Gonadotoxic effect of antiretroviral therapy in hiv-infected women
Objective. To study mitochondrial DNA (mtDNA) copy number in cumulus cells in HIV-infected women receiving antiretroviral therapy (ARVT).Mityurina Е.V., Perminova S.G., Selimova F.N., Burmenskaya О.V., Kozyrina N.V., Kravchenko А.V.
Materials and methods. This prospective clinical study included 191 patients who presented for assisted reproductive technologies (ART) treatment. The main group consisted of 95 HIV-infected women; the control group included 96 HIV-seronegative patients. There were 89 and 113 treatment cycles, respectively. The quantitative assessment of mtDNA copy number was carried out in 78 and 111 samples of cumulus cells, respectively, using real-time polymerase chain reaction (PCR) method.
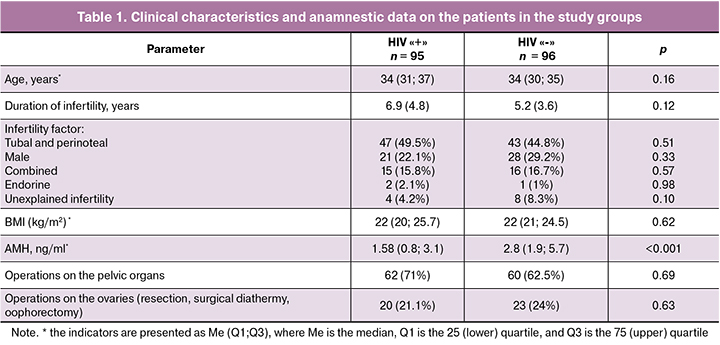
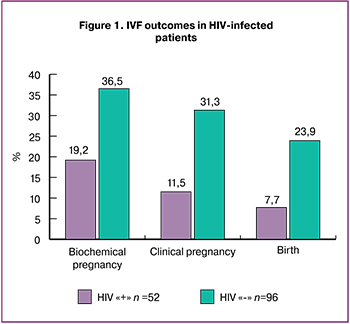
Results. Duration of HIV infection in patients was 8 (6; 11) years, third subclinical stage of the disease prevailed (61.05%). All patients received ARVT, the median treatment duration was 4 years (2; 6.5). The patients of the groups were similar in age (34 and 34 years; p=0.16), body mass index (22 and 22 kg/m2; p=0.62), duration (6.9 and 5.2; p=0.12) and factors of infertility. The assessment of outcomes of IVF programs showed lower rate of biochemical pregnancy (19.2% and 36.5%), р=0.03), clinical pregnancy (11.5% and 31.3%; р=0.008) and live birth (7.7% and 23.9%; р=0.007) in HIV-infected patients in comparison with HIV-seronegative women. mtDNA copy number in cumulus cells in HIV-infected women was statistically significantly lower than one in patients without HIV infection (566.7 (229.1) and 639.7 (197); р=0.02). The obtained data revealed inverse relationship between mtDNA level in cumulus cells and duration of ARVT (r=-0.228; р=0.04). Patients receiving ARVT more than one year showed a statistically significantly lower mtDNA content in cumulus cells than women from the control group (505.3(175) and (639.7 (197.9), respectively; р=0.001).
Conclusion. Lower rate of achieving pregnancy through IVF programs in HIV-infected women is due to gonadotoxic effect of ARVT.
Keywords
In the Russian Federation the cumulative number of registered HIV cases is 1,408,264; among them there are 510,495 HIV-positive people who receive antiretroviral therapy (ARVT) (dated October 31, 2019) [1]. The highest level of HIV infection is observed in the age group of 30-44 years. Male dominance was observed in the gender structure (62%), but in recent years there has been an increase in the proportion of women living with HIV. According to some data, about 500,000 women in Russia were diagnosed with HIV infection by the end of 2019; many of them are planning to have a child [2, 3]. In addition to the improvement of quality of life and significant increase in life expectancy, administration of ARVT allows the patients of this group to achieve pregnancy, even without using assisted reproductive technologies (ART) in case of preserved fertility in both partners [4]. However, a number of studies showed a decrease in fertility in women with HIV infection [2, 5, 6]. For example, Parsons et al. (2000) demonstrated that the incidence of infertility in patients with HIV infection is 37% higher than one in women without HIV infection [7].
ART treatment can be considered as a measure to prevent infection of a healthy partner in HIV-discordant couples, and also as an opportunity to achieve parenthood. However, according to various data, pregnancy rate in the in vitro fertilization (IVF) program in HIVinfected patients ranges from 6.7% to 24.1%, which is significantly lower in comparison with the women without HIV infection [2, 6, 8]. Most scientists explain this by the negative impact of ARVT medications on the quality of oocytes and their ability to be fertilized. It is known that nucleotide reverse transcriptase inhibitors (NRTIs) which are always used in ARVT schemes, inhibit the activity of HIV reverse transcriptase and impair the process of viral replication. However, this group of medications can also cause the suppression of human DNA polymerase gamma, which is necessary for mitochondrial DNA (mtDNA) replication; as a result, there is a decrease in the content of mtDNA and an increase in the level of its mutation [9, 10, 11].
Few studies have addressed issues related to oocyte mtDNA copy number in HIV-positive women receiving ARVT. Thus, Lopez et al. (2008) were the first to show that oocytes of HIV-positive infertile patients receiving ARVT had mtDNA depletion in 32% of cases in comparison with the controls [12]. In a study by Boston et al. (2010), NRTI stavudine induces mtDNA depletions in mouse oocytes, which may impair their ability to be fertilized and affect the viability of embryos [13].
A small number of studies may be related to the ethical problems of using a mature oocyte since the evaluation of oocyte mtDNA copy number is an invasive procedure. A non-invasive marker that reflects the quality of oocytes is mitochondria in cumulus cells. In the work of Pawlak et al. (2015) it was found that the number of mtDNA copies in porcine oocytes correlates with the number of mtDNA copies in cumulus cells. Moreover, mtDNA of cumulus cells contributes to the normal development of egg cell [14]. Studies on mtDNA copy number in cumulus cells in HIV-infected women have not been published.
To date, there is no sufficient data on the impact of HIV infection and/or ARVT on the quality of gametes, as an indicator of the effectiveness of ART programs in HIV-infected women.
Therefore, the aim of this research was to study mtDNA copy number in cumulus cells in HIV-infected women receiving ARVT.
Materials and Methods
This prospective clinical study included 191 patients who presented for assisted reproductive technologies (ART) treatment. The main group consisted of 95 HIVinfected women, the control group included 96 HIVseronegative patients. There were 89 and 113 treatment cycles, respectively. When IVF outcomes were analyzed, 25 patients with HIV and hepatitis C coinfection and 19 concordant pairs were excluded. The quantitative assessment of mtDNA copy number was carried out in 78 and 111 samples of cumulus cells, respectively. The criteria for inclusion in the main group were as follows: HIV infection, 3rd subclinical stage, stages 4A, 4B, 4C, remission phase; administration of ARVT, undetectable viral load in two consecutive studies which were performed at least 3 months apart; informed consent to participate in this study. Exclusion criteria were viral hepatitis B, C and HIV coinfection, HIV-positive status of the partner. Non-inclusion criteria were contraindications to ART treatment. Criteria for inclusion in the control group were as follows: HIV-seronegative status of both partners, selective transfer of one embryo.
The status of HIV-infected patients was assessed on the basis of the data on the stage and phase of the disease, the level of viral load, CD4+, CD8+ lymphocytes, its duration, as well as ARVT components.
Ovarian stimulation was performed according to the protocol involving gonadotropin-releasing hormone (GnRH) antagonists using recombinant follicle-stimulating hormone and human menopausal gonadotropin. When the follicles reached a diameter of 14-15 mm, GnRH antagonist was injected at a dose of 0.25 mg/day subcutaneously to prevent the endogenous LH surge. For the final maturation of oocytes, human chorionic gonadotropin (HCG) at a dose of 10,000 IU was administered when three or more follicles greater than or equal to 17 mm were visualized. Oocyte aspiration was performed 35 or 36 hours after the injection of the ovulation trigger. Oocyte fertilization was performed using the ICSI method. The transfer of the first embryo was performed on days 3-5, the remaining embryos of grade 1-2 quality were cryopreserved. After embryo transfer the patient was injected micronized progesterone preparation into the vagina at a daily dose of 600 mg from the day after the puncture. The effectiveness of the treatment was assessed using the indicator of pregnancy rate per embryo transfer. Biochemical pregnancy was diagnosed on days 12-14 after embryo transfer if the level of β-human chorionic gonadotropin in the serum was more than 20 IU/L. Clinical pregnancy was confirmed when a gestational sac containing a live embryo was visualized in the uterine cavity 5-6 weeks after the transfer.
The study of mtDNA copy number in cumulus cells was performed using the following technique: cumulus cells were washed in a sterile phosphate-buffered saline and then placed in individual test tubes containing this buffer under aseptic conditions using an inverted microscope. The absolute mtDNA copy number was determined using the method of polymerase chain reaction (PCR) in real time. For this purpose, DNA was extracted from cumulus cells. The cells were lysed in a buffer containing guanidine thiocyanate for 10 minutes at 65°C. Afterwards isopropanol precipitation of DNA was performed, the samples were centrifuged at 13000 rpm for 10 minutes (Kit Proba-NK-plus, DNA-Technology LLC, Russia). Then the precipitate was washed with two washing solutions, dried, and resuspended in 50 ml of eluting solution. mtDNA copies were counted using real-time PCR with oligonucleotides and TaqMan samples for amplification and quantification of specific mtDNA fragments (MTND2 gene - mitochondrially encoded NADH dehydrogenase 2 and MTND4 gene - mitochondrially encoded NADH dehydrogenase 4). The use of primers for the MTND2 gene allowed us to estimate the content of the total mtDNA pool (mtDNA total), and the primers for the MTND4 gene made it possible to evaluate the content of full-length mtDNA pool deprived of deletions (mtDNAdel -), particularly del mtDNA4977. TaqMan samples for mitochondrial and genomic DNA fragments were labeled with different fluorophores (FAM and HEX) which allowed us to perform reaction in one test tube (multiplex PCR) with two repeats for each sample. “Hot start” PCR was performed using paraffin. Reagents, oligonucleotides, TaqMan samples and detecting amplifiers “DTprime” (LLC “DNA-Technology”, Russia) were used in the study. Amplification was performed according to the following program: 80° C for 1 min, incubation at 95° C for 1 min followed by 50 cycles: 94° C for 15 sec and 64° C for 20 sec with the measurement of fluorescence level in each cycle. Normalization was performed for genomic DNA (LTC4S gene - leukotriene C4-synthase). The ratio between mtDNA number and genomic DNA was determined by comparing threshold cycles (2∆Сt) and represented in relative amounts using formula 1: mtDNA/gDNA = 2Ct gDNA - Ct mtDNA, where Ct gDNA is the threshold cycle of amplification of genomic DNA, and Ct mtDNA is the threshold cycle of amplification of mitochondrial DNA.
Statistical processing of the data was performed using the IBM SPSS Statistics version 22 software package. In normal distribution of the data, the mean value and standard deviation were determined, parametric statistics methods (t-test) were used to evaluate differences in groups. When the data distribution was different from the normal one, the median and quartiles (Me(Q1;Q3)) were determined, and nonparametric statistical methods (the Mann-Whitney U test) were used to assess differences in the groups. The χ2 test was used to compare categorical variables, as well as to evaluate significant differences between them. Dependent data were evaluated using the Pearson correlation coefficient. At a significance level of p < 0.05 the results were considered statistically significant.
Results
The median age of women with HIV infection was 34 years (31;37). Duration of HIV infection in patients was 8 (6; 11) years, third subclinical stage of the disease prevailed (61.05%). Patients with stages 4A (32.63%), 4B (4.21%) and 4C (2.11%) had a remission for at least 6 months. HIV with hepatitis C coinfection was detected in 25 out of 95 (26.3%) HIV-seropositive women, in 19 cases (20%) the sexual partner also had an HIV-positive status. All patients received combined ARVT, including 84 (88.4%) patients who took NRTI medications in combination with protease inhibitors, 9 (9.5%) patients took NTRI in combination with non–nucleoside reverse transcriptase inhibitor, and 2 (2.1%) patients were administered three medications of NTRI group. In 48 (50.5%) patients, NTRI with high mitochondrial toxicity (didanosine, stavudine, zidovudine) was used, while the other 52 (49.5%) patients received drugs with little toxic effect on the mitochondria (phosphazide, lamivudine, abacavir, tenofovir, emtricitabine). The median duration of drug administration was 4 years (2-6.5 years). The viral load in the blood was undetectable in 100% of cases. Levels of CD4+ lymphocytes (622.5 (446.7;808.7), CD8+ lymphocytes (741 (613;990)) indicated a “satisfactory” condition of the immune system.
The patients of the groups were similar in age (34 and 34 years; p = 0.16), body mass index (22 and 22 kg/m2; p = 0.62), duration (6.9 and 5.2; p = 0.12) and factors of infertility (Table 1). The most common factor causing infertility was tubal and peritoneal factor (49.5% and 44.8%, р = 0.51). Infertility was not diagnosed in 6 out of 95 (6.3%) HIV-seropositive patients, IVF program was performed for epidemiological indications.
The patients of the groups were evaluated for the number of pelvic surgeries (60.9% and 76.5%; p = 0.21), including the frequency of operations on the ovaries (21.1% and 24%; p = 0.63). At the same time, particular attention was given to the lower concentration of AMH (1.5 and 3.5 ng/ml; p < 0.001) in the main group, compared to HIV-seronegative patients.
 As a result, HIV-infected patients received fewer oocytes (Me 6 (3; 11) and 10 (7;15.5); p < 0.001), embryos at the stage of cleavage (Me 4 (2;7) and 6 (4;10); p < 0.0001) and blastocysts (Me 1(0;3) and 3 (1;6); p < 0.0001), compared to the control group.
As a result, HIV-infected patients received fewer oocytes (Me 6 (3; 11) and 10 (7;15.5); p < 0.001), embryos at the stage of cleavage (Me 4 (2;7) and 6 (4;10); p < 0.0001) and blastocysts (Me 1(0;3) and 3 (1;6); p < 0.0001), compared to the control group.
Evaluation of IVF outcomes showed a lower rate of biochemical pregnancy (19.2% and 36.5%), p = 0.03), clinical pregnancy (11.5% and 31.3%; p = 0.008) and live birth (7.7% and 23.9%; p = 0.007) in HIV-infected patients, compared with HIV-seronegative women (Figure 1).
In the next stage of our study, we analyzed mtDNA copy number in 110 samples of cumulus cells obtained from HIV-infected women and 111 samples obtained from women without HIV infection. Identical results were obtained when using both pairs of primers to MTND2 and MTND4 genes. Thus, deletions in the MTND2 and MTND4 regions of mtDNA were not detected.
 It was revealed that mtDNA copy number in cumulus cells in HIV-infected patients was significantly lower than one in women without HIV infection (566.7 (229.1) and 639.7 (197); p = 0.02) (Figure 2).
It was revealed that mtDNA copy number in cumulus cells in HIV-infected patients was significantly lower than one in women without HIV infection (566.7 (229.1) and 639.7 (197); p = 0.02) (Figure 2).
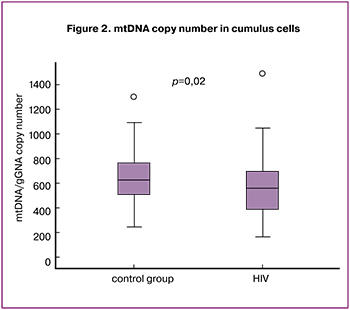
The evaluation of the association did not reveal any correlation between mtDNA copy number in cumulus cells with the age of patients in both groups (r = 0.153; p = 0.18 and r = 0.164; p = 0.08, respectively), as well as with the duration of infertility (r = 0.300; p = 0.07 and r = -0.041; p = 0.67) and BMI (r = 0.168; p = 0.35 and r = 0.260; p = 0.09). In the main group, mtDNA copy number in cumulus cells did not depend on the duration of HIV infection (r = -0.132; p = 0.26), the level of CD4+ (r = 0.037; p = 0.74) and CD8+lymphocytes (r = 0.020; p = 0.91). The inverse relationship between mtDNA level in cumulus cells and the duration of ARVT (r = -0,228; p = 0.04) was revealed (Figure 3).
Moreover, if ARVT was administered less than one year, mtDNA copy number in cumulus cells was comparable to the one in patients of the control group (677.1 (292.7) and 639.7 (197.9); p = 0.46). When administering ARVT for the period from 1 to 5 years, mtDNA content in cumulus cells was 505.3 (175), which was statistically significantly lower than one in the control group (639.7 (197.9); p = 0.001). If the course of the therapy lasted more than 5 years, this indicator was also significantly lower than one in the group of patients without HIV infection (522.7 (203.1); p = 0.04).
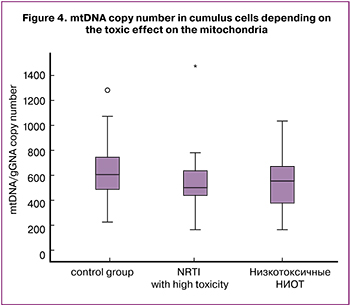
Next, we performed a comparative analysis of the mtDNA level in cumulus cells depending on the toxic effect of NRTI on mitochondria.
mtDNA copy number in cumulus cells in patients taking NRTI with high mitochondrial toxicity was significantly lower (554.6 (239.5) and 639 (197.9); p = 0.04); while mtDNA copy number in women taking medications with a minor toxic effect on mitochondria was comparable to healthy controls (574.3 (224.6) and 639 (197.9); p = 0.06).
 Discussion
Discussion
The use of ART treatment has solved many relevant problems of HIV-infected patients, including achievement of parenthood and prevention of infection in a healthy partner. However, IVF program shows a low efficiency in this group of patients, which according to many researchers, is associated with the gonadotoxic effect of ARVT due to the depletion of mtDNA in oocytes [5, 6, 8, 12]. An indirect evidence of the negative impact of ARVT on the quality of oocytes is pregnancy rate which is comparable to healthy controls when using donor oocytes [6, 15].
In this study, HIV-infected patients were reproductiveaged (34 years (31; 37)), their disease was not long-term, namely 8 years (6; 11) and all patients (100%) received combined ARVT (4 years (2; 6.5). The levels of CD4+ lymphocytes (622.5 (446.7; 808.7) in the blood and an undetectable viral load before the IVF program indicated a “satisfactory” condition of the immune system, despite the presence of HIV infection.
In both groups tubal and peritoneal factor of infertility prevailed (49.5% and 44.8%, p = 0.51), which in HIVinfected women can be explained by the higher incidence of sexually transmitted infections and purulent and inflammatory diseases of the pelvic organs in HIVinfected women in comparison with the patients without HIV infection. The results of our study are consistent with the findings of other researchers [2, 5, 8].
It is known that one of the factors negatively affecting the ART outcomes is the patient’s high BMI. In our study, women with HIV infection had a BMI comparable to healthy patients (22 and 22 kg/m2; p = 0.62). There is no agreement about it in the literature. Thus, in the study of Santulli et al. (2011) it was shown that HIV-infected women had a higher BMI than HIV-seronegative patients (24.2 and 22.9, = 0.03) [16]. Nurudeen et al. (2013), on the contrary, showed that HIV-infected patients and women without HIV infection had a similar BMI [17].
Thought the concentration of AMH in HIVinfected women was within the normative values, it was significantly lower (1.5 and 3.5 ng/ml; p < 0.0001) compared to HIV-seronegative patients. Both groups were similar in age (34 and 34 years; p = 0.16) and the number of ovarian surgeries (21.1% and 24%; p = 0.63). The data obtained may indicate a gonadotoxic effect of HIV and/or ARVT in the main group. However, we cannot separate the effect of HIV and ARVT on the concentration of AMH, since all the patients received the medications in this study. There is currently no consensus that HIV infection and/ or ARVT affects the ovarian reserve. Thus, research by Ohl et al. (2010) showed that women with HIV infection had lower levels of AMH by 23% than in the control group [18]. In the study by Scherzer (2015), the concentration of AMH was 16% lower if viral load was undetectable and 26% lower if viral load was detectable compared to uninfected women. The authors associated the obtained data with viral effect, since there was an evidence of a direct relationship between the level of AMH and CD4+ lymphocytes in the blood [19].
HIV-infected patients received fewer oocytes (Me 6 (3; 11) and 10 (7; 15.5); p < 0.001), embryos at the stage of cleavage (Me 4 (2; 7) and 6 (4; 10); p < 0.001) and blastocysts (Me 1 (0; 3) and 3 (1; 6); p < 0.001), compared to the control group. The lower number of oocytes obtained in women with HIV infection can be explained by initially lower levels of AMH.
There was a low rate of biochemical (19.2% and 36.5%), p = 0.03), clinical pregnancy (11.5% and 31.3%; p = 0.008) and live birth (7.7% and 23.9%; p = 0.007) in HIV-infected patients, compared to HIVseronegative women. The data obtained by our study are consistent with the results of other scientists [6, 15, 18]. In contrast, in studies by Martinet et al. (2006), Santulli et al. (2011), Nurudeen et al. ((2013), Prisant et al. (2010) it was shown that the rate of clinical pregnancy in women with HIV infection was comparable to the one in the control group of HIV-seronegative patients [16, 17, 20, 21]. The cause of the low effectiveness remains unclear. The possible negative influence of ARVT on the mitochondrial potential of oocytes is discussed.
Our study revealed significantly lower levels of mtDNA in cumulus cells in HIV-infected women in comparison with healthy controls (566.7 (229.1) and 639.7 (197); p = 0.02). These data can explain the low pregnancy rate in the IVF program in HIV-infected women. It is known that there is a relationship between the number of mtDNA copies, the production of adenosine triphosphate by mitochondria and the quality of oocytes, as well as the potential of embryo development. It has been shown that oocytes with fewer mtDNA copies have a lower fertilization rate [22].
Our study has detected an inverse relationship between mtDNA copy number in cumulus cells with the duration of ARVT (r = -0.228; p = 0.04). The obtained data confirm the information that toxic damage to mitochondria depends on the duration of exposure to drugs [23]. Moreover, mtDNA copy number in cumulus cells began to decrease after one year of taking ARVT. Similar results were presented in studies by Masyeni S. et al. (2018) [24], Z. Dai et al. (2015) when they studied the content of mtDNA in plasma and mononuclear cells of peripheral blood. The authors showed a decrease in mtDNA after 12 months of ARVT with NRTI medications [25].
It should be noted that NRTI medications differ significantly from each other in the severity of toxic effects on mitochondria. Thus, abacavir and lamivudine are significantly less toxic than zidovudine, stavudine or didanosine; as for zalcitabine, due to its high toxicity, it has ceased to be administered at all [26]. Among modern NRTI medications which are recommended as the first line therapy (tenofovir, abacavir, emtricitabine, lamivudine), manifestations of mitochondrial toxicity are minimal. It is important to emphasize that ARVT medications with high mitochondrial toxicity are currently not recommended by specialists in the treatment of HIV infection in ARVT schemes. The only exception is zidovudine, which is still recommended in some cases [27, 28].
In our study, every second patient (50.5%) was administered NRTI medications with a confirmed high toxic effect on mitochondria. As a result, in this group of women, mtDNA copy number in cumulus cells was significantly lower than one in the control group (554.6 (239.5) and 639 (197.9); p = 0.04); and in case of administration of NRTI medications with a minor toxic effect on mitochondria, it was comparable to the healthy controls (574.3(224.6) and 639 (197.9); p = 0.06).
Conclusion
Thus, infertility in HIV-infected women is more frequently caused by tubal and peritoneal factor. In this group of patients, there is a decrease in the concentration of AMH, so in performing IVF there can be a smaller number of oocytes and embryos. Low rate of pregnancy and live birth is noted, probably due to the gonadotoxic effects of AVRT on the function of oocyte mitochondria and it depends on the duration of therapy. Patients with HIV infection should not postpone motherhood, and when an undetectable level of viral load is reached, it is recommended to perform an IVF program for epidemiological indications or for the purpose of treating infertility. In administration of ARVA treatment, preference should be given to medications with a confirmed lower toxic effect on mitochondria. HIV-infected women who postpone motherhood should consider the possibility of performing an IVF program with cryopreservation of oocytes/embryos when the patients reach undetectable level of viral load in the blood.
References
- Федеральный научно-методический центр по профилактике и борьбе со СПИДом ФБУН Центрального НИИ эпидемиологии Роспотребнадзора. Доступно по: http//www.hivrussia.info [Federal'nyi nauchno-metodicheskii tsentr po profilaktike i bor'be so SPIDom FBUN Tsentral'nogo NII epidemiologii Rospotrebnadzora. Available at: http//www.hivrussia.info (in Russian).]
- Marques C., Guerreiro C., Soares S.R. Lights and shadows about the effectiveness of IVF in HIV infected women: a systematic review. Infect. Dis. Obstet. Gynecol. 2015; 2015: 517208. https://dx.doi.org/10.1155/2015/517208.
- Lampe M.A., Smith D.K., Anderson G.J., Edwards A.E., Nesheim S.R. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. Am. J. Obstet. Gynecol. 2011; 204(6): 488. e1-8. https://dx.doi.org/10.1016/j.ajog.2011.02.026.
- Bujan L., Pasquier C. People living with HIV and procreation:30 years of progress from prohibition to freedom? Hum. Reprod. 2016; 31(5): 918-25. https://dx.doi.org/10.1093/humrep/dew036.
- Savasi V., Mandia L., Laoreti A., Cetin I. Reproductive assistance in HIV serodiscordant couples. Hum. Reprod. Update. 2013; 19(2): 136-50. https://dx.doi.org/10.1093/humupd/dms046.
- Stora C., Epelboin S., Devouche E., Matheron S., Epelboin L., Yazbeck C. et al. Women infected with human immunodeficiency virus type 1 have poorer assisted reproduction outcomes: a case-control study. Fertil. Steril. 2016; 105(5): 1193-201. https://dx.doi.org/10.1016/j.fertnstert.2015.12.138.
- Johns Hopkins Bloomberg School of Public Health. News Releases. Fertility rate of HIV-infected women is 37 percent less than that of healthy women. May 15, 2000. Available at: http://www.jhsph.edu/news/news-releases/2000/hiv-fertility.html
- Santulli P., Chopin N., Patrat C., Marcellin L., Wolf J.P., Chapron C. et al. IVF-ICSI in HIV positive and sero-discordant couples: results of five-years of experience. Hum. Reprod. 2009; 24: Abstract Book 1: 342.
- Payne B.A., Wilson I.J., Hateley C.A., Horvath R., Santibanez-Koref M., Samuels D.C. et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat. Genet. 2011; 43(8): 806-10. https://dx.doi.org/10.1038/ng.863.
- Jitratkosol M.H., Sattha B., Maan E.J., Gadawski I., Harrigan P.R., Forbes J.C. et al. Blood mitochondrial DNA mutations in HIV-infected women and their infants exposed to HAART during pregnancy. AIDS. 2012; 26(6): 675-83. https://dx.doi.org/10.1097/QAD.0b013e32835142eb.
- Morén C., Noguera-Julian A., Garrabou G., Rovira N., Catalán M., Bañó M. et al. Mitochondrial disturbances in HIV pregnancies. AIDS. 2015; 29(1): 5-12. https://dx.doi.org/10.1097/QAD.0000000000000486PMID: 25268887.
- López S., Coll O., Durban M., Hernàndez S., Vidal R., Suy A. et al. Mitochondrial DNA depletion in oocytes of HIV-infected antiretroviral-treated infertile women. Antivir. Ther. 2008; 13(6): 833-8.
- Bostan A., Demeestere I., Vanderwinden J.M., Devreker F., Englert Y. Nucleoside analog stavudine depletes mitochondrial DNA with no organelle loss in mouse oocytes. Curr. HIV Res. 2010; 8(2): 127-33. https://dx.doi.org/10.2174/157016210790442678.
- Pawlak P., Chabowska A., Malyszka N., Lechniak D. Mitochondria and mitochondrial DNA in porcine oocytes and cumulus cells - A search for developmental competence marker. Mitochondrion. 2016; 27: 48-55. https://dx.doi.org/10.1016/j.mito.2015.12.008.
- Coll O., Suy A., Figueras F., Vernaeve V., Martı´nez E., Mataro´ D. et al. Decreased pregnancy rate after in-vitro fertilization in HIV-infected women receiving HAART. AIDS. 2006; 20(1): 121-3. https://dx.doi.org/10.1097/01.aids.0000196161.25647.35.
- Santulli P., Gayet V., Fauque P., Chopin N., Dulioust E., Wolf J.P. et al. HIV-positive patients undertaking ART have longer infertility histories than agematched control subjects. Fertil. Steril. 2011; 95(2): 507-12. https://dx.doi.org/10.1016/j.fertnstert.2010.09.018.
- Nurudeen S.K., Grossman L.S., Bourne L., Guarnaccia M.M., Sauer M.V., Douglas N.C. Reproductive outcomes of HIV seropositive women treated by assisted reproduction. J. Women’s Health. (Larchmt). 2013; 22(3): 243-9. https://dx.doi.org/10.1089/jwh.2012.3855.
- Ohl J., Partisani M., Demangeat C., Binder-Foucard F., Nisand I., Lang G.M. Alterations of ovarian reserve testsin Human Immunodeficiency Virus (HIV)-infected women. Gynecol. Obstet. Fertil. 2010; 38(5): 313-7. https://dx.doi.org/10.1016/j.gyobfe.2009.07.019.
- Scherzer R., Bacchetti P., Messerlian G., Goderre J., Maki P.M., Seifer D.B. et al. Impact of CD4+ lymphocytes and HIV infection on anti-Müllerian hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am. J. Reprod. Immunol. 2015; 73(3): 273-84. https://dx.doi.org/10.1111/aji.12332.
- Martinet V., Manigart Y., Rozenberg S., Becker B., Gerard M., Delvigne A. Ovarian response to stimulation of HIV-positive patients during IVF treatment: a matched, controlled study. Hum. Reprod. 2006; 21(5): 1212-7. https://dx.doi.org/10.1093/humrep/dei493.
- Prisant N., Tubiana R., Lefebvre G., Lebray P., Marcelin A.G., Thibault V. et al. HIV-1 or hepatitis C chronic infection in serodiscordant infertile couples has no impact on infertility treatment outcome. Fertil. Steril. 2010; 93(3): 1020-3. https://dx.doi.org/10.1016/j.fertnstert.2009.07.1663.
- Reynier P., May-Panloup P., Chrétien M.F., Morgan C.J., Jean M., Savagner F.et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol. Hum. Reprod. 2001; 7(5): 425-9. https://dx.doi.org/10.1093/molehr/7.5.425.
- Dhakshinamoorthy Subashini, Thongadi Ramesh Dinesha, Rao B. Srirama, Jayaseelan Boobalan, Selvamuthu Poongulali, Devaraj A. Chitra et al. Mitochondrial DNA content of peripheral blood mononuclear cells in ART untreated & stavudine/zidovudine treated HIV-1-infected patients. Indian J. Med. Res. 2018; 148(2): 207-14. https://dx.doi.org/10.4103/ijmr.IJMR_1144_16.
- Masyeni S., Sintya E., Megawati D., Sukmawati N.M.H., Budiyasa D.G., Aryastuti S.A. et al. Evaluation of antiretroviral effect on mitochondrial DNA depletion among HIV-infected patients in Bali. HIV AIDS (Auckl). 2018; 10: 145-50. https://dx.doi.org/10.2147/HIV.S166245.
- Dai Z., Cai W., Hu F., Lan Y., Li L., Chung C. et al. Plasma mitochondrial DNA levels as a biomarker of lipodystrophy among HIV-infected patients treated with highly active antiretroviral therapy (HAART). Curr. Mol. Med. 2015; 15(10): 975-9. https://dx.doi.org/10.2174/1566524016666151123114401.
- Bolhaar M.G., Karstaedt A.S. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin. Infect. Dis. 2007; 45(2): 254-60. https://dx.doi.org/10.1086/518976.
- EACS Guidelines version 10.0 Available at: https://eacs.sanfordguide.com/contents/changes-v9-1-to-v10-0
- Покровский В.В., Юрин О.Г., Кравченко А.В., Беляева В.В., Ермак Т.Н.,Канестри В.Г., Шахгильдян В.И., Козырина Н.В., Буравцова В.В., Нарсия Р.С., Хохлова О.Н., Покровская А.В., Ефремова О.С., Коннов В.В.,Куимова У.А., Попова А.А., Воронин Е.Е., Афонина Л.Ю., Васильева И.А., Зимина В.Н. и др. Рекомендации по лечению ВИЧ-инфекции и связанных с ней заболеваний, химиопрофилактике заражения ВИЧ. Эпидемиология и инфекционные болезни, актуальные вопросы. 2018; (4, Прил.).[Pokrovskii V.V., Yurin O.G., Kravchenko A.V., Belyaeva V.V., Ermak T.N., Kanestri V.G., Shakhgil'dyan V.I., Kozyrina N.V., Buravtsova V.V., Narsiya R.S.,Khokhlova O.N., Pokrovskaya A.V., Efremova O.S., Konnov V.V.,Kuimova U.A., Popova A.A., Voronin E.E., Afonina L.Yu., Vasil'eva I.A., Zimina V.N. et al. Rekomendatsii po lecheniyu VICh-infektsii i svyazannykh s nei zabolevanii, khimioprofilaktike zarazheniya VICh. Epidemiology and Infectious Diseases. Current Items/Èpidemiologiâ i infekcionnye bolezni. Aktual’nye voprosy, aktual'nye voprosy. 2018; (4, Suppl.).(in Russian).]
Received 04.02.2020
Accepted 07.02.2020
About the Authors
Elena V. Mityurina, PhD, researcher, the 1st Gynecological Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79647967465. E-mail: mity-elena@yandex.ru,Svetlana G. Perminova, MD, leading researcher, the 1st Gynecological Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79162021687. E-mail: perisvet@list.ru
Fatima N. Selimova, postgraduate student, the 1st Gynecological Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79268887755. E-mail: doc.fselimova@mail.ru
Olga V. Burmenskaya, Doctor of Biological Sciences, head of the Laboratory of Oncological Genetics, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954382292.
E-mail: o_bourmenskaya@oparina4.ru
Nadezhda V. Kozyrina, PhD, researcher, Central Research Institute of Epidemiology, Federal Service on Customers’ Rights Protection and Human Well-being Surveillance. 111123, Russia, Moscow, Novogireevskaya str., bldg. 3a.
Tel.: +79167151018. E-mail: nad-kozyrina@yandex.ru
Aleksey V. Kravchenko, MD, professor, leading researcher, Federal Scientific-Methodological Center for the Prevention and Control of AIDS, Central Research Institute of Epidemiology, Federal Service on Customers’ Rights Protection and Human Well-being Surveillance. 111123, Russia, Moscow, Novogireevskaya str., bldg. 3a.
Tel.: + 74953660518. E-mail: alexey-ravtchenko@yandex.ru
For citation: Mityurina Е.V., Perminova S.G., Selimova F.N., Burmenskaya О.V., Kozyrina N.V., Kravchenko А.V. Gonadotoxic effect of antiretroviral therapy in hiv-infected women. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 111-119. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.111-119



