Chronic cervicitis associated with human papillomavirus and markers of inflammation in women of reproductive age
Objective. To investigate the incidence of chronic cervicitis (CC), detection rates of various types of human papillomavirus (HPV), the significance of the viral load, expression of mRNA genes involved in regulating the cell cycle, apoptosis, immunity, matrix metalloproteinases, and the methylation of the WIF1 gene promoter region among patients with CC associated with HPV infection. Material and methods. The study comprised 202 patients aged from 25 to 49 years. A comprehensive clinical and laboratory examination included patient history taking, gynecological examination, colposcopy, HPV testing, liquid-based cytology and targeted cervical biopsy (according to indications), vaginal smear microscopy, assessment of vaginal microbiocenosis, analysis of the transcriptional profile of KI67 genes KI67, CDKN2A, BCL2, PGR, IFNAR1, IL1RN, CCND1, EGFR, IL1B, IL10, IL18, TNFA, GATA3, TLR4, CD68, TGFB1, CXCL5, CXCL10 CXCL13, MMP8, MMP9, and the determination of the methylation level of the WIF1 gene promoter region. Results. Among patients with cervical pathology, 25.7% were found to have HPV-associated CC. The most commonly detected types were 16, 33, 35, and 66. The presence of HPV infection in endocervical scrapings was associated with a 1.8- and 2.4-fold higher expression of mRNA of CDKN2A/p16 gene and IL10, respectively (p < 0.05). The inflammatory process was accompanied by a 1.5 to 2.9 – fold increase in the expression of mRNA of IFNAR1, IL1B, IL10, TNFA, TLR4, CD68, TGFB1 and MMP9 genes (p <0.05). In the study cohort, the methylation of the WIF1 gene was either not observed or weakly expressed. Conclusion. The genes IFNAR1, IL1B, IL10, TNFA, TLR4, CD68, TGFB1, and MMP9, may be suggested as markers for CC. The presence of HPV contributes to an increase in the expression of mRNA of the CDKN2A/p16 genes and the immunosuppressive IL10.Amirkhanyan A.S., Bairamova G.R., Kiselev V.I., Prilepskaya V.N., Poloznikov A.A., Burmenskaya O.V., Babkina I.O., Asaturova A.V.
Keywords
Currently, research on investigating chronic cervicitis (CC) associated with the human papillomavirus (HPV) is one of the research priorities in gynecology due to its high incidence among women of reproductive age (15–40% of cases) [1]. HPV is the most common sexually transmitted infection and a major factor in the induction and maintenance of chronic cervical inflammation [2]. HPV - associated CC has several clinical, colposcopic and morphological features that largely depend on whether the infection in persistent or transient [3]. The development of cervicitis is associated with the complex relationship of the vaginal microbiota - pathogens of sexually transmitted infections (Chlamydia trachomatis, Trichomonas vaginalis, Mycoplasma genitalium) with fungi, conditionally pathogenic aerobic and anaerobic microorganisms [4]. In recent years, HPV-associated chronic inflammation along with HPV infection has been proven to play a significant role in the initiation or promotion of dysplasia and cervical cancer [5]. The search for more accurate diagnostic tools to detect molecular genetic markers of chronic inflammation may help personalize the approach and determine the best management strategy for patients [6].
This study aimed to investigate the incidence of CC, detection rates of various types of HPV, the significance of the viral load, expression of mRNA genes involved in regulating the cell cycle, apoptosis, immunity, matrix metalloproteinases (MMP), and the methylation of the WIF1 gene promoter region among patients with CC associated with HPV infection.
Material and methods
The study comprised 202 patients with mean age 31.8 (5.8) years, ranging from 25 to 49 years, who sought diagnosis and treatment of cervical pathology at the Research and Outpatient Department of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia from October 2016 to September 2018.
The inclusion criteria for the study patient selection were as follows: women aged 25 to 49 years with a diagnosis of CC, confirmed by cytological and/or histological examination and HPV - positive test; patients diagnosed with CC with HPV - negative test; patients with a positive HPV test with no signs of CC; women with HPV - negative test in the absence of signs of CC (control group). All patients signed informed consent to participate in the study.
Exclusion criteria were as follows: pregnancy and lactation, cervical dysplasia and cancer, failure to meet the study protocol requirements.
Clinical evaluation included patient history taking, general physical and gynecological examination, HPV typing for quantitative assessment of HPV, liquid-based cytology and/or targeted cervical biopsy subsequent histological examination (if indicated), extended colposcopy, and molecular biological studies.
Real time polymerase chain reaction (PCR) (DNA-Technology LLC, Russia, Roszdravnadzor RU No. FSR 2010/08811) was used to detect HPV 21 types (6, 11, 16, 18, 26, 31, 33, 35, 39, 44 (55), 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) with viral load determination. Using a special probe, biological material was taken from the cervical canal in 1.5 ml Eppendorf tubes with 0.9% sodium chloride solution [7].
The findings of liquid-based cytology were reported according to the 2014 Bethesda System (TBS). Cervical biopsy and histological examination were performed based on indications and evaluated according to the WHO classification (2014).
The findings of extended colposcopy reported using colposcopic terminology recommended by the International Federation of Cervical Pathology and Colposcopy (IFCPC) 16th World Congress (Orlando, 2017). The extended colposcopic examination included assessment of the cervical epithelium, blood vessels, colposcopic signs such as hypertrophy, deformity, multiple Nabothian cysts, pronounced vascular pattern, detection of acetowhite epithelium by applying a 3% acetic acid solution, uneven iodine positive or negative staining of the cervix (Schiller test) [8].
The mRNA expression of innate immune response genes IFNAR1, IL1RN, IL1B, IL10, IL18, TNFA, GATA3, TLR4, CD68, TGFB1, CXCL5, CXCL10, and CXCL13, cell cycle and apoptosis regulatory genes KI67, CCND1, CDKN2A, BCL2, MMP8, and MMP9, progesterone receptors PGR and epidermal growth factor EGFR was measured by quantitative real-time PCR with preliminary reverse transcription (reagents and amplifiers manufactured by the DNA-Technology LLC, Russia). In reverse transcription and amplification reactions, specific oligonucleotides and fluorescent FAM-labeled samples were used. The relative expression level was calculated by comparing indicator cycles (∆Cp) normalized by the reference B2M, GUSB, TBP genes [9].
The methylation level of the WIF1 gene promoter region was determined in DNA isolated from homogenized D cervical cell samples, the concentration of which was measured by fluorimetry using a Qubit dsDNA HS Assay Kit on a Qubit 2.0 fluorometer (Life Technologies, USA) standard set. Using the innuCONVERT Bisulfite Basic Kit (Analytik Jena, Germany), 150 ng of isolated DNA was subjected to bisulfite conversion and photometric determination of the amount of converted DNA using the CLARIOstar multi-detector (BMG Labtech, Germany). 20 ng of bisulfite-converted DNA was isolated for “touchdown” PCR amplification using GoTaq Hot Start Green Master Mix (Promega, USA) and primers to increase the number of copies of the promoter region of the WIF1 gene from -554 to -140 nucleotides before the start -codon and containing complementary and universal sequence M13 at the 5’-end: WIF1-M13F and WIF1-M13R. Using electrophoresis in a 2.5% agarose gel with molecular weight markers of 100 bp Molecular Ruler (Bio-Rad, USA), the presence and size (length) of PCR products were determined [10].
Further sequenced using the universal primers M13: M13F and M13R and the analysis of the reaction products were performed on an Applied Biosystems 3730 DNA Analyzer (USA) automated sequencer [11] at the Center of Collective Use «GENOM» at the V.A. Engelhardt Institute of Molecular Biology of the RAS.
Statistical analysis was performed using the SPSS for Windows v. 22 package. IBM, USA. All quantitative variables were assessed for normal distribution using the Kolmogorov-Smirnov test. Quantitative variables with a normal distribution were expressed as arithmetic means (M) and standard deviation (SD) presented as M(SD). Otherwise, the median and quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Qualitative variables were summarized as counts and percentages. Kruskal–Wallis test was used for comparing numerical data between three and more groups followed by pairwise comparisons using the Mann-Whitney U-test with the Bonferroni correction for multiple comparisons. Categorical variables were compared by the χ2 test with the Yates correction (for counts less than 10) was used; if it could not be used, the two proportion Z-test adjusted for the endpoints was used.
Results and discussion
The study comprised 202 patients with mean age 31.8 (5.8) years, ranging from 25 to 49 years, who were divided into two groups based on cytology findings and HPV-typing. Each group was divided into 2 subgroups depending on the presence or absence of HPV infection co-occurring with CC: group 1 (n = 108) included women with CC, of which 52 (25.7%) HPV positive women with CC comprised subgroup 1a and 56 (27.7%) HPV negative women were included subgroup 1b. Ninety four women without CC (group 2) were divided into subgroup 2a (n = 44; 21.8%) with HPV positive women and subgroup 2b (control group, n = 50; 24.8%) with HPV negative women. HPV-associated CC constituted 25.7% of all cervical pathology among women of reproductive age (Fig. 1)
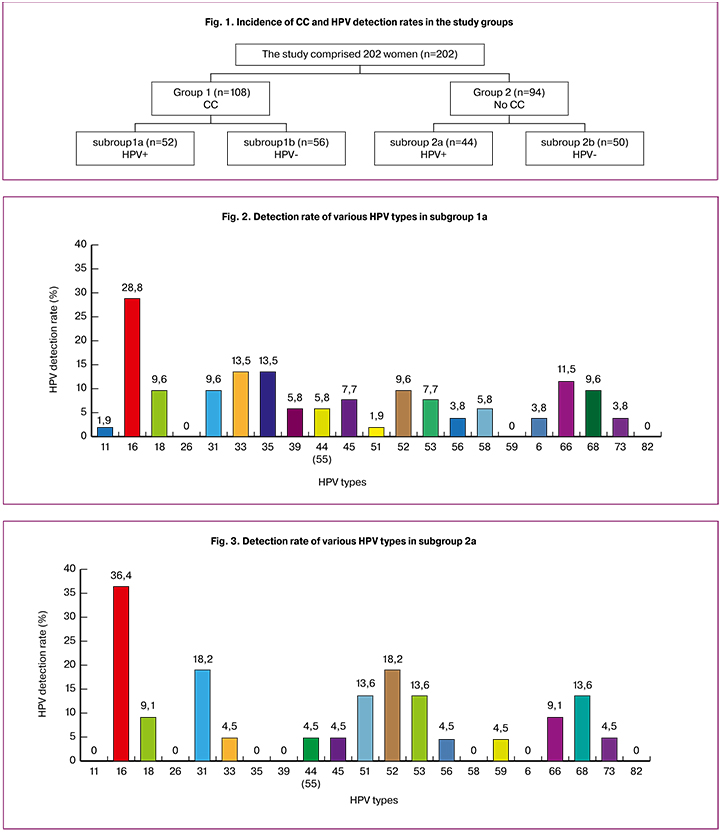
The mean age of patients in the subgroups is presented in Table 1; there were no statistically significant differences according to the Kruskal-Wallis test (p> 0.05).

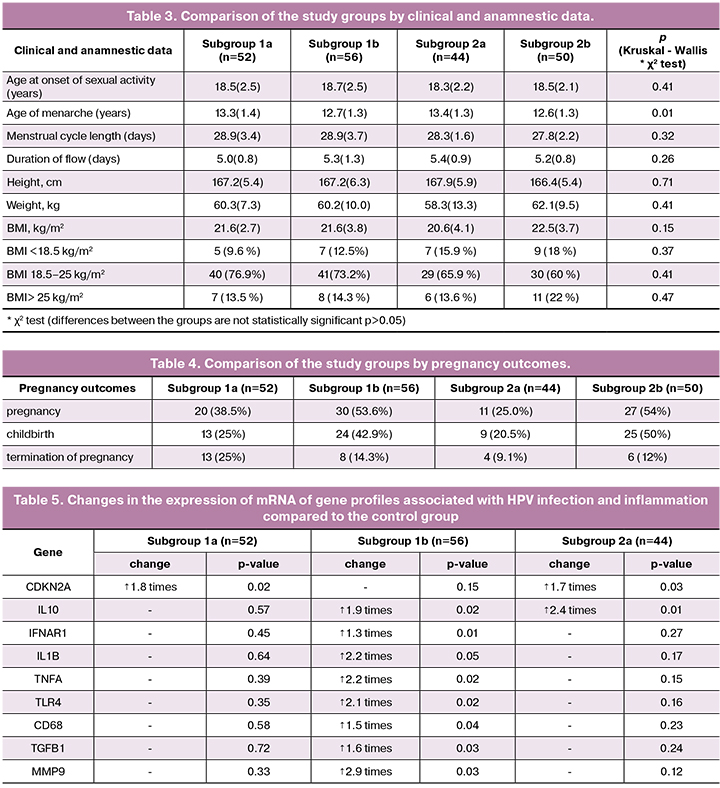
In subgroup 1a, the most commonly detected types of HPV were 16, 33, 35, 66, 18, 31, 52, 68, 53, 45 39, 44 (55), and 58. HPV type 16 was detected in 15 women (28.8%); 33 and 35 in 7 (13.5%); 66 in 6 (11.5%); 18, 31, 68 and 52 in 5 (9.6%); 53 and 45 in 4 (7.7%); 39, 44 (55) and 58 in 3 (5.8%). The remaining HPV genotypes 11, 56, 6, and 73 were found in less than 5.0% of cases (Fig. 2). HPV 16 was the most common type among oncogenic genotypes of HPV. One, 2, 3 and 5 types of HPV virus were detected in 71.2% (n=37), 21.1% (n = 11), 5.8% (n = 3), and 1.9% (n = 1) patients [12].
In subgroup 2a, the most commonly detected types of HPV were 16, 31, 52, 51, 53, 68, 18, 66, 33, 44 (55), 45, 56, 59 and 73. HPV type 16 was detected in 16 women (36.4%); 31 and 52 in 8 (18.2%); 51, 53 and 68 in 6 (13.6%); 18 and 66 in 4 (9.1%). The remaining HPV genotypes 33, 44 (55), 45, 56, 59, and 73 were detected in less than 5.0% of patients (Fig. 3). HPV 16 was the most common type among oncogenic genotypes of HPV [13].
The detection rate of HPV type 51 type was statistically significantly higher among women in subgroup 2b without CC, compared with women with CC in subgroup 1b (p = 0.04) (Table 2).
One, 2, 3 and 4 types of HPV virus were detected in 63.6% (n = 28), 22.7% (n = 10), 4.6% (n = 2), and 9.1% (n = 2) patients [12]. The mean viral load in subgroup 1a at baseline was 4.9 log copies of the virus in the sample (IQR 3.6–6.6), and 4.5 log copies of the virus in the sample (IQR 4.2 - 8.8) in subgroup 2a (without CC). There were no significant differences between the subgroups in the viral loads, and it cannot be used as a factor for predicting the development of CC.
Analysis of clinical and anamnestic data showed that the mean age at the onset of regular sexual activity among women in subgroup 1a, 1b, 2a, and 2b (control group) was 18.5 (2.5), 18.7 (2.5), 18.3 (2, 2), and 18.5 (2.1) years without statistically significant differences between the subgroups.
The mean age of menarche in subgroup 1a, 1b, 2a, and 2b (control group) was 13.3 (1.4), 12.7 (1.3), 13.4 (1.3), and 12.6 (1.3) years, respectively. There were statistically significant differences in the mean age of menarche between subgroups 1a and 2b (p = 0.03), 1b and 2a (p = 0.02), and 2a and 2b (p = 0.01). The mean duration of menstrual flow was 5.2 (0.9) days with a menstrual cycle length of 28.4 (2.7) days. There were no significant differences between the subgroups regarding menstrual function parameters.
Anthropometric parameters such as height [167.2 (5.7) cm], weight [60.2 (10) kg], and body mass index (BMI) [21.6 (3.6) kg/m2] did not differ statistically significantly between the subgroups (Table 3).
The total number of past pregnancies in subgroup 1a, 1b, 2a, and 2b was 20 (38.5%), 30 (53.6%), 11 (25.0%), and 27 (54%). There was a statistically significantly greater total number of pregnancies in subgroup 1b compared with the subgroup 2a (p = 0.01). The total number of spontaneous births in subgroup 1a, 1b, and 2a was 13 (25%), 24 (42.9%), and 9 (20.5%).
The greatest number of spontaneous births (25; 50%) was in subgroup 2b (control group). There were statistically significant differences in the number of spontaneous births between subgroups 1a and 2a compared with the control group (p = 0.01 and p = 0.01, respectively), and statistically significant differences (p = 0.01) between subgroups 1a and 1b. The total number of terminations of pregnancy was in subgroup 1a, 1b, 2a, and 2b was 13 (25%), 8 (14.3%), 4 (9.1%), and 6 (12%). There was a statistically significant difference (p = 0.03) in the total number of terminations of pregnancy between subgroups 2a and 1a (Table 4).
Cytologic examination was reported as chronic cervicitis in 61.5% (n = 32) and 69.6% (n = 39) women in subgroup 1a and 1b, respectively. NILM (Negative for Intraepithelial Lesion or Malignancy) was reported in 100% (n = 44) and (n = 50) women in subgroup 2a and 2b (control), respectively.
All patients (n = 202) underwent extended colposcopy. Women with HPV - associated CC were found to have ectopia, cervical hypertrophy, and deformity with a pronounced vascular pattern, multiple Nabothian cysts with an overstretched vascular pattern, mild colposcopic changes, such as thin acetowhite epithelium, fine punctation, fine mosaic and iodine-negative areas with irregular outer borders.
In 78 (38.6%) cases, chronic inflammation was accompanied by abnormal colposcopic findings. In subgroup 1, 18 (34.6%), 17 (32.3%), 14 (26.9%), and 11 (21, 1%) patients had multiple Nabothian cysts, cervical hypertrophy, pronounced vascular pattern, and thin acetowhite epithelium with fine punctation, respectively. The results of the Schiller test showed that 47 (90.3%) of women had an irregular iodine-positive and iodine-negative staining of the cervical epithelium. In subgroup 2b (control), all 50 women (100%) had a normal colposcopic picture, while among the patients in subgroups 1a and 1b mild cervical abnormalities (thin acetowhite epithelium) were significantly more common (71% and 65.8%, respectively).
Patients in subgroup 1a and 2a with persistent HPV, who had mild colposcopic changes (presence of acetowhite epithelium with fine mosaic in 23 (23.9%) and punctation - in 27 (28.1%)) and major colposcopic changes (dense acetowhite epithelium and coarse mosaic 5 (5.1%)) underwent targeted cervical biopsy. According to the results of histological examination, 10 (18%), 1 (1.8%), 6 (10.9%), and 22 (40%) patients had LSIL (low-grade squamous intraepithelial lesion), HSIL (high-grade squamous intraepithelial lesion), cervical leukoplakia, and CC, respectively. Patients with a histologically confirmed LSIL and HSIL were further excluded from the study (according to the exclusion criteria).
HPV - associated CC was most often represented by 16, 52, 45, 31 types of high oncogenic risk HPV; in combination with Candida fungi in 68 (33.7%), with herpes viruses in 29 (14.4%) and with another conditionally pathogenic microflora, among which Escherichia coli was dominant, in 42 (20.8%), Staphylococcus sarophoticus and aureus (33.7 and 14.4%, respectively). Quantitative and qualitative analysis of the vaginal microbiocenosis composition in women with CC (subgroups 1a and 1b) revealed that 45 (41.6%), 21 (19.4%), 36 (33%), and 1 (0.9%) patient had high titers of obligate anaerobic microorganisms, facultative anaerobic microorganisms, Candida fungi, and Trichomonas infection, respectively.
Analysis of the transcriptional profile of genes associated with HPV infection and inflammation (genes regulating the cell cycle, apoptosis, immunity, matrix metalloproteinases) demonstrated that the presence of HPV infection in endocervical scrapings was associated with a 1.8-fold higher expression of mRNA of CDKN2A/p16 gene (p = 0.02) (Table 5). HPV has an immunosuppressive effect mediated by anti-inflammatory IL10, which had a 2.4 -fold higher expression (p = 0.01) in the presence of HPV infection (subgroup 2a). The immunosuppressive effect of HPV can lead to the formation of an inadequate pro-inflammatory immune response (absence of significant pro-inflammatory changes in subgroup 1a), an imbalance of the normal and conditionally pathogenic flora, which ultimately is a risk factor for long-term viral persistence and further dysplasia.
CC without HPV infection is accompanied by a 1.5 to 2.9-fold increase in the level of mRNA expression of immunity genes IFNAR1, IL1B, IL10, TNFA, TLR4, CD68, TGFB1 and proinflammatory MMP9 gene (p = 0.03).
The occurrence of hypermethylation sites of WIF1 gene indicates the initial stages of the cervical dysplasia formation. In the study cohort, WIF-1 gene methylation was either not observed or weakly expressed. No statistically significant differences were found between the study groups in the levels of methylation of the WIF1 gene promoter region. The results of DNA sequencing are presented in Figure 4 [14].

The level of hypermethylation of the WIF1 gene promoter region is a diagnostic and prognostic marker for the early detection and treatment of HPV-associated cervical diseases.
Summary
Among women of reproductive age with cervical pathology, 52 (25.7%) were found to have HPV-associated CC.
No statistically significant differences in colposcopy findings were found between subgroups 1a and 2a. The most common colposcopic changes in CC included multiple Nabothian cysts (n = 64; 59.2%), cervical hypertrophy (n = 62; 57.4%) and deformity in (n = 58; 53.7%), and marked vascular pattern (n = 39; 36, 1%). In subgroups 1a and 2a, 38.9% of women with CC had abnormal colposcopic findings.
In subgroup 1a, the most commonly detected HPV types were type 16 (28.8%), 33 and 35 (13.5%), 66 (11.5%), 18, 31, 68 and 52 (9.6 %), 53 and 45 (7.7%), 39, 44 (55%), and 58 (5.8%). In subgroup 2a, the most commonly detected HPV types were 16 (36.4%), 31 and 52 (19.1%), 51, 53 and 68 (13.5%), 18 and 66 (9.5%). In subgroups 1a and 2a, HPV 16 was the most common type among oncogenic genotypes of HPV. The remaining HPV genotypes were detected in less than 5.0% of cases. In subgroup 1a, one type of HPV was detected in 71.2% and two or more HPV types in 28.8% of cases. In subgroup 2a, one type of HPV was detected in 63.6% and two or more HPV types in 36.4% of cases.
The genes IFNAR1, IL1B, IL10, TNFA, TLR4, CD68, TGFB1, and MMP9, may be suggested as markers for CC. The presence of HPV increases the expression of mRNA of the CDKN2A/p16 genes and the immunosuppressive IL10, which can lead to an inadequate immune response, an imbalance of the normal and conditionally pathogenic flora thus contributing to the long-term viral persistence and the development of dysplasia.
No statistically significant differences were found between the study groups in the levels of methylation of the WIF1 gene promoter region.
Conclusion
The study findings showed a high incidence of HPV-associated CC, which was found in every fourth patient. At the same time, colposcopic signs of CC are not specific, and every third patient had abnormal colposcopic findings characteristic of LSIL though not confirmed by morphological examination. The absence or weak expression of aberrant hypermethylation of the WIF1 gene promoter region in patients with CC may be regarded as a favorable prognostic sign for carcinogenesis. Almost a 3–fold increase in the expression of mRNA of IFNAR1, IL1B, IL10, TNFA, TLR4, CD68, TGFB1, and MMP9 genes may be viewed as a factor associated with the inflammatory process.
References
- Серов В.Н., Сухих Г.Т., Прилепская В.Н., Радзинский В.Е., ред. Руководство по амбулаторно-поликлинической помощи в акушерстве и гинекологии. М.: ГЭОТАР-Медиа; 2016. 1136с. [Serov V.N., Sukhikh G.T., Prilepskaya V.N., Radzinsky V.E., ed. Guidelines for outpatient care in obstetrics and gynecology. M .: GEOTAR-Media; 2016. 1136p. (in Russian)].
- Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. February 2013.
- Амирханян А.С., Прилепская В.Н., Байрамова Г.Р., Бурменская О.В., Костава М.Н., Асатурова А.В. Хронический цервицит: современные возможности диагностики и лечения. Акушерство и гинекология. 2018; 4: 22-7. [Chronic cervicitis: modern possibilities of diagnosis and treatment. A.S. Amirkhanyan, V.N. Prilepskaya, G.R. Bayramova, O.V. Burmenskaya, M.N. Kostava, A.V. Asaturov. Obstetrics and gynecology. - 2018. - №4 - S .- 22-27. (in Russian)].
- Ollendorff A.T. Cervicitis. Updated: Feb 09, 2017.
- World Health Organization. Comprehensive cervical cancer control. A guide to essential practice. 2nd ed. Geneva: WHO; 2014. Available at: http//www.who.int/repro ductivehealth/ publications/ cancers/cervical-cancer-guide/en
- Jayakumar N.K. Cervicitis: how often is it non-specific! J. Clin. Diagn. Res. 2015; 9(3): EC11-2. https://dx.doi.org/10.7860/JCDR/2015/11594.5673.
- Burd E.M. Human papillomavirus laboratory testing: the changing paradigm. Clin. Microbiol. Rev. 2016; 29(2): 291-319. https://dx.doi.org/10.1128/CMR.00013-15.
- Quaas J., Reich O., Frey Tirri B., Küppers V. Explanation and use of the colposcopy terminology of the IFCPC (International Federation for Cervical Pathology and Colposcopy) Rio 2011. Geburtshilfe Frauenheilkd. 2013; 73(9): 904-7. https://dx.doi.org/10.1055/s-0033-1350824.
- Bourmenskaya O., Bayramova G., Nepsha O., Rebrikov D., Trofimov D., Muravieva V., Sukhikh G. Vaginal smear TNF-alpha, IL18, and GATA3 mRNA levels correlate with local inflammation. Int. J. Biomed. 2014; 4(4): 204-8.
- Vasiljević N., Scibior-Bentkowska D., Brentnall A.R., Cuzick J., Lorincz A.T. Credentialing of DNA methylation assays for human genes as diagnostic biomarkers of cervical intraepithelial neoplasia in high-risk HPV positive women. Gynecol. Oncol. 2014; 132(3): 709-14. https://dx.doi.org/10.1016/j.ygyno.2014.02.001.
- Kan Y.Y., Liou Y.L., Wang H.J., Chen C.Y., Sung L.C., Chang C.F., Liao C.I. PAX1 methylation as a potential biomarker for cervical cancer screening. Int. J. Gynecol. Cancer. 2014; 24(5): 928-34. https://dx.doi.org/10.1097/IGC.0000000000000155.
- Arbyn M., Tommasino M., Depuydt C., Dillner J. Are 20 human papillomavirus types causing cervical cancer? J. Pathol. 2014; 234(4): 431-5. https://dx.doi.org/10.1002/path.4424.
- Piroozmand A., Mostafavi Zadeh S.M., Madani A., Soleimani R., Nedaeinia R., Niakan M. et al. The association of high risk human papillomaviruses in patients with cervical cancer: an evidence based study on patients with squamous cell dysplasia or carcinoma for evaluation of 23 human papilloma virus genotypes. Jundishapur J. Microbiol. 2016; 9(4): e32728. https://dx.doi.org/10.5812/jjm.32728.
- Сухих Г.Т., Ашрафян Л.А., Байрамова Г.Р., Бабкина И.О., Чернова В.Ф., Осипьянц А.И., Королькова А.И., Полозников А.А., Асфарова Г.Р., Муллабаева С.М., Коган Е.А., Муйжнек Е.Л., Друх В.М., Киселев В.И. Метилирования гена WIF 1 при цервикальных плоскоклеточных интраэпителиальных поражениях. Акушерство и гинекология. 2017; 5: 114-23. [Methylation of the WIF 1 gene in cervical squamous and epithelial lesions / G.T. Sukhikh, L.A. Ashrafyan, G.R. Bayramova, I.O. Babkina, V.F. Chernova, A.I. Osipyants, A.I. Korolkova, A.A. Poloznikov., G.R. Asfarova, S.M. Mullabaeva, E.A. Kogan., E.L. Muiznek, V.M. Druh., V.I. Kiselev // Obstetrics and Gynecology. - 2017.– №5.– S.– 114-123. (on Russian)].
Received 15.02.2019
Accepted 22.02.2019
About the Authors
Amirkhanyan, Armine S., PhD student at the Research and Outpatient Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79266050048. Е-mail:07062005@mail
Bairamova, Giuldana R., MD, clinical care supervisor at Research and Outpatient Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79265266905. Е-mail:bayramova@mail.ru
Kiselev Vsevolod I., Dr.Bio.Sci.,, professor, corr. member of the RAS, deputy director of the Institute of Oncology and Mammology, V.I. Kulakov NMRC for OG&P
of Minzdrav of Russia. 117997, Russia, Moscow, ul. Academician Oparin, 4. E-mail: vkis10@mail.ru
Prilepslaya, Vera N., MD, professor, deputy director, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954386934. E-mail: VPrilepskaya@mail.ru
Burmenskaya, Olga V., Dr.Bio.Sci., head of the Laboratory of Oncological Genetics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954382292. E-mail: o_bourmenskaya@oparina4.ru
Asaturova, Alexandra V., PhD, senior researcher at the 1st Pathology Department, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954382292. E-mail: a_asaturova @oparina4.ru
Poloznikov Andrey A., PhD in Chemistry, head of the Laboratory of Biochemistry and Enzymology, D. Rogachev NMRC for PHO&I of Minzdrav of Russia.
117997, Russia, Moscow, GSP-7, Samora Machel str., 1. Tel.: +74952876570 ext. 5509. E-mail: andrey.poloznikov@fccho-moscow.ru
Babkina Irina O., junior researcher at the Laboratory of Biochemistry and Enzymology, D. Rogachev NMRC for PHO&I of Minzdrav of Russia.
117997 Moscow, GSP-7, Samora Machel str., 1. Tel.: +74952876570 ext. 5509. E-mail: iobabkina@gmail.com
For citation: Amirkhanyan A.S., Bairamova G.R., Kiselev V.I., Prilepskaya V.N., Poloznikov A.A., Burmenskaya O.V., Babkina I.O., Asaturova A.V. Chronic cervicitis associated with human papillomavirus and markers of inflammation in women of reproductive age. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (4): 49-57. (in Russian)
https://dx.doi.org/10.18565/aig.2019.4.49-57



