Preeclampsia as a separate gestational clinical and pathogenetic form of insulin resistance syndrome
Objective: To develop the concept of preeclampsia based on the similar clinical and laboratory features of preeclampsia and insulin resistance syndrome (IRS).Tezikov Yu.V., Lipatov I.S., Azamatov A.R., Tyutyunnik V.L., Kan N.E., Zumorina E.M., Kuzmina A.I.
Materials and methods: This prospective study included 292 pregnant women retrospectively divided into four groups. Group 1 comprised 89 high-risk women with preeclampsia, Group 2 consisted of 50 women with IRS and preeclampsia, Group 3 consisted of 32 pregnant women with IRS without preeclampsia, Group 4 (control group) included 30 pregnant women with a healthy pregnancy. The examination was conducted at weeks 11–14, 18–21, and 30–34 of pregnancy and the onset of preeclampsia.
Results: There was a similarity of clinical manifestations of preeclampsia and IRS in the form of arterial hypertension, proteinuria/microalbuminuria, abnormal diurnal blood pressure pattern, gestational /obstructive sleep apnea syndrome, abdominal visceral adipose tissue accumulation and insomnia. There were no significant differences in the incidence of preeclampsia at different times of its onset and severity in the high-risk group without somatic pathology (49.4%) and the group with IRS (61.0%) [χ2=2.56, p=0.11]. Preeclampsia and IRS had common underlying mechanisms, characterized by an increase in pathological IR and hyperinsulinemia (HI) from the early stages of pregnancy and associated with them dyslipidemia, leptinemia, uricemia, abdominal visceral adipose tissue accumulation, inflammatory state, endothelial and hemostasis disorders, due to disruption of gestational adaptation to placental anti-insulin factors that provide stable fetal nutritional and energy supply. The time of preeclampsia onset is determined by processes associated with pathological IR, HI, and alteration in the fetal-placental unit.
Conclusion: Comprehensive evaluation of pregnant women with preeclampsia and without somatic comorbidities, with preeclampsia and IRS, and with an uncomplicated pregnancy allowed for creating a scientific concept of preeclampsia development, according to which preeclampsia is considered as a separate gestational clinical and pathogenetic form of IRS, which defines the prospect of the prediction and prevention strategy as the priority directions of medical management of high-risk pregnant women.
Keywords
Rapid advances in medical technologies resulted in discovering new molecular bases of the pathogenesis of various conditions. From this perspective, there is a need for new approaches to investigating insulin resistance syndrome (IRS) or metabolic syndrome, diabetes, arterial hypertension (AH), obesity, which are the leading risk factors for gestational and perinatal complications [1–3].
Insulin and insulin resistance (IR) plays the most critical role in the autoregulation of energy storage and expenditure [4, 5]. The latter accompanies us at all stages of life and is realized in many physiological processes, including the sleep-wake cycle, the menstrual cycle, tissue repair, and, most importantly, during pregnancy [6]. An essential function of pregnancy is fetal nutritional and energy supply, and the critical regulator of this function is IR. It induces molecular signaling pathways of energy redirection by limiting the supply of nutrient substrates to the cells of the pregnant body, with the saved energy ensuring fetal growth and development [7, 8].
In recent decades, clinicians have increasingly observed the pathological nature of IR due to the influence of risk factors associated with lifestyle and diet, unhealthy habits, ecology, and epigenetic disorders, which has led to an increase in the incidence of dysmetabolic diseases [1, 9, 10]. Pathological IR plays a key role in impaired antenatal programming of metabolic processes, acts as a leading cause of type 2 diabetes mellitus, obesity, and, most importantly, endothelial dysfunction in essential hypertension and IRS [4, 11]. It should be noted that endothelial dysfunction is also a well-studied pathogenetic mechanism of preeclampsia that determines its clinical manifestations [12, 13]. However, the question: "What leads to gestational endotheliosis, what are its causes and mechanisms?" is still a stumbling block in obstetrics. Therefore, given the changes associated with pregnancy, similar to IRS [6], the connection of dysmetabolic mechanisms with the formation of endothelial dysfunction in essential hypertension, it is relevant to determine the place and role of metabolic disorders in the pathogenesis of preeclampsia.
This study aimed to develop the concept of preeclampsia based on the similar clinical and laboratory features of preeclampsia and insulin resistance syndrome.
Materials and methods
This prospective study was conducted at the Perinatal Center of V.D. Seredavin Samara Regional Clinical Hospital. The study included 292 women, 180 of whom had an increased risk of preeclampsia (presence of independent risk factors, including preeclampsia in the obstetric history – 72 women (40.0%), family history – 71 (39.4%), first pregnancy of older reproductive age (>35 years) – 37 (20.6%) observations), 82 women with IRS and 30 women with uncomplicated pregnancy. The patients were retrospectively allocated into groups depending on the occurrence of preeclampsia. Group 1 included 89 women with preeclampsia; Group 2 included 50 women with IRS and preeclampsia; Group 3 consisted of 32 pregnant women with IRS without preeclampsia; Group 4 (control) was represented by 30 pregnant women with a healthy progressive pregnancy.
The criteria for inclusion in Group 1 were preeclampsia in women with independent risk factors, prenatal B.P. less than 130 and 85 mm Hg, body mass index of 18.5 to 24.9 kg/m2, and the absence of metabolic abnormalities. International Diabetes Federation (2005) criteria were used to identify women with IRS, also registered at the pre-pregnancy stage (waist circumference as a manifestation of visceral obesity and two additional of the following criteria – triglyceride (T.G.), high-density lipoprotein (HDL) concentration, B.P. level – were taken into account as mandatory criteria). To offset the effect of baseline hyperglycemia on gestational change processes, the plasma glucose concentration in women with IRS was <6.1 mmol/L. The exclusion criteria for all groups were pre-pregnancy carbohydrate metabolism disorders, somatic (except for IRS in Groups 2 and 3), infectious and autoimmune pathology, gestational diabetes, PCOS, fetal and genital malformations of the pregnant woman, induced pregnancy, and violation of the examination protocol in the course of pregnancy.
The women were examined at weeks 11–14, 18–21, 30–34 of pregnancy and during the onset of preeclampsia and included assessment of metabolic parameters, including venous blood glucose, HDL, T.G., uric acid, calculation of I.R. (HOMA-IR) and TG/HDL), hormones (leptin, insulin, placental lactogen (P.L.), prothrombotic markers (platelet aggregation, mean platelet volume) and proinflammatory (tumor necrosis factor-α (TNF-α), leukocyte activation index (LAI)) statuses, vascular endothelial destabilization (desquamated endothelial cells (DEC), nitric oxide (NO) metabolites), placental α-microglobulin-1 (PAMG-1), placental growth factor (PGF). The clinical examination consisted of determining diurnal BP patterns, assessing adipose tissue deposition, subjective evaluation of sleep characteristics, and periods of gestational sleep apnea.
Laboratory and instrumental investigations were performed using Sysmex XN-1000 analyzers (Sysmex Corporation, Japan), Alat-2 (Biola, Russia), Architect c4000, Architect i1000 SR (Abbotte, USA), Voluson E6 ultrasound system GE Healthcare (GE, USA). Automatic BP-Lab system (Petr Telegin, Russia) was used to assess diurnal blood pressure patterns. Subjective assessment of sleep characteristics was performed according to the recommendations of J.I. Levin (1995) [14]. Sleep apnea periods were diagnosed using SomnoCheck cardiorespiratory sleep monitoring device (Weinmann, Germany). Adipose tissue distribution was evaluated according to K. Tayama et al. (1999) by measuring preperitoneal (P-fat) and subcutaneous (S-fat) fat deposition. The abdominal wall fat index (AWFI) was calculated as the ratio of the maximum thickness of preperitoneal fat to the minimum thickness of subcutaneous fat. AWFI >1.0 shows visceral type of abdominal wall fat deposition [15]. The diagnosis of preeclampsia was made according to the recommendations of the clinical protocol [16]. The severity of placental insufficiency was assessed, considering the recommendations of A.N. Strizhakov et al. (2014) [17].
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 25 HC IMAGO 5.0 (IBM, USA). The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. Variables not meeting normality assumptions were reported as the median (Me) with interquartile range Q1 (25% quartile) to Q3 (75% quartile). Independent samples were compared using the Mann–Whitney U-test. Categorical variables were compared using the Pearson's χ² test and Pearson's χ² with the Yates correction for 2×2 tables. Paired samples (changes during pregnancy) were compared by Wilcoxon W-test. Spearman correlation analysis was used to assess the relationships between the variables.
Results and discussion
A systemic view of the preeclamptic problem allows us to note a pronounced clinical similarity between preeclampsia and IRS, and it concerns AH and proteinuria as main criteria of preeclampsia, and circadian changes in BP, obstructive sleep apnea syndrome, insomnia, and adipose tissue deposition (Table 1). The choice of these clinical manifestations is related to the fact that IRS has characteristic features [9, 18, 19], which were also detected in preeclampsia. Abnormal types of BP variability, characterized by elevated BP at night (night -picker) or by its insufficient decrease (<10% compared to the average daytime level, non-dipper) at night, were found not only in Groups 2 and 3 women with IRS but also in 53.9% of Group 1 women with preeclampsia (p˃0.05).
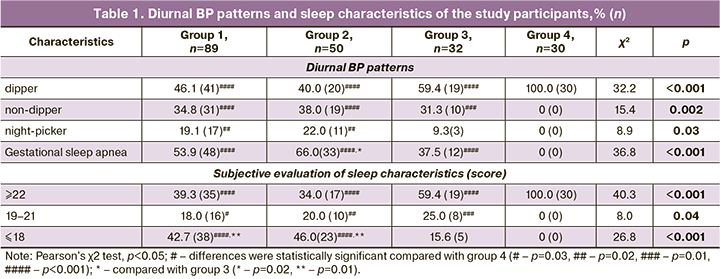
Obstructive sleep apnea syndrome is a separate clinical and pathogenic variant of IRS and is characterized by IR potentiated by oxidative stress and elevated catecholamine levels at night during periods of hypoxemia and hypoxia [19, 20]. Such respiratory dysfunction in pregnant women, termed gestational sleep apnea, occurs in 53.9% of preeclampsia without concurrent extragenital pathology, in 66.0% and 37.5% of observations in Groups 2 and 3 (χ21-2=1.92, p1-2=0.17; χ21-3=2.54, p1-3=0.11; χ22-3=6.40, p2-3=0.01). Associated with IRS insomnia is characterized by a poor quality of nighttime sleep, negative dreams, daytime sleepiness, and occurs in 42.7% of women with preeclampsia in Group 1 and 46.0% in Group 2, is also associated with elevated BP and periods of sleep apnea at night, which reflects similarity of impaired central mechanism in preeclampsia and IRS.
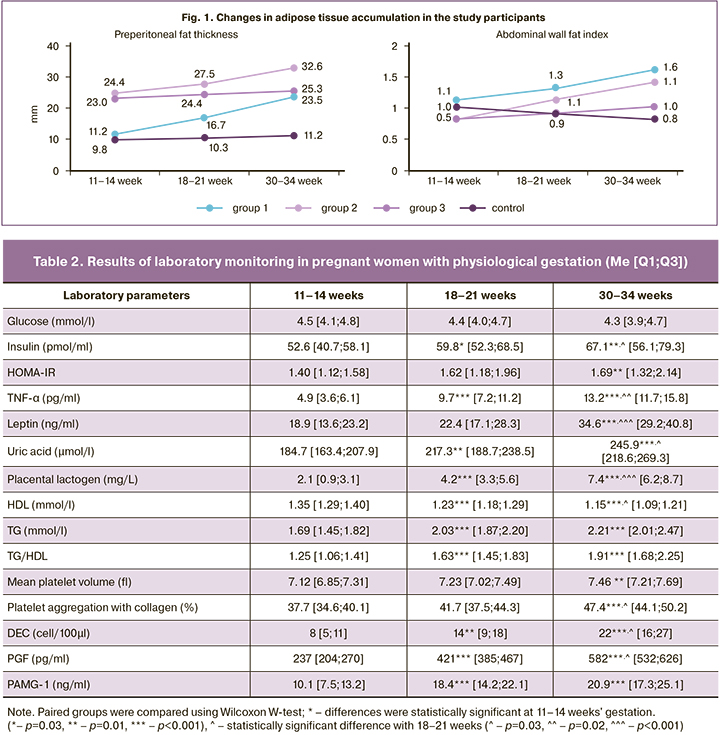
Of interest are results from an analysis of adipose tissue deposition in patients with preeclampsia, the volume and functional activity of which determine the leading IRS criteria suggested by most of the international guidelines [9]. Thus, data from dynamic ultrasonography showed that in women with preeclampsia, the increase in adipose tissue is mainly because of preperitoneal (visceral) fat. The changes presented in Fig. 1 graphically show a statistically significant 1- and 1,3-fold increase of P-fat during gestation in Groups 1 and 2, respectively (p<0,001) and also the dynamics of AWFI; AWFI >1.0 shows visceral type of abdominal wall fat deposition. This confirms the similar pattern of adipose tissue distribution in both IRS and preeclampsia, with a predominance of visceral fat.
There is no doubt that the clinical similarity between preeclampsia and IRS has a pathogenic basis. This fact is supported by the rates of preeclampsia, which occurred in 49.4% (89/180) of women in the group with independent risk factors, and reached 61.0% (50/82) in the IRS group [χ2=2.56, p=0.11]. The high incidence of preeclampsia and its comparability in the study groups undoubtedly reflects the similarity of IRS and preeclampsia pathogenesis.
When analyzing the development of physiological gestation, it was noted that healthy pregnancy causes changes inherent to IRS. A woman's organism during pregnancy is characterized by a switch of cellular energy supply from the carbohydrate component, glucose, to fat. This process of natural selection is fixed to create the most favorable conditions for fetal growth and development [6, 7]. According to our findings (Table 2), physiological gestation is accompanied by a statistically significant increase in HOMA-IR, insulin levels, lipid profile changes (p<0.05 for all indicators). These changes indicate a diabetogenic and atherogenic metabolic shift with the formation of physiological IR and hyperinsulinemia (HI), which is consistent with the literature [8]. This indicates the similarity of physiological pregnancy with the functional phase of the metabolic syndrome [21]. The metabolic pattern detected in the control group, as well as moderate proinflammatory and hypercoagulable states, activation of the endothelial-platelet link in the disruption of permanent adaptation mechanisms may exceed the physiological nature of the increase with implementation in structural disorders and act as preconditions for the onset of preeclampsia.
The results of laboratory monitoring in pregnant women with preeclampsia and IRS showed marked similarity in all parameters (Fig. 2). IR and HI, the key components of IRS, increased significantly in women with preeclampsia from the first trimester of pregnancy compared with controls, becoming pathological (p<0.001). The high intensity of atherogenic and hyperuricemic shifts in patients with preeclampsia, which are also characteristic of IRS, is reflected in the changes of lipid profile and uric acid (p<0.001). Blood glucose levels in all groups were within the reference range, due to the chosen inclusion and exclusion criteria (p>0.05).
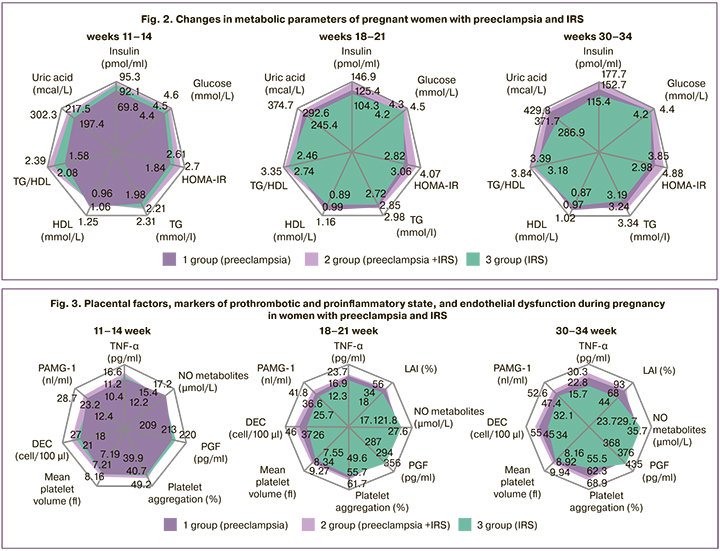
Preeclampsia, like IRS, is accompanied by a pronounced counter-insular shift in hormonal profile, which is generated by the placenta to generate IR for the energy supply of the developing fetus. The concentration of PL, which has the most pronounced counter-insular effect of placental hormones, is significantly higher in pregnant women with preeclampsia during gestation than in women in Groups 3 and controls (Group 1 – 5.7 [4.1;7.0], 9.3 [7.6;10.2] and 12.5 [11.2;13.4] mg/l; Group 2 –7.5 [6.3;8.9], 11.7 [10.4;13.1] and 14.6 [13.6;15.3] mg/L; Group 3 – 2.5 [1.4;3.7], 4.8 [3.9;6.0] and 7.9 [6.5; 9.1] mg/L at 11–14, 18–21, and at 30–34 weeks of gestation, respectively, p1-3, p1-4, p2-3, p2-4<0.001 at each time-point of examination), which determines the pathological increase in IR and HI levels. Levels of leptin, which plays an essential role in the pathogenesis of IRS and is closely related to IR [22], also had the largest increases in the groups with preeclampsia (Group 1 – 32.5 [25.8;36.4], 62.4 [56.4;69.6], 83.9 [76.3;88.1] ng/mL; Group 2 – 46.7 [41.1;54.8], 77.6 [72.3;81.4], 86.2 [81.9;92.3] ng/ml; Group 3 – 41.4 [37.6;50.1], 48.3 [41.2;54.1], 59.8 [51.3 67.7] ng/mL at 11–14, 18–21, and 30–34 weeks of gestation, respectively, p1-3, p1-4, p2-3, p2-4<0.001 at each term of examination).
It is widely known that in IRS, the body develops a state of chronic low-intensity inflammation, which is maintained by the synthesis in adipose tissue of TNF-α, the most important mediator of inflammation and IR [23]. Pregnant women with preeclampsia also showed a significant increase in TNF-α compared with the control group at all gestational ages (p<0.001) (Fig. 3). The dynamics of IAL, which characterizes the functional activity of leukocytes, showed similar changes, which also confirms the formation of immune-metabolic disorders in preeclampsia and IRS. The laboratory features of IRS include the formation of a prothrombotic state, and the findings showed similar changes in pregnant women with preeclampsia and IRS; they included an increase in the average platelet volume at the expense of young and activated forms, and an increase in platelet aggregation activity. In pregnant women with preeclampsia and IRS, compared with controls, we found statistically significantly lower levels of PGF, which belongs to one of the proteins of the vascular endothelial growth factor family. At the same time, according to the literature, a similar deficiency of these factors is described in IRS outside pregnancy, indicating similar anti-angiogenic changes in preeclampsia and IRS [24].
The data on a statistically significant decrease in the concentration of NO metabolites in pregnant women of groups 1, 2, and 3 compared with controls favor similarity of the mechanisms of AH formation in preeclampsia and IRS. Moreover, the dynamics of the parameters indicate the determinacy of NO synthesis abnormalities in the second and third trimesters by the increase in pathological IR and HI. In addition to decreased NO metabolites, desquamated epithelial cells suggest the morphofunctional damage to vascular endotheliocytes. It should be noted that pregnant women with preeclampsia and IRS showed a similar increase in DECs, exceeding the control values (p<0.001).
The changes in the studied parameters and the results of correlation analysis indicate the primacy of dysmetabolic processes associated with pathological IR and HI, relative to other, later, links in the pathogenesis of preeclampsia. There was a strong positive relationship between insulin hormone concentration, IR index and TNF-α content (k=0.83; k=0.85, at p<0.001), DEC (k=0.86; k=0.89, at p<0.001), platelet aggregation (k=0.81; k=0.87, at p<0.001), P-fat (k=0.85; k=0.89, at p<0.001). There was a strong negative relationship with the concentration of NO metabolites (k=-0.81; k=-0.88, at p<0.001), FRP (k=-0.87; k=-0.92, at p<0.001). There was also a positive association of moderate strength between insulin levels, HOMA-IR, and uric acid concentration (k=0.69; k=0.70, at p<0.05).
Consequently, the identified clinical and laboratory parallels reflecting the common changes of pathogenetically significant laboratory parameters with implementation in similar clinical manifestations of preeclampsia and IRS indicate that preeclampsia and IRS are formed by common mechanisms, in which IR is triggered either by the fat tissue – in IRS, or by the placenta – in preeclampsia.
The question of pathogenic heterogeneity of early and late preeclampsia remains extremely relevant. At the same time, the differences observed at the population level are essentially due only to the rate of manifestation of common clinical manifestations – AH and proteinuria, as well as the frequency of complications that mask the pathophysiological changes inherent to preeclampsia [25–27]. An analysis of the examination results of women with early and late uncomplicated preeclampsia from the group with independent risk factors (Group 1) allowed us to objectify the clinical and laboratory features of different gestational ages of the onset of preeclampsia. Analysis of the clinical and laboratory features of uncomplicated early and late preeclampsia showed no significant differences in the frequency of complications related to the functioning of the fetal-placental unit, with a tendency for these complications to occur mainly in women with early preeclampsia (Table 3). We can conclude that the pathology of the fetal-placental unit, including threatened pregnancy termination, placental insufficiency of varying severity, and preterm labor is not specific and of primary importance in the mechanisms of early preeclampsia development.
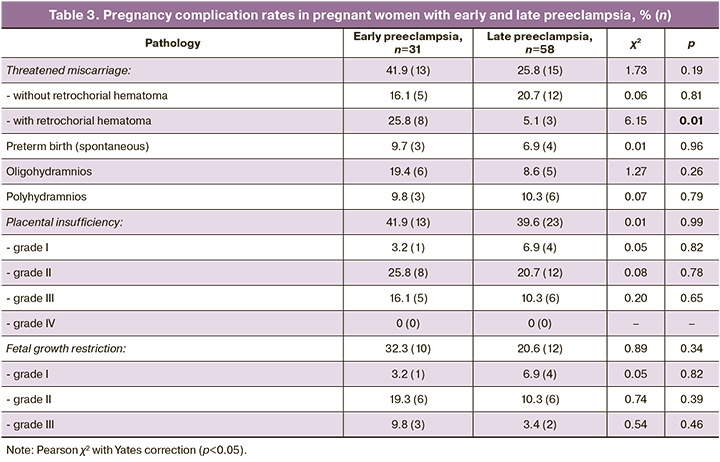
Analysis of circadian BP profile and subjective sleep characteristics in women with early and late preeclampsia also showed no significant differences (p>0.05).
In early and late uncomplicated preeclampsia, we found no differences in most of the studied markers (p>0.05), indicating the same involvement of pathological IR and HI and related disorders in the underlying mechanisms of both early and late preeclampsia (Fig. 4). This indicates that there is no underlying pathogenic heterogeneity of preeclampsia of different times of clinical manifestation.
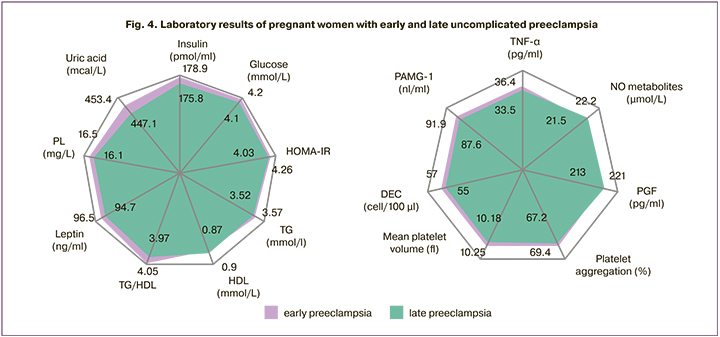
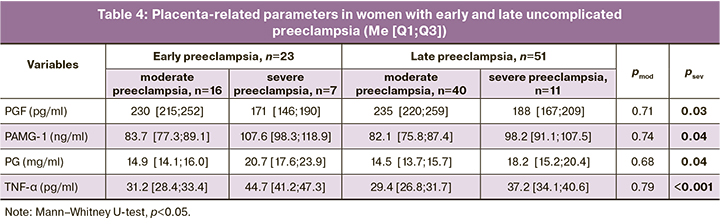
At the same time, there was a statistically significant predominance of a number of placental-associated parameters in early severe preeclampsia against the observed tendency to develop more severe forms of placental insufficiency (Table 4). This fact confirms that the dysfunction of the fetal-placental system as an additional destabilizing factor, along with pre- and periconceptional disorders, gene network deviations and epigenetic mechanisms, immune reactions accelerate the development and increase in the common underlying mechanisms of pathological IR and HI, which determines the individual time of preeclampsia manifestation [28, 29].
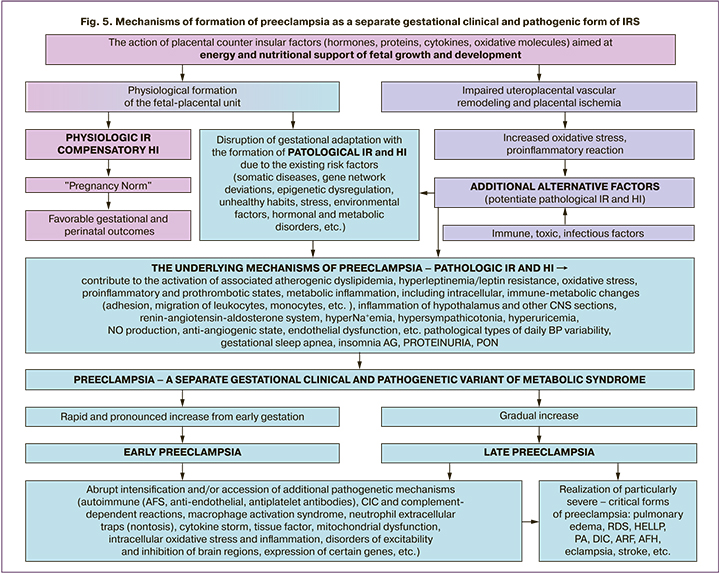
Consequently, the presented arguments confirming the clinical and laboratory identity of preeclampsia and IRS allow us to consider preeclampsia as a separate gestational clinical and pathogenic form of insulin resistance syndrome. Their underlying mechanisms are unified and universal, regardless of the gestational age of clinical manifestation of preeclampsia. A generalized scheme of the developed scientific concept is shown in Figure 5. The buildup of pathological IR and HI from early gestation determines the direction of pathological changes in high-risk pregnancy with the realization of gestational AH, proteinuria, and multiple organ failure.
Conclusion
Our findings have allowed us to develop a new concept of the preeclampsia pathogenesis with the preconditions for its formation that are inherent to the patterns of healthy pregnancy. Disruption of gestational adaptation to placental counter-insulator factors leads to a marked increase in physiological changes with a transformation in the pathogenic links of preeclampsia. Pathophysiological mechanisms of preeclampsia development, as well as IRS, are pathological IR, HI, which achieves its effect through secondary atherogenic dyslipidemia, hyperleptinemia and hyperuricemia, metastases and immune-metabolic disorders, oxidative stress, prothrombotic status, antiangiogenic state. They lead to structural and functional destabilization of the vascular endothelium, acting only as the tip of the iceberg in the chain of pathogenic events of preeclampsia, and are implemented in the complex of AH, proteinuria, and multiple organ failure.
The presence of additional damaging factors, such as placental ischemia, immune, infectious, toxic, gene network deviations, epigenetic dysregulation, potentiates the basic mechanisms of pathogenesis, which determines the term of clinical manifestation of preeclampsia.
Complicated severe preeclampsia is associated with a sharp intensification or accession of additional pathogenic mechanisms, including the development of the so-called cytokine storm, antiphospholipid syndrome, endothelial dysfunction, complement-dependent reactions, leukocyte trap activation (netosis), mitochondrial dysfunction, oxidative stress, etc.
Our results prove the identity of the pathogenic mechanisms of preeclampsia and metabolic syndrome, which allows us to consider this pregnancy complication as a separate clinical and pathogenic variant of IRS.
Pregnancy, as a powerful stress factor, damasks latent disorders associated with phenotypic features of energy supply, particularly with IR, leading to their clinical realization in the form of preeclampsia and gestational diabetes mellitus. The end of pregnancy does not lead to a complete regression of the activated pathological process. Preservation of these genes is associated with a high incidence of cardiovascular and metabolic disorders in women with preeclampsia in later stages of life.
In conclusion, the concept of preeclampsia as a clinical and pathogenic variant of IRS explains its clinical and pathogenic variability and determines the feasibility of developing new diagnostic and prognostic markers to reduce maternal and perinatal morbidity.
References
- Драпкина О.М., Гамбарян М.Г., Горный Б.Э., Карамнова Н.С., Концевая А.В., Новикова Н.К., Попович М.В., Рыбаков И.А., Калинина А.М., Попович М.В., Рыбаков И.А., Калинина А.М. Укрепление здоровья и профилактика хронических неинфекционных заболеваний в условиях пандемии и самоизоляции. Консенсус экспертов Национального медицинского исследовательского центра терапии и профилактической медицины и Российского общества профилактики неинфекционных заболеваний. Кардиоваскулярная терапия и профилактика. 2020; 19(3): 270-94. [Drapkina O.M., Gambaryan M.G., Gorny B.E., Karamnova N.S., Kontsevaya A.V., Novikova N.K. et al. Health promotion and prevention of chronic non-communicable diseases in the context of the COVID-19 pandemic. Consensus of experts of the National society of evidence-based pharmacotherapy and the Russian society of the prevention of non-communicable diseases. Cardiovascular therapy and prevention. 2020; 19(3): 270-94 (in Russian)]. https://dx.doi.org/10.15829/1728-8800-2020-2605.
- Серов В.Н. Метаболический синдром (нейрообменно-эндокринный синдром). Medica mente. Лечим с умом. 2015; 1: 16-9. [Serov V.N. Metabolic syndrome (neuro-endocrine syndrome). Medica mente. Lechim s umom. 2015; 1: 16-9 (in Russian)].
- Chikowore T., Kamiza A.B., Oduaran O.H., Machipisa T., Fatumo S. Non-communicable diseases pandemic and precision medicine: Is Africa ready? EBioMedicine. 2021; 65: 103260. https://dx.doi.org/10.1016/j.ebiom.2021.103260.
- Nolan C.J., Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 2019; 16(2): 118-27. https://dx.doi.org/10.1177/1479164119827611.
- Kolb H., Kempf K., Röhling M., Martin S. Insulin: too much of a good thing is bad. BMC Med. 2020; 18: 224-36. https://dx.doi.org/10.1186/s12916-020-01688-6.
- Гордюнина С.В. Инсулинорезистентность при беременности. Проблемы эндокринологии. 2013; 59(5): 61-6. [Gordyunina S.V. Pregnancy insulin resistance. Problemy endokrinologii/ Problems of Endocrinology. 2013; 59(5): 61-6 (in Russian)].
- Napso T., Yong H.E.J., Lopez-Tello J., Sferruzzi-Perri A.N. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front. Physiol. 2018; 9: 1091. https://dx.doi.org/10.3389/fphys.2018.01091.
- Sun Y.Y., Juan J., Xu Q.Q., Su R.N., Hirst J.E., Yang H.X. Increasing insulin resistance predicts adverse pregnancy outcomes in women with gestational diabetes mellitus. J. Diabetes. 2020; 12(6): 438-46. https://dx.doi.org/10.1111/1753-0407.13013.
- Zafar U., Khaliq S., Ahmad H.U., Manzoor S., Lone K.P. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones (Athens). 2018; 17(3): 299‐313. https://dx.doi.org/10.1007/s42000-018-0051-3.
- Saklayen M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018; 20(2): 12. https://dx.doi.org/10.1007/s11906-018-0812-z.
- Saxena T., Ali A.O., Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev. Cardiovasc. Ther. 2018; 16(12): 879-87. https://dx.doi.org/10.1080/14779072.2018.1540301.
- Сухих Г.Т., Силачев Д.Н., Горюнов К.В., Волочаева М.В., Шмаков Р.Г. Роль дисфункции стволовых клеток в развитии больших акушерских синдромов. Акушерство и гинекология. 2018; 7: 5-11. [Sukhikh G.T., Silachev D.N., Goryunov K.V., Volochaeva M.V., Shmakov R.G. Role of stem cell dysfunction in the development of great obstetrical syndromes. Obstetrics and Gynecology. 2018; 7: 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.7.5-11.
- Кан Н.Е., Ломова Н.А., Амирасланов Э.Ю., Чаговец В.В., Тютюнник В.Л., Хачатрян З.В., Стародубцева Н.Л., Кициловская Н.А., Франкевич В.Е. Особенности метаболомного профиля при преэклампсии. Акушерство и гинекология. 2019; 11: 82-8. [Kan N.E., Lomova N.A., Amiraslanov E.Yu., Chagovets V.V., Tyutyunnik V.L., Khachatryan Z.V. et al. Specific features of a metabolomic profile in preeclampsia. Obstetrics and Gynecology. 2019; 11: 82-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.82-88.
- Ляшенко Е.А., Левин О.С. Расстройства сна в клинической практике. Современная терапия в психиатрии и неврологии. 2017; 1: 22-8. [Lyashenko E.A., Levin O.S. Sleep disorders in clinical practice. Sovremennaya terapiya v psikhiatrii i nevrologii/ Modern therapy in Psychiatry and Neurology. 2017; 1: 22-8. (in Russian)].
- Чабанова Н.Б., Василькова Т.Н. Особенности жирового обмена у беременных в зависимости от срока гестации, массы тела и характера жироотложения. Современные проблемы науки и образования. 2018; 5: 27-9. [Chabanova N.B., Vasil'kova T.N. Features of fat metabolism in pregnant women depending on gestational age, body weight and the nature of fat deposition. Sovremennye problemy nauki i obrazovaniya/ Modern problems of science and education. 2018; (5): 27-9. (in Russian)].
- Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Клинические рекомендации (Протокол лечения). 2021. [Pre-eclampsia. Eclampsia. Hypertensive disorders during pregnancy, childbirth and postpartum Clinical Guidelines (Treatment Protocol). 2021. (in Russian)].
- Стрижаков А.Н., Тезиков Ю.В., Липатов И.С., Шарыпова М.А., Анпилогова И.В., Азизов К.У., Костянова Е.В. Стандартизация диагностики и клиническая классификация хронической плацентарной недостаточности. Вопросы гинекологии, акушерства и перинатологии. 2014; 13(3): 5-12. [Strizhakov A.N., Tezikov Yu.V., Lipatov I.S., Sharypova M.A., Anpilogova I.V., Azizov K.U., Kostyanova E.V. Diagnosis standardization and clinical classification of chronic placental insufficiency. Voprosy ginekologii, akusherstva i perinatologii/ Issues of Gynecology, Obstetrics and Perinatology. 2014; 13 (3): 5-12. (in Russian)].
- Altikardes Z.A., Kayikli A., Korkmaz H., Erdal H., Baba A.F., Fak A.S. A novel method for dipper/non-dipper pattern classification in hypertensive and non-diabetic patients. Technol. Health Care. 2019; 27(Suppl. 1): 47-57. https://dx.doi.org/10.3233/THC-199006.
- Karan S., Ginosar Y. Gestational sleep apnea: have we been caught napping? Int. J. Obstet. Anesth. 2016; 26: 1-3. https://dx.doi.org/10.1016/j. ijoa.2016.03.001.
- Калачин К.А., Пырегов А.В., Шмаков Р.Г. Гестационное сонное апноэ. Связь беременности и преэклампсии с синдромом обструктивного апноэ сна. Альманах клинической медицины. 2019; 47(3): 266-75. [Kalachin K.A., Pyregov A.V., Shmakov R.G. Gestational sleep apnea. The relationship of pregnancy and preeclampsia with obstructive sleep apnea syndrome. Al'manakh klinicheskoi meditsiny/ Clinical Medicine Almanac. 2019; 47(3): 266-75. (in Russian)]. https://dx.doi.org/10.18786/2072-0505-2019-47-031.
- Бунятин А.А., Мизиков В.М., ред. Анестезиология. Национальное руководство. М.: ГЭОТАР-Медиа; 2017. 656с. [Bunyatin A.A., Mizikov V.M., ed. Anesthesiology: National guidance. Moscow: GEOTAR-Media. 2017; 656 p. (in Russian)].
- Ökdemir D., Hatipoğlu N., Kurtoğlu S., Siraz Ü.G., Akar H.H., Muhtaroğlu S. et al. The role of irisin, insulin and leptin in maternal and fetal interaction. J. Clin. Res. Pediatr. Endocrinol. 2018; 10(4): 307-15. https://dx.doi.org/10.4274/jcrpe.0096.
- Романцова Т.И., Сыч Ю.П. Иммунометаболизм и метавоспаление при ожирении. Ожирение и метаболизм. 2019; 16(4): 3-17. [Romantsova T.I., Sych Yu.P. Immunometabolism and metainflammation in obesity. Obesity and metabolism. 2019; 16(4): 3-17 (in Russian)].
- Шепель Р.Н., Драпкина О.М. Новые векторы в диагностике метаболического синдрома: оценка уровня сосудистого эндотелиального фактора роста, пентраксина-3 и трансформирующего фактора роста бета. Кардиоваскулярная терапия и профилактика. 2019; 18(6): 57-61. [Shepel R.N., Drapkina O.M. New directions in metabolic syndrome diagnosis: assessment of vascular endothelial growth factor, pentraxin-3 and transforming growth factor beta levels. Kardiovaskulyarnaya terapiya i profilaktika/ Cardiovascular therapy and prevention. 2019; 18(6): 57-61. (in Russian)]. https://dx.doi.org/10.15829/1728-8800-2019-6-57-61.
- Савельева Г.М., Шалина Р.И., Коноплянников А.Г., Симухина М.А. Преэклампсия и эклампсия: новые подходы к диагностике и оценке степени тяжести. Акушерство и гинекология: новости, мнения, обучение. 2018; 6(4): 25-30. [Savelyeva G.M., Shalina R.I., Konoplyannikov A.G., Simukhina M.A. Preeclampsia and eclampsia: new approaches in diagnosis and evaluation of severity. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie/ Obstetrics and Gynecology: News, Opinions, Training. 2018; 6(4): 25-30. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2018-14002.
- Сидорова И.С., Никитина Н.А., Унанян А.Л., Агеев М.Б., Кокин А.А. Система комплемента при беременности, осложненной преэклампсией. Акушерство и гинекология. 2021; 8: 5-12. [Sidorova I.S., Nikitina N.A., Unanyan A.L., Ageev M.B., Kokin A.A. The complement system in preeclampsia-complicated pregnancy. Obstetrics and Gynecology. 2021; 8: 5-12 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.8.5-12.
- Макацария А.Д., Бицадзе В.О., Акиньшина С.В. Тяжелые формы преэклампсии как проявление тромботической микроангиопатии. Акушерство и гинекология. 2017; 4: 21-6. [Makatsaria A.D., Bitsadze V.O., Akinshina S.V. Severe forms of preeclampsia as a manifestation of thrombotic microangiopathy. Obstetrics and Gynecology. 2017; (4): 21-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.4.21-6.
- Smith A.N., Wang X., Thomas D.G., Tatum R.E., Booz G.W., Cunningham M.W. The role of mitochondrial dysfunction in preeclampsia: causative factor or collateral damage? Am. J. Hypertens. 2021; 34(5): 442-52. https://dx.doi.org/10.1093/ajh/hpab003.
- Wu P., Haththotuwa R., Kwok C.S., Babu A., Kotronias R.A., Rushton C. et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes. 2017; 10(2): e003497. https://dx.doi.org/10.1161/CIRCOUTCOMES.116.003497.
Received 20.12.2021
Accepted 26.01.2022
About the Authors
Yurii V. Tezikov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University,Ministry of Health of Russia, +7(927)685-44-85, yra.75@inbox.ru, https://orcid.org/0000-0002-8946-501X, Researcher ID: С-6187-2018, SPIN-код: 2896-6986,
Author ID: 161372, Scopus Author ID: 6603787595, 443099, Russia, Samara, Chapaevskaya str., 89.
Igor S. Lipatov, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(927)262-92-70, i.lipatoff2012@yandex.ru, https://orcid.org/0000-0001-7277-7431, Researcher ID: С-5060-2018, SPIN-код: 9625-2947,
Author ID: 161371, Scopus Author ID: 6603787595, 443099, Russia, Samara, Chapaevskaya str., 89.
Amir R. Azamatov, obstetrician-gynecologist, Perinatal Center, Seredavin Samara Regional Clinical Hospital, +7(846)958-24-18, azamatov.amir@yandex.ru,
https://orcid.org/0000-0003-0372-6889, SPIN-код: 9261-9264, Author ID: 1013819, 443095, Russia, Samara, Tashkentskaya str., 159.
Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher at the Department of Research Administration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; +7(903)969-50-41, tioutiounnik@mail.ru, https://orcid.org/0000-0002-5830-5099,
Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Author ID: 213217, Scopus Author ID: 56190621500, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)220-86-55, kan-med@mail.ru, https://orcid.org/0000-0001-5087-5946, Researcher ID: B-2370-2015,
SPIN-код: 5378-8437, Author ID: 624900, Scopus Author ID: 57008835600, 117997, Russia, Moscow, Ac. Oparina str., 4.
Ellina M. Zumorina, obstetrician-gynecologist, Perinatal Center, Seredavin Samara Regional Clinical Hospital, +7(846)958-24-18, ellina.zumorina@yandex.ru,
https://orcid.org/0000-0002-0140-5566, SPIN-код: 9924-2273, Author ID: 1105503, 443095, Russia, Samara, Tashkentskaya str., 159.
Alina I. Kuzmina, 6th year student at the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, +7(846)958-24-18,
alina.cuzmina555@mail.ru, https://orcid.org/0000-0003-1354-1626, 443099, Russia, Samara, Chapaevskaya str., 89.
Corresponding author: Yurii V. Tezikov, yra.75@inbox.ru
Authors’ contributions: Tezikov Yu.V., Lipatov I.S. – the concept and design of the study; Azamatov A.R., Zumorina E.M., Kuzmina A.I. – data collection, processing and analysis; Lipatov I.S., Tezikov Yu.V., Azamatov A.R., Tyutyunnik V.L.,
Kan N.E. – manuscript drafting and editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was approved by the Ethics Committee of the Samara State Medical University, Ministry of Health of Russia.
Patient Сonsent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Tezikov Yu.V., Lipatov I.S., Azamatov A.R., Tyutyunnik V.L.,
Kan N.E., Zumorina E.M., Kuzmina A.I. Preeclampsia as a separate gestational clinical and pathogenetic form of insulin resistance syndrome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 4: 64-74 (in Russian)
https://dx.doi.org/10.18565/aig.2022.4.64-74



