blood vessel formation occurs in the endometrium due to the existing vessels with subsequent maturation and restructuring. During embryo attachment, expression of angiogenic factors includes differentiation of angioblasts with formation of vessels from progenitor stem cells [3]. Currently, a number of activating and inhibiting endogenous regulators of angiogenic markers have been studied. Among them, the most important are VEGF, integrins, matrix metalloproteinases (MMPs) and a number of others [5–7].
Vascular endothelial growth factor (VEGF) and its receptors play a fundamental role in regulation of angiogenesis. The VEGF family includes several factors (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E). VEGF-A is a major regulator of angiogenesis. VEGF-B is involved in the degradation of the extracellular matrix, cell adhesion and migration. VEGF-C and VEGF-D are required for lymphangiogenesis [6, 7]. VEGF-A is one of the most studied factors of angiogenesis and is considered a potential target for the targeted therapy of a number of diseases [6].
Integrins are heterodimeric transmembrane proteins composed of 2 subjunits non-covalently linked to each other. They interact with the components of the extracellular matrix and transmit intracellular signals. The major integrins that are expressed on endothelial cells surface include α1β1, α2β1, α5β1, αvβ3, α6β1 and α6β4. Integrin αvβ3 is a receptor for a wide range of molecules of extracellular tissue structures. There is an insignificant expression level of integrin αvβ3 in the membrane of inactive endothelial cells, but in endothelial cells activated during angiogenesis its level is significantly high [7]. Complete angiogenic program implementation is possible in obligatory contact between integrin αvβ3 and vascular endothelial growth-factor receptor VEGF-2 at the molecular level during the growth of new vessels [5].
MMPs belong to the family of zinc metalloprotenases associated with protein metabolism of the extracellular matrix. These enzymes determine the development, degradation and changes in the structure of tissues, coordinated migration, coming into contact, differentiation and cell proliferation, as well as are involved in a number of pathological conditions. Much research has been devoted to study their role in various processes. Significant changes in expression of MMPs were noted in the structures that were subjected to intense cyclic restructuring, such as human endometrium. In epithelial cells of human endometrium, the activity of MMPs increases in the proliferative late secretory and menstrual phases of the cycle, when the structure of the endometrium changes along with a high level of estrogen compared to progesterone level [6, 8].
Being key enzymes in the metabolism of connective tissue components, MMPs are involved in many physiological and pathological processes, which require cell proliferation and migration, and therefore, remodeling of the extracellular matrix [8, 9]. MMPs regulate activation of various growth factors: vascular endothelial growth factor, fibroblast growth factor receptor, epithelial growth factor and insulin-like growth factor. It was found, that adequate levels of MMP-2 and MMP-9 expression in the endometrium are necessary for successful implantation and normal pregnancy.
Until recently, the main method for assessment of angiogenesis was microvessel optical density measurement using standard immunoperoxidase technique for staining vessels with specific markers for endothelial cells. However, another technique – dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was put into practice, and the parameters of this technique reflected angiogenesis and correlated with VEGF expression [10].
Introduction and development of technologies in diagnostic imaging comprise the steps from the study of the anatomical structure to the study of the functional state. Modern techniques of MRI make it possible to assess the speed and direction of diffusion of water molecules, to identify tissues that differ in chemical and molecular structure, to assess perfusion, oxygenation and metabolic activity of tissues. Perfusion MRI with the use of contrast agents significantly increase the results of research [11].
Standard contrast-enhanced MRI (DCE-MRI) with acquisition of sequential images before and after contrasting due to the effect of accumulation of contrast allows to assess the size, boundaries of various neoplasms, invasion degree and characteristic features of blood circulation [12–14].
Recently, dynamic contrast-enhanced MRI (DCE-MRI) – a technique for sequential acquisition of multiple visual field maps in the zones of clinical interest prior to and after injection of the contrast agent is widely used [14–16].
Research results can be presented both in the form of parametric color maps and in the form of line charts. DCE-MRI and non-contrast arterial spin labelling (ASL) MRI) allow to vizualize tissue perfusion. Blood oxygen level-dependent (BOLD) contrast-based functional MRI (fMRI) and oxygen-enhanced MRI (OE-MRI) allow to assess tissue oxygenation. Diffusion-weighted magnetic resonance imaging (DWI) measures diffusion of the water molecules in the tissue. Multidirectional diffusion-weighted MRI (MDDW) allows to assess not only the speed, but also the direction of diffusion [11].
Dynamic contrast-enhanced MRI is one of the techniques for studying perfusion. It is based on intravenous bolus injection of a paramagnetic contrast agent to evaluate tissue perfusion, which quantifies blood flow in tissues.
Aim of the study: to evaluate characteristic features of blood circulation and angiogenesis in patients of reproductive age with uterine myoma, nodular adenomyosis and intrauterine septum using functional MRI-based assessment of the level of expression of angiogenesis markers in the endometrium.
Material and methods
862 patients underwent endoscopic surgery before the IVF program in the Gynecologic Department (Head of the Department – Adamyan L.V., Academician of RAS, M.D., Professor) of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Among them, 200 patients were with nodular adenomyosis, 510 patients – with uterine myoma, 152 patients – with intrauterine septum. Histological and immunohistochemical (IHC) analysis of endometrium was performed in the 1st Department of Anatomical Pathology (Head of the Department – Shchegolev A.I., M.D., Professor).
Immunohistochemical analysis was performed by the standard method using monoclonal antibodies to integrins αV/β3, VEGF-A, MMP9, MMP2 [18].
MRI was performed using magnetic induction field of 3T in X-ray Department (Head of the Department – Bychenko V.G., Ph.D.). Gadolinium-based paramagnetic contrast agent – Gadodiamide was used according to the standard technique.
Treatment of all patients for infertility was performed according to IVF protocol with controlled ovarian stimulation and embryo transfer into the uterine cavity or cryoprotocol with transfer of previously cryopreserved embryos into the uterine cavity in a cycle. Clinical and laboratory tests and infertility treatment were performed in accordance with the Order No 107n of 30.09.2019 of the Ministry of Health of Russia in B.V. Leonov Department of Assisted Reproductive Technologies (Head of the Department – Professor Kalinina E.A.) [17].
Statistical analysis
Statistica 10.0 software (USA) for statistical analysis in Microsoft Excel was used in compliance with the guidelines for medical and biological research. The Shapiro–Wilk test was used to evaluate a normal distribution. Aggregate data of quantitative indicators, the distribution of which differed from normal were described using the values of the median (Me) and the lower and upper quartiles (Q1–Q3). The Mann– Whitney U-test was used to compare independent samples, and the Wilcoxon W-test was used to compare dependent samples. The differences were statistically significant at p <0.05 [17].
Results
The mean age of the patients with nodular adenomyosis (ADM) was 35.9±0.5 years, with uterine myoma (UM) – 36.2±5.68 years, with uterine malformations (intrauterine septum) – 31.2±5.4 years.
During examination and treatment, combined gynecologic pathology with benign uterine diseases was detected in majority of patients: adenomyosis was in 67% of patients, myoma – in 58% of patients, uterine malformations – in 48.6% of patients. Most often, external genital endometriosis and different endometrial pathologies were detected in all groups.
Indications for surgical treatment of patients with adenomyosis were: heavy menstrual period (70.8%), persistent pain syndrome (48%), infertility (100%), as well as ineffectiveness of previously performed conservative therapy, contraindications for IVF and in medical history. After surgery and subsequent IVF program pregnancy occurred in 45.6% of patients resulting in childbirth in 36.7% of cases.
Indications for surgical treatment of patients with myoma were: specific clinical manifestations of the disease: heavy (73.1%) and long (54.9%) menstrual periods, lower abdominal pain (39%), marked growth of myoma nodules (29.2%). large sizes of myoma nodules or uterus (25.8%), including in combination with dysfunction of pelvic organs (22.2%). After surgery and subsequent IVF program pregnancy occurred in 44.3% of patients. Total pregnancy rate was higher in the group of patients who underwent endoscopic myomectomy (45%) compared to the rate of pregnancies in the group of patients, who underwent laparoscopic myomectomy (40.0%). In most cases, pregnancies resulted in childbirths (58.8%). This exceeded by 1.4 times the overall proportion (41.6%) of unfavorable pregnancy outcomes (ectopic pregnancy, abortion, spontaneous miscarriage at different terms).
The patients with intrauterine septum complained of infertility (61.8%), miscarriage in medical history (85.5%), painful menstrual periods (13.8%). All patients with intrauterine septum underwent laparoscopic and hysteroscopic surgery for verification and correction of uterine malformations, as well as elimination of concomitant gynecological pathology. Histeroscopic surgery for the dissection of intrauterine septum was performed using monopolar loop electrodes until a regular uterine cavity was formed. After surgical treatment and IVF program, pregnancy occurred in 77% of patients. 57.9% of pregnancies resulted in childbirths. The results of IHC analysis of the angiogenesis markers before and after surgery compared to the control group are shown in the Table [17].
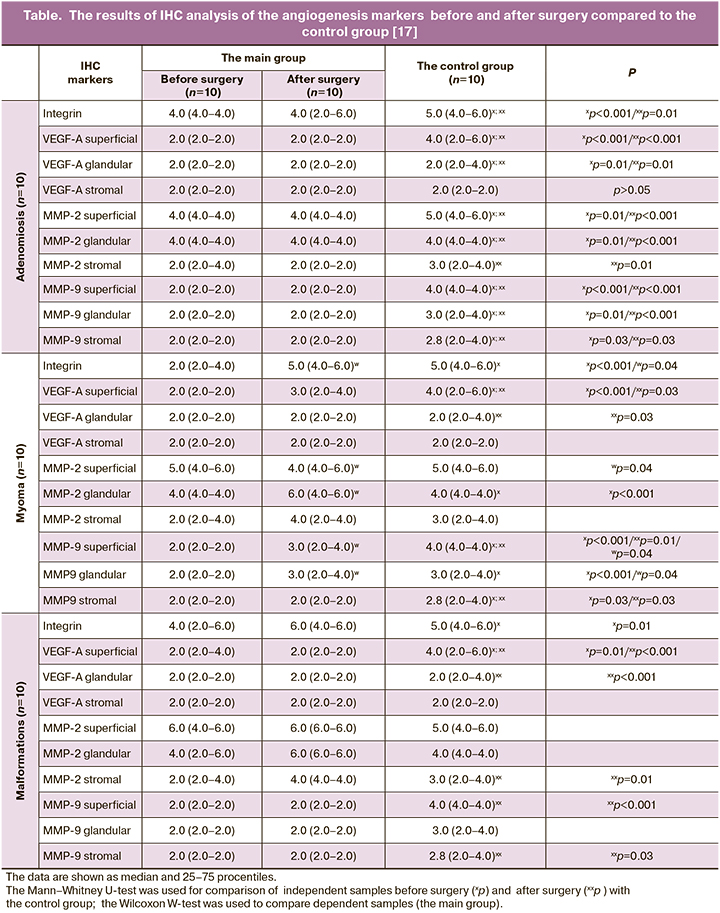
In the group of patients with adenomyosis, the analysis of integrin αvβ3 expression showed that the expression level in the main groups was average (4.0) and statistically significantly lower before surgery compared to the control (5.0, p=0.005). Expression of superficial VEGF-A was weak in the main groups compared to the control group (2.0; 2.0; 4.0, respectively, p=0.001), while cytoplasmic expression in the glandular epithelium and in the vascular endothelium did not differ. Moderate MMP-2 expression was observed in the cytoplasm of epithelial superficial (4.0) and glandular epithelial cells (4.0). Weak expression (2.0) was in the cytoplasm of epithelial stromal cells in patients in the main groups compared to the control group. Moderate expression of MMP-9 marker was in the cytoplasm of epithelial superficial (5.0) and glandular epithelial cells (4.0), and slightly reduced expression was in the cytoplasm of epithelial stromal cells (3.0).
In the group of patients with uterine myoma, integrin expression was low (2.0) before surgery compared to the other groups (6.0, p=0.001). Expression of VEGF-A on the cell surface was statistically significantly weak compared to the control group (2.0; 3.0; 4.0, respectively, p=0.001). Low expression of matrix metalloproteinases in the cytoplasm of epithelial superficial and glandular epithelian cells was in patients before surgery compared to the other groups (p=0.01).
In the group of patients with intrauterine septum, integrin expression was moderate before surgery (4.0) and reduced in comparison to expression in the control group (6.0, p=0.025), and it was low compared to the group of patients after surgery (p>0.05). Low expression of superficial VEGF-A was observed compared to its average expression level in the control group (2.0; 2.0; 4.0, respectively, p=0.01 and p=0.001). In the group of patients with uterine malformations before and after surgery, ММР-2 expression was high (6.0) in the cytoplasm of epithelial superficial cells; after surgery, expression (6.0) of ММР-2 was high (6.0) in the cytoplasm of glandular epithelial cells, and it was moderate (4.0) in the cytoplasm of epithelial stromal cells. The difference was statistically significant in comparison of the results before surgery: average expression (4.0) of ММР-2 was in the cytoplasm of epithelial superficial cells and low expression (2.0) was in the cytoplasm of epithelial stromal cells (p=0.01). ММР-9 expression was low in the main group compared to moderate expression in the control group. Comparison of ММР-9 expression in epithelial superficial and stromal cells in the group before surgery and in the control group was statistically significant (p=0.01).
 Clinical case 1
Clinical case 1
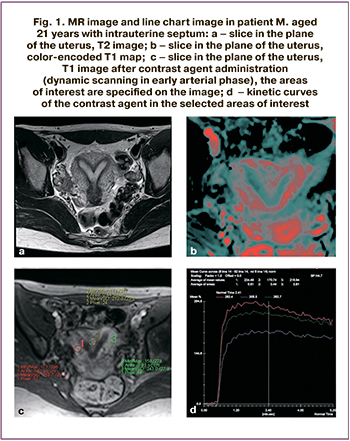
The patient M, aged 21 years referred with complains of the absence of pregnancy, painful menstrual periods, spotting in the middle of the cycle, lower abdominal pain. MRI was used to clarify the type of uterine malformation. Intrauterine septum with a wide base was visualized. Conclusion: MRI visualization of incomplete intrauterine septum.
DCE-MRI was performed. The zones of interest were specified (Fig. 1c), and blood flow was analyzed. The zone of interest in the intrauterine septum is marked with red marker, and zone of interest in myometrium – with yellow marker. The chart (Fig. 1d) shows blood flow parameters in the selected zones of interest and the rates of accumulation and excretion of contrast agent. During the study, a decrease in blood flow parameters by 30% or more was observed in the area of the intrauterine septum (red curve) compared to the myometrium (yellow curve). This is also seen in color map (Fig. 1b).
Clinical case №2
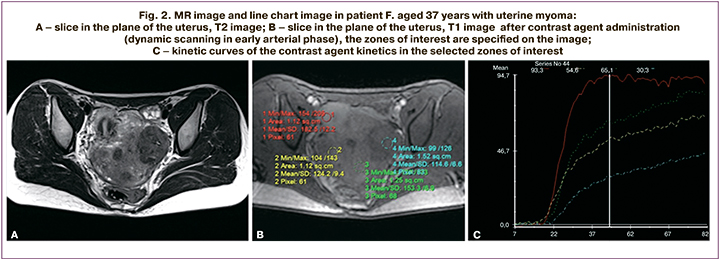
The patient F., aged 37 years was worried about infertility, heavy menstrual bleeding, failed IVF in medical history. The uterus was anteverted, it had irregular shape, and was of size 48×46×52 mm. Thickness of functional layer was up to 4 mm, and thickness of mucous membrane was up to 5 mm. In the thickness of the myometrium and submucosal layer (mainly in the area of the uterine fundus and posterior wall), multiple myoma nodules of size 29×28×28 mm were found (the largest intramural node in the posterior wall deformed the uterine cavity). Besides, in the uterine isthmus along the right side of anterior wall, a single subserous nodule of size 21×20×23 mm was found. The right ovary of size 20×20×13 mm was normally located and had single fluid inclusions. The left ovary of size 20×20×13 mm was normally located and had multiple liquid inclusions. Conclusion: MR imaging visualized multiple intramural, submucosal and subserosal myomas.
Axial, frontal and slice orientations were used. The slices were 3–4 mm thick. The study was carried out in the patient with incomplete bladder. The contrast agent was evaluated using graphs (Fig. 2). The readings of the images of contrast agent kinetics in myoma correlate with the kinetics in the unchanged myometrium (blue curve) depending on blood flow velocity estimation and, accordingly, contrast agent accumulation and washout in the pathological tissue and the patient-specific blood flow. Figures 2a and 2b show clinical cases with blood flow estimation in myoma nodules. Visual color maps were built (Fig. 2c) and estimated with regard to the unchanged myometrium, contrast agent accumulation rate (color-coded WashIn map), contrast agent washout rate (WashOut), time maximum of intensity projection (color-coded MIP map), positive enhancement integral (color map of PEI). Myoma nodules accumulate the contrast agent (the nodule in the posterior wall with the curve of contrast agent kinetics specific for hypervascular nodules, the subserous nodule with the curve of contrast agent kinetics specific for of intermediate vascularization nodes). There were no other areas of pathological accumulation of the contrast agent in the studied zone.
Discussion
Low uterine blood flow in patients with uterine myoma due to adenomyosis and intrauterine septum leads to impairment of microcirculation, endometrial receptivity and implantation of chorion and causes reproductive disorders (loss of pregnancy and infertility).
In cases of adenomyosis, uterine myoma and intrauterine septum, low expression of angiogenesis markers (VEGF, MMPs 2,9 and integrin αvβ3) was in the endometrium before surgery compared to expression of these markers in patients after surgery and in the control group (without endometrial and myometrial pathology). The data were consistent with the results obtained in other studies [6, 18].
DCE-MRI allows evaluation of the blood flow using kinetic curves of the contrast agent and visualization using color maps.
In cases of intrauterine septum, DCE-MRI allows to study the blood flow loci in the myometrium and intrauterine septum, and choose the tactics for surgical treatment and management of pregnancy depending on status indicators. In the previous study we found, that reduced blood flow to the intrauterine septum by more than 30% was associated with an increased frequency of reproductive losses in most cases (72%) [11].
Conclusion
DCE-MRI reliably allows to assess the blood flow in the myometrium in different pathologies, including uterine myoma, adenomyosis, and to determine the structure and mapping of myoma and adenomyosis nodules. This technique allows to develop recommendations for treatment tactics and planning, depending on the assessed tissue perfusion.
This study is being continued.
References
- Hickey M., Fraser I. Human uterine vascular structures in normal and diseased states. Microsc. Res. Tech. 2003; 60(4): 377-89. https://dx.doi.org/10.1002/jemt.10276.
- Украинец Р.В., Корнева Ю.С. Ремоделирование сосудистого русла эндометрия у женщин репродуктивного возраста в норме и при патологии (обзор литературы). Проблемы репродукции. 2018; 24(5): 27-32. [Ukrainets R.V., Korneva Yu.S. Remodeling of endometrial vascular system in woman of reproductive age in normal and pathological condition (a review). Problemy Reproduktsii/ Problems of reproduction. 2018; 24(5): 27-32. (in Russian)]. https://doi.org/10.17116/repro20182405127.
- Кушлинский Д.Н., Терешкина И.В., Дегтярь В.Г., Лактионов К.П., Адамян Л.В. Фактор роста эндотелия сосудов и его рецепторы при раке яичников. Молекулярная медицина. 2013; 1: 3-11. [Kushlinsky D.N., Tereshkina I.V., Degtyar V.G., Laktionov K.P., Adamyan L.V. Vascular endothelial growth factor and its receptors in ovarian cancer. Molecular medicine. 2013; 1: 3-11. (in Russian)
- Светозарский Н.Л., Артифексова А.А., Светозарский С.Н. Фактор роста эндотелия сосудов: биологические свойства и практическое значение (обзор литературы). Медицина и образование в Сибири. 2015; 5: 24. [Svetozarsky N.L., Artifeksova A.A., Svetozarsky S.N. Vascular endothelial growth factor: biological properties and practical significance (literature review). Journal of Siberian Medical Sciences. 2015; 5: 24. URL: https://cyberleninka.ru/article/n/faktor-rosta-endoteliya-sosudov-biologicheskie-svoystva-i-prakticheskoe-znachenie-obzor-literatury/ (in Russian)].
- Риппа А.Л., Воротеляк Е.А., Васильев А.В., Терских В.В. Роль интегринов в формировании и гомеостазе эпидермиса и придатков кожи. Acta Naturae. 2013; 5(4): 24-36. [Rippa A.L., Vorotelyak E.A., Vasiliev A.V., Terskikh V.V. The role of integrins in the formation and homeostasis of the epidermis and skin appendages. Acta Naturae. 2013; 5(4): 24-36. URL: https://cyberleninka.ru/article/n/rol-integrinov-v-formirovanii-i-gomeostaze-epidermisa-i-pridatkov-kozhi. (in Russian)].
- Никитина Л.А., Демидова Е.М., Радзинский В.Е., Демидов Б.С., Самоходская Л.М. Молекулярные основы регуляции имплантации и плацентации. Вопросы гинекологии, акушерства и перинатологии. 2007; 6(3): 43-8. [Nikitina L.A., Demidova E.M., Radzinsky V.E. et al. Molecular basis of implantation and placentation regulation. Questions of gynecology, obstetrics and perinatology. 2007; 6(3): 43-8. (in Russian)].
- Иванов А.Н., Норкин И.А., Пучиньян Д.М., Широков В.Ю., Жданова О.Ю. Адгезивные молекулы эндотелия сосудистой стенки. Успехи физиологических наук. 2014; 45(4): 35-49. [Ivanov A.N., Norkin I.A., Puchinyan D.M. et al. Adhesive molecules of the vascular wall endothelium. Uspekhi fiziologicheskikh nauk/Advances in physiological sciences. 2014; 45(4): 35-49. (in Russian)].
- Рогова Л.Н., Шестернина Н.В., Замечник Т.В., Фастова И.А. Матриксные металлопротеиназы, их роль в физиологических и патологических процессах (обзор). Вестник новых медицинских технологий. 2011; 18(2): 86-9. [Rogova L.N., Shesternina N.V., Zametnik T.V., Fastova I.A. Matrix metalloproteinases, their role in physiological and pathological processes (review). Bulletin of new medical technologies. 2011; 18(2): 86-9. URL: https://cyberleninka.ru/article/n/matriksnye-metalloproteinazy-ih-rol-v-fiziologicheskih-i-patologicheskih-protsessah-obzor. (in Russian)].
- Потеряева О.Н. Матриксные металлопротеиназы: строение, регуляция, роль в развитии патологических состояний (обзор литературы). Медицина и образование в Сибири. 2010; 5: 7. [Poteryaeva O.N. Matrix metalloproteinases: structure, regulation, role in the development of pathological conditions (literature review). Journal of Siberian Medical Sciences. 2010; 5: 7. URL: https://cyberleninka.ru/article/n/matriksnye-metalloproteinazy-stroenie-regulyatsiya-rol-v-razvitii-patologicheskih-sostoyaniy-obzor-literatury. (in Russian)].
- Thomassin-Naggara I., Bazot M., Daraï E., Callard P., Thomassin J., Cuenod C.A. Epithelial ovarian tumors: value of dynamic contrast-enhanced MR imaging and correlation with tumor angiogenesis. Radiology. 2008; 248(1): 148-59. https://dx.doi.org/10.1148/radiol.2481071120.
- Макиян З.Н., Адамян Л.В., Быченко В.Г., Мирошникова Н.А., Козлова А.В. Функциональная магнитно-резонансная томография для определения кровотока при симметричных аномалиях матки. Акушерство и гинекология. 2016; 10: 73-9. [Makiyan Z.N., Adamyan L.V., Bychenko V.G., Miroshnikova N.A., Kozlova A.V. Functional magnetic resonance imaging for determining blood flow in symmetrical uterine anomalies. Obstetrics and gynecology. 2016; 10: 73-9. (in Russian)]. http://dx.doi.org/10.18565/aig.2016.10.73-9.
- Thomassin-Naggara I., Siles P., Balvay D., Cuenod C.A., Carette M.F., Bazot M. MR perfusion for pelvic female imaging. Diagn. Interv. Imaging. 2013; 94(12): 1291-8. https://dx.doi.org/10.1016/j.diii.2013.06.004.
- Wu L.M., Xu J.R., Gu H.Y., Hua J., Haacke E.M., Hu J. Predictive value of T2-weighted imaging and contrast-enhanced MR imaging in assessing myometrial invasion in endometrial cancer: a pooled analysis of prospective studies. Eur. Radiol. 2013; 23(2): 435-49. https://dx.doi.org/10.1007/s00330-012-2609-9.
- Kundu S., Chopra S., Verma A., Mahantshetty U., Engineer R., Shrivastava S.K. Functional magnetic resonance imaging in cervical cancer: current evidence and future directions. J. Cancer Res. Ther. 2012; 8(1): 11-8. https://dx.doi.org/10.4103/0973-1482.95167.
- Wu L.M., Xu J.R., Gu H.Y., Hua J., Haacke E.M., Hu J. Predictive value of T2-weighted imaging and contrast-enhanced MR imaging in assessing myometrial invasion in endometrial cancer: a pooled analysis of prospective studies. Eur. Radiol. 2013; 23(2): 435-49. https://dx.doi.org/10.1007/s00330-012-2609-9.
- Punwani S. Contrast enhanced MR imaging of female pelvic cancers: established methods and emerging applications. Eur. J. Radiol. 2011; 78(1): 2-11. https://dx.doi.org/10.1016/j.ejrad.2010.03.010.
- Козаченко И.Ф., Файзуллина Н.М., Щеголев А.И., Адамян Л.В. Рецептивность эндометрия у больных с доброкачественными заболеваниями матки в сочетании с бесплодием до и после оперативного лечения. Акушерство и гинекология. 2020; 11: 147-58. [Kozachenko I.F., Fayzullina N.M., Shchegolev A.I., Adamyan L.V. Endometrial receptivity in patients with benign uterine diseases in combination with infertility before and after surgical treatment. Obstetrics and gynecology. 2020; 11: 147-58. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.147-158.
- Ниаури Д.А., Гзгзян А.М., Кветной И.М., Коган И.Ю., Джемлиханова Л.Х., Крихели И.О., Федорова И.Д., Лесик Е.А., Шарфи Ю.Н., Крылова Ю.С., Шильникова Е.М. Иммуногистохимическая характеристика рецептивности эндометрия в циклах ЭКО. Акушерство и гинекология. 2014; 9: 44-50. [Niauri D.A., Gzgzyan A.M. et al. Immunohistochemical characteristics of endometrial receptivity in IVF cycles. Obstetrics and Gynecology. 2014; 9: 44-50. (in Russian)].
Received 26.02.2021
Accepted 02.03.2021
About the Authors
Irena F. Kozachenko, Ph.D., leading researcher of the Gynecological Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(910)419-97-14. E-mail: i_kozachenko@oparina4.ru. ORCID: 0000-0003-1822-9164. 117997, Russia, Moscow, Ac. Oparin str., 4.Zograb N. Makiyan, Dr. Med. Sci., leading researcher of the Gynecological Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-77-83. E-mail: z_makiyan@oparina4.ru. ORCID: 0000-0002-0463-1913.
117997, Russia, Moscow, Ac. Oparin str., 4.
Nafisa M. Fayzullina, PhD, senior researcher of the Pathology Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-23-11. E-mail: n_faizullina@oparina4.ru. ORCID: 0000-0003-1804-8523. 117997, Russia, Moscow, Ac. Oparin str., 4.
Vladimir G. Bychenko, PhD, Head of the Department of Radiation Diagnostics, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-23-11. E-mail v_bychenko@oparina4.ru. ORCID: 0000-0002-1459-4124.
117997, Russia, Moscow, Ac. Oparin str., 4.
Aleksandr I. Shchegolev, Dr. Med. Sci., Professor, Head of 2nd Pathology Department, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)531-44-44. E-mail: ashegolev@oparina4.ru. ORCID: 0000-0002-2111-1530.
117997, Russia, Moscow, Ac. Oparin str., 4.
Leila V. Adamyan, Dr. Med. Sci., Professor, Academician of the Russian Academy of Sciences, Head of the Gynecological Department, Deputy Scientific Director, V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(495)438-7783. E-mail: aleila@inbox.ru.
ORCID: 0000-0002-3253-4512. 117997, Russia, Moscow, Ac. Oparin str., 4.
For citation: Kozachenko I.F., Makiyan Z.N., Fayzullina N.M., Bychenko V.G., Shchegolev A.I., Adamyan L.V. Characteristic features of blood circulation and angiogenesis in patients of reproductive age with uterine myoma, adenomyosis and intrauterine septum: functional MRI-based assessment of expression levels of angiogenesis markers in the endometrium.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 101-109 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.101-109



