Characteristics and management of cancer patients who wish to preserve their reproductive capacity
Aim. To develop a differentiated management strategy for cancer patients referred for retrieval and cryopreservation of reproductive material based on the structure of oncological diseases by referral, oncological and reproductive status, individual patient characteristics, and the planned/conducted treatment.Nazarenko T.A., Ashrafyan L.A., Biryukova A.M., Kirillova A.O., Martirosyan Ya.O., Dzhanashvili L.G., Bunyaeva E.S.
Materials and methods. We analyzed cancer patients’ medical records, including a complete description of the disease, the stage of the process, and the examination results. The planned treatment, prognosis for the course, and curability of the disease were outlined. Patients referred during stable remission had detailed medical case notes describing the treatment, the results of the examination, and the oncologist's conclusion about the feasibility of therapy to achieve pregnancy. Clinical evaluation at admission included assessing the patient's reproductive system, ovarian reserve, general and gynecological health. Based on the totality of analyzed data, individual treatment algorithms were developed.
Results. Between February and December 2019, 259 patients were consulted at the V.I. Kulakov NMRC for OG&P to determine the feasibility and methods of retrieval and preservation of reproductive material and assess the prospects for achieving pregnancy during stable remission. Fifty-six patients completed cancer treatment, and 203 were planned to have gonadotoxic therapy and/or radical treatment. Eighty-five controlled ovarian stimulation programs were conducted, including 68 before the onset of gonadotoxic therapy and 17 after cancer treatment completion. Sixty-one patients underwent retrieval and cryopreservation of ovarian tissue with the collection of immature oocytes, followed by in vitro maturation of oocytes and conservation, or fertilization, and embryo cryopreservation. Treatment was not administered to 113 referred patients due to a sharp decrease in the ovarian reserve and the inability to obtain their oocyte or for other reasons.
Conclusion. There is no doubt in need for joint counseling of cancer patients wishing to preserve fertility or in cases when oncologists allow a woman to become pregnant after treatment. The retrieval of reproductive material before the onset of cancer treatment undoubtedly increases patients' chances for subsequent childbirth, including, if necessary, using a surrogate mother. The choice of programs aimed at retrieval and cryopreservation of reproductive material was made on a case-by-case basis taking into account the location, type, and stage of the malignancy and the state of the patient's ovarian reserve.
Keywords
Over the past few years, there has been a growing interest in preserving fertility in female cancer patients [1]. This interest is motivated by increased survival rates in cancer patients, improved quality of life among cancer survivors in remission, and rapid advances in the field of assisted reproduction [2].
One of the most essential steps in preliminary retrieval and storage of cryopreserved reproductive material to preserve the potential for conception for cancer survivors is developing a comprehensive multidisciplinary strategy for treating this subset of patients based on their individual cancer and reproductive characteristics. First of all, it is necessary to timely provide patients with conclusive information about the disease curability and loss of ovarian function due to gonadotoxic therapy. Secondly, to familiarize them with available methods aimed at preserving reproductive material, their effectiveness, and safety. Most international clinical guidelines recommend that patients should make decisions about their treatment based on principles of shared decision-making and complete information provided them by oncologists and fertility specialist [3–5].
There are several problems associated with decision making regarding the preservation of reproductive material in cancer patients. The key ones are the time limit and issues related to the safety and the impact of assisted reproductive technologies on the underlying disease [6]. Discussion of options for preserving reproductive material in cancer patients is accompanied by specific difficulties since each option (cryopreservation of oocytes, embryos, and ovarian tissue) carries a specific risk [7]. Many specialists quite reasonably believe that female cancer patients need to use all the available means of fertility preservation and combinations of various techniques [8–10].
In recent years, there have been reports on the use of ovarian tissue cryopreservation and in vitro maturation of immature oocytes, which are still considered experimental [11, 12]. Patients should receive as quickly and thoroughly as possible necessary information about their cancer and the state of the reproductive system, which may help make the best decision. In this regard, it is essential to create patient registries to determine the structure of oncological diseases in cancer patients seeking fertility preservation. Similar measures are underway in many countries and are necessary for advancements in this direction [13–15].
The present study was aimed to develop a differentiated management strategy for cancer patients referred for retrieval and cryopreservation of reproductive material based on the structure of oncological diseases by referral, oncological and reproductive status, individual patient characteristics, and the planned/conducted treatment.
Materials and methods
We collected and analyzed data of female cancer patients faced with potential gonadotoxic therapy or radical surgery and requiring retrieval of reproductive material for fertility preservation and those in a stable remission who planned to achieve pregnancy. These patients were referred by an oncologist and were managed at the V.I. Kulakov NMRC for OG&P from February to December 2019. We analyzed patients’ medical records, including a complete description of the disease, the stage of the process, the examination results, the nature of the forthcoming treatment, and the prognosis of loss or a sharp decline in reproductive function. The state of the reproductive function was evaluated based on the reproductive history, the characteristics of the menstrual cycle, the serum levels of hormones, and the ovarian reserve state. The totality of the analyzed data enabled the determination of the prospects of programs aimed at preserving reproductive material and to choose optimal methods on a case-by-case basis. The decision was made within two days from the day of the patients’ referral.
All patients signed informed consent providing details about the possible consequences of the planned treatment for the reproductive system, the methods of reproductive material preservation, and the potential risks associated with the treatment.
Group I (n=203) included female cancer patients requiring gonadotoxic therapy or radical surgery. Of them, 68 underwent ovarian stimulation, retrieval, and cryopreservation of oocytes/embryos. In 61 patients ovarian tissue was sampled during surgery with the extraction of immature oocytes from the ovaries, followed by in vitro maturation of immature oocytes and their cryopreservation. Group II (n=56) included patients who completed cancer treatment; however, only 17 of them received an IVF program. Treatment was not administered to 113 referred patients due to diminished ovarian reserve, the inability to retrieve their oocytes, or personal reasons.
Statistical analysis
Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. Qualitative variables were summarized as counts and percentages. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Equality of variance was assessed by Fisher’s test. Since the quantitative variable did not meet the normality assumption, they were compared using the nonparametric Mann–Whitney test. Categorical variables were compared using Fisher’s exact test. The differences were considered statistically significant at the p<0.05. Statistical analysis was performed using R (version 3.2, R Foundation for Statistical Computing, Vienna, Austria).
Results and discussion
During the study, 259 female cancer patients were referred by oncologists for fertility preservation to achieve a pregnancy after cancer treatment. The table summarizes the clinical and anamnestic characteristics of the patients. Group I (n=203) included patients who were admitted before their gonadotoxic cancer therapy. Group II (n=56) comprised patients who completed cancer treatment.
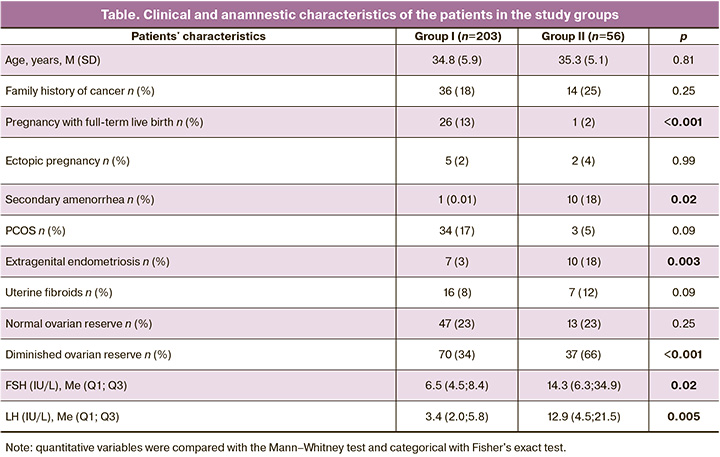
The study findings emphasize the need for measures aimed at preserving reproductive material for delayed childbirth in this category of patients because the vast majority of women were young and had no children. In group I, 26 (13%) patients gave birth to one child before the onset of the disease, and the only one gave birth after treatment. It is noteworthy that many patients had a family history of cancer and menstrual irregularities, particularly PCOS [n=34 (17%)]. Besides, 3% and 8% of patients had extragenital endometriosis and uterine fibroids, respectively. These observations suggest that the onset of cancer is associated with an unfavorable gynecological and reproductive history. Significant differences between the groups were found in the state of the ovarian reserve. Despite similar age, 66% of women admitted after cancer treatment had diminished ovarian reserve, while among those referred for preliminary cryopreservation of reproductive material, a diminished ovarian function was found in 34% of patients aged 38–40. The presented data unambiguously confirm the need to treat patients to preserve their reproductive material before cancer treatment, which is recommended in international documents [1–3].
Preliminary retrieval and cryopreservation of reproductive material are appropriate in cancer patients with favorable estimated survival. Figure 1 shows the distribution of patients by the type of malignancy.
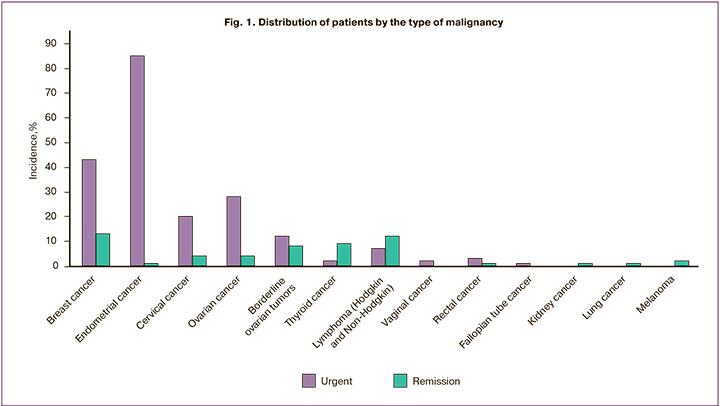
The most common malignancies among the study participants were gynecological cancers and breast cancer. As emphasized by other researchers, breast cancer patients planning to receive chemotherapy is a priority group among those who need to preserve reproductive capacity [9]. Besides, due to the gynecologic oncology specialization of our institution, most study participants had gynecological cancers. Only a small proportion of them had hematological malignancies. At the same time, in international registries, they rank second after breast cancer and have good prospects for survival but are at a high risk of treatment-related infertility [10]. This is confirmed by the high referral rates of patients after hematological and thyroid cancer treatment with a good prognosis of survival.
Preliminary retrieval and cryopreservation of reproductive material are appropriate in cancer patients with favorable estimated survival. Statistical analysis of the cancer stages in patients of the two groups revealed some differences that were not of significant importance. The study findings showed that the majority of patients had stage I and II cancers and a favorable estimated survival. All women showed a strong desire to preserve their oocytes and hoped to have children after completing treatment.
Analysis of breast cancer molecular biological subtypes showed higher detection rates of the luminal A and luminal B subtypes.
The choice of patient management strategy aimed at preserving reproductive material or achieving pregnancy after completion of treatment was based on the combination of oncological and reproductive characteristics with an assessment of the ovarian reserve. A – 68 women who underwent ovarian stimulation, retrieval, and cryopreservation of oocytes/embryos. Types of cancers included breast cancer, cervical cancer, cancer of the lower third of the vagina, rectal cancer, lymphomas, borderline ovarian tumors, stage I uterine cancer, and atypical endometrial hyperplasia. The mean age of the patients was 33.38 (3.73) years. All participants had a normal female body type with normal secondary sexual characteristics. The mean body mass index was 22.1 ± 2.0 kg/m2. Analysis of FSH, LH, estradiol, and AMH concentrations and evaluating antral follicle count revealed normal ovarian reserve and, consequently, the feasibility of ovarian stimulation and obtaining oocytes in the IVF program. Depending on the day of referral and the urgency of the situation, the patients underwent ovarian stimulation with conventional GnRH-antagonist-based ovarian stimulation or random-start controlled ovarian stimulation. According to international clinical guidelines, in breast cancer patients with high estrogen receptor expression, the stimulation regimen included 2.5–5 mg aromatase inhibitors (Letrozoles).
B – 61 women who underwent retrieval and cryopreservation of ovarian tissue with the harvesting of immature oocytes from the ovary, followed by in vitro maturation of immature oocytes and their cryopreservation, or fertilization and cryopreservation of embryos. Ovarian tissue was sampled during laparoscopic surgery by complete or partial ovarian resection. Based on malignancy type and the motivation for particular treatment type, the patients were distributed as follows: surgery for oncological indications in patients with recurrent borderline ovarian tumors (n=8), patients with ovarian cancer (n=19), breast cancer (n=8), uterine cancer (n=10), and cervical cancer (n=12). Thirty one ovarian tissue samples, 161 oocytes, and 13 blastocysts were cryopreserved.
C – 56 patients in stable remission who were referred to achieve pregnancy using assisted reproductive technologies. In most women who underwent chemotherapy/radiation therapy, diminished ovarian reserve, and ovarian insufficiency precludeв retrieval of oocytes. Ten out of 27 patients who received chemotherapy did not return to regular ovulatory menstrual cycles despite pharmacological protection of ovarian function using gonadotropin-releasing hormone agonists, combined oral contraceptives, etc.
Attempts to stimulate ovarian function were carried out in 17 out of 56 patients in whom regular menstruation was observed, and the AMH level was > 0.7 ng/ml. However, patients undergoing chemotherapy had extremely low potential for obtaining competent oocytes and embryos even when they had a preserved menstrual cycle and ovarian reserve. In most of the programs carried out after gonadotoxic therapy, we could not obtain embryos suitable for transfer, namely in 10 (58.8%) programs. In 7 (41.2%) programs, the embryo was transferred into the uterine cavity, but pregnancy did not occur in any case.
Undoubtedly, these facts confirm the need for preliminary cryopreservation of oocytes, even in those cases when the planned therapy is not gonadotoxic, such as long-term tamoxifen therapy.
 D – 113 women received only a consultation. Of them, 39 (69.6% of those referred after treatment) were in stable remission, and 74 (36.8% of those referred by oncologists) were planned to undergo cancer treatment. The main reason for precluding retrieval and preservation of reproductive material was a sharp decrease in the ovarian reserve up to its depletion in the vast majority of patients in this group. Also, there were subjective reasons, including a lack of material resources and fear resulting from communications with oncologists or relatives.
D – 113 women received only a consultation. Of them, 39 (69.6% of those referred after treatment) were in stable remission, and 74 (36.8% of those referred by oncologists) were planned to undergo cancer treatment. The main reason for precluding retrieval and preservation of reproductive material was a sharp decrease in the ovarian reserve up to its depletion in the vast majority of patients in this group. Also, there were subjective reasons, including a lack of material resources and fear resulting from communications with oncologists or relatives.
Conclusion
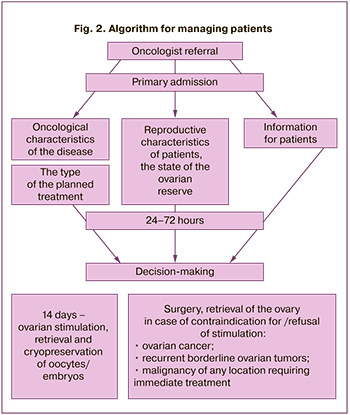
The study findings enabled developing a management strategy for cancer patients who need to preserve reproductive material for delayed childbirth (Fig. 2).
1. The decision on the feasibility of preserving reproductive material should be made jointly by oncologists and fertility specialists, based on oncological indications. They include the stage of malignancy, the prognosis of survival, a high risk of losing reproductive function, and reproductive characteristics – the state of the ovarian reserve, ensuring the oocyte procurement.
2. The woman makes the final decision based on shared decision-making and the doctors' full information.
3. The retrieval and cryopreservation of reproductive material are carried out before starting treatment for the underlying disease.
4. In case of a decision on the possibility of ovarian stimulation for retrieval and cryopreservation of oocytes/embryos, the stimulation protocol, the choice and dose of ovulation inducers are determined by the reproductive specialist; the oncologist can limit the duration of the treatment, but not less than 14 days.
5. If ovarian stimulation is contraindicated (ovarian cancer), or the patient refuses this treatment, it is necessary to discuss obtaining and cryopreservation of ovarian tissue with a collection of immature oocytes from ovarian tissue followed by oocyte in vitro maturation. The patient should be informed that ovarian tissue is obtained during laparoscopic surgery by complete or partial ovarian resection. It is also necessary to inform the patient that this method is currently experimental and may be used when other methods cannot be applied.
References
- Woodruff T.K., Clayman M.L., Waimey K.E. Oncofertility communication: sharing information and building relationships across disciplines. New York: Springer; 2013.
- Rashedi A.S., de Roo S.F., Ataman L.M., Edmonds M.E., Silva A.A., Scarella A. et al. Survey of fertility preservation options available to patients with cancer around the globe. J. Glob. Oncol. 2018; 4: 1-16. https//dx.doi.org/10.1200/JGO.2016.008144.
- Anazodo A., Laws P., Logan S., Saunders C., Travaglia J., Gerstl B. et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum. Reprod. Update. 2018; 25(2): 159-79. https://dx.doi.org/10.1093/humupd/dmy038.
- Oktay K., Harvey B.E., Partridge A.H., Quinn G.P., Reinecke J., Taylor H.S. et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018; 36(19): 1994-2001. https://dx.doi.org/10.1200/JCO.2018.78.1914.
- von Wolff M., Germeyer A., Liebenthron J., Korell M., Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: fertility preservation techniques. Arch. Gynecol. Obstet. 2017; 297(1): 257-67. https://dx.doi.org/10.1007/s00404-017-4595-2.
- Loren A.W., Mangu P.B., Beck L.N., Brennan L., Magdalinski A.J., Partridge A.H. et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013; 31(19): 2500-10. https://dx.doi.org/10.1200/jco.2013.49.2678.
- Matthews M.L., Hurst B.S., Marshburn P.B., Usadi R.S., Papadakis M.A., Sarantou T. Cancer, fertility preservation, and future pregnancy: a comprehensive review. Obstet. Gynecol. Int. 2012; 2012: 953937. https://dx.doi.org/10.1200/jop.18.00160.
- Donnez J., Dolmans M.-M. Fertility preservation in women. N. Engl. J. Med. 2017; 377(17): 1657-65.https://dx.doi.org/10.1056/NEJMra1614676.
- Kedem A., Yerushalmi G.M., Brengauz M., Raanani H., Orvieto R., Hourvitz A., Meirow D. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J. Assist. Reprod. Genet. 2018; 35(5): 851-6. https://dx.doi.org/10.1007/s10815-018-1153-1.
- Woodruff T.K. From the bench to bedside to babies: translational medicine made possible by funding multidisciplinary team science. J. Assist. Reprod. Genet. 2013; 30(10): 1249-53. https://dx.doi.org/10.1007/s10815-013-0082-2.
- Yasmin E., Balachandren N., Davies M.C., Jones G.L., Lane S., Mathur R. et al. Fertility preservation for medical reasons in girls and women: British fertility society policy and practice guideline. Hum. Fertil. (Camb.). 2018; 21(1): 3-26. https:/dx./doi.org/10.1080/14647273.2017.1422297.
- Galvão A., Segers I., Smitz J., Tournaye H., De Vos M. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J. Assist. Reprod. Genet. 2018; 35(12): 2161-71. https://dx.doi.org/10.1007/s10815-018-1317-z.
- Rodriguez-Wallberg K.A., Oktay K. Fertility preservation in women with breast cancer. Clin. Obstet. Gynecol. 2010; 53(4): 753-62. https://dx.doi.org/10.1097/GRF.0b013e3181f96e00.Kim S.S., Donnez J., Barri P., Pellicer A., Patrizio P., Rosenwaks Z. et al.;
- ISFP Practice Committee. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J. Assist. Reprod. Genet. 2012; 29(6): 465-8. https://dx.doi.org/10.1007/s10815-012-9786-y.
- Woodruff T.K., Snyder K.S. Oncofertility. New York: Springer; 2007.
- De Vos M., Smitz J., Woodruff T.K. Fertility preservation in women with cancer. Lancet. 2014; 384(9950): 1302-10. https://dx.doi.org/10.1016/s0140-6736(14)60834-5.
Received 07.09.2020
Accepted 17.09.2020
About the Authors
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Technologies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.Tel.: +7(915)322-08-79. E-mail: t.nazarenko@mail.ru. ORCID: 0000-0002-5823-1667. 117997, Russia, Moscow, Ac. Oparina str., 4.
Lev A. Ashrafyan, Dr. Med. Sci., Professor, Academician of the RAS, Director of the Institute of Oncogynecology and Mammology, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia. Tel.: +7(495)531-44-44. E-mail: levaa2004@yahoo.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Almina M. Birukova, Ph.D., Clinical Supervisor at the F. Paulsen SEC for ART, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: alma21@list.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Anastasia O. Kirillova, Ph.D. (bio.sci.), Senior Researcher at the 1st Department of Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(926)781-55-24. E-mail: stasia.kozyreva@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Yana O. Martirosyan, Junior Researcher at the F. Paulsen SEC for ART, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(925)124-99-99.
E-mail: marti-yana@index.ru. ORCID: 0000-0002-9304-4410. 117997, Russia, Moscow, Ac. Oparina str., 4.
Lana G. Dzhanashvili, Ph.D. student at the, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(962)918-56-19. E-mail: lana.janashvili@gmail.com.
ORCID: 0000-0002-2891-3974. 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina S. Bunyaeva, Ph.D. student at the 1st Department of Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-26-22. E-mail: es_bunyaeva@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Nazarenko T.A., Ashrafyan L.A., Biryukova A.M., Kirillova A.O., Martirosyan Ya.O., Dzhanashvili L.G., Bunyaeva E.S. Characteristics and management of cancer patients who wish to preserve their reproductive capacity.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 11: 93-99 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.93-99



