Программы вспомогательных репродуктивных технологий (ВРТ) занимают ведущую позицию среди методов лечения бесплодия и их количество с каждым годом неуклонно растет. В связи с чем в современных реалиях крайне актуален вопрос эффективности ВРТ. В общей сложности, начиная с 1997 г., в результате программ экстракорпорального оплодотворения (ЭКО) на свет появилось более 8 млн детей. Согласно данным РАРЧ, с 1995 по 2020 гг. в России было проведено 902 578 циклов ВРТ, в результате которых родилось 225 354 детей. За 2020 г. в России было проведено 77 845 циклов ЭКО/ИКСИ. Первая успешная беременность в результате ВРТ наступила в результате программы ЭКО в естественном цикле еще в 1978 г. [1]. Увеличить результативность программ ЭКО позволило применение гонадотропинов с целью стимуляции суперовуляции и получения большего количества ооцитов и эмбрионов. В настоящее время доля программ в естественном цикле составляет лишь 3–5% среди всех циклов ЭКО по разным данным. Данные программы в основном ведутся при наличии противопоказаний к гормональной стимуляции и в редких случаях при желании женщины минимизировать гормональную нагрузку на организм либо нежелании проводить селекцию и криоконсервацию эмбрионов [1]. Таким образом, стимуляция суперовуляции является одним из важнейших этапов программы ЭКО. Данный этап подразумевает под собой получение адекватного количества генетического материала для последующего оплодотворения и получения эмбрионов и, как следствие, достижения высокой частоты наступления беременности. Возможность криоконсервации эмбрионов, в свою очередь, позволяет увеличить кумулятивную частоту наступления беременности в программах ВРТ.
В настоящее время в арсенале репродуктологов имеется широкий спектр гонадотропинов, которые подразделяются на 2 основные группы – менопаузальные (чМГ) и рекомбинантные гонадотропины (рФСГ). Менопаузальные гонадотропины синтезируются на основе мочи постменопаузальных женщин. После первых лет использования чМГ было предположено, что избыточное содержание ЛГ влияет на снижение эффективности применения данных препаратов. Однако к середине 80-х гг. прошлого века накопились данные о безопасности и высокой эффективности применения препаратов чМГ. В настоящее время Менопур остается «золотым стандартом» среди менопаузальных гонадотропинов, широко применяются в программах ЭКО у пациенток старшего репродуктивного возраста, а также остается незаменимым при наличии у пациентки гипогонадотропной аменореи. Тем не менее, производство данной группы препаратов имеет технические сложности, в связи с чем целью дальнейших исследований стала разработка синтезированных гонадотропинов и появилась технология получения рекомбинантных препаратов [2]. Препараты гонадотропинов, включающие только ФСГ, различаются по альфа, бета и дельта субъединицам. В практике репродуктивной медицины до сегодняшнего дня среди рекомбинантных гонадотропинов в основном применялись фоллитропины альфа и бета. Для производства фоллитропинов альфа используется культура клеток яичников китайского хомячка с рекомбинантным наследственным материалом, что снижает долю присутствующих примесей белкового, углеводного и стероидного характера [3]. Прорывом в производстве рекомбинантных гонадотропинов, стала разработка фоллитропина дельта. В отличие от прочих препаратов рСФГ для получения фоллитропина дельта применяются клетки человека линии PER.C6, что в еще большей степени позволяет нивелировать вероятность развития индивидуальной реакции организма на сопутствующие вещества, имеющиеся в ранее используемых менопаузальных и рекомбинантных гонадотропинах, и повысить безопасность использования. PER.C6 относится к клеточной линии сетчатки плода человека с аминокислотной последовательностью, идентичной последовательности человеческого ФСГ, а также существующих препаратов рекомбинантного ФСГ, полученных из клеточных линий китайского хомячка. Благодаря использованию клеток линии PER.C6 профиль гликозилирования фоллитропина дельта отличен от фоллитропинов альфа и бета. Фоллитропин дельта имеет более высокую долю три- и тетра-сиалированных гликанов, чем фоллитропин альфа, а также содержит как α2,3-, так и α2,6-связанную сиаловую кислоту, в то время как фоллитропин альфа содержит лишь α2,3-связанную сиаловую кислоту [4]. Более высокое общее содержание сиаловых кислот приводит к снижению клиренса и более выраженному ответу яичников у пациенток по сравнению с аналогами в тех же суточных дозировках [4]. Данная особенность препарата позволяет получить оптимальный ответ яичников на стимуляцию при использовании меньших доз фоллитропина дельта по сравнению с прочими гонадотропинами, что делает программу ЭКО более выгодной с экономической точки зрения [5, 6]. При сопоставлении назначаемых доз гонадотропинов по критериям эффективности программ ЭКО 10 мкг фоллитропина дельта приблизительно соответствует 150 МЕ фоллитропина альфа [7]. Назначение меньшей суточной дозировки препарата позволяет снизить гормональную нагрузку на организм, при этом не повышая частоты бедного ответа на стимуляцию суперовуляции.
Необходимость подбора индивидуальных доз препаратов для стимуляции суперовуляции является неоспоримой. Отличительной особенностью применения фоллитропинов альфа и бета является то, что подбор дозы производится эмпирическим путем на основании возраста пациентки, массы тела и профиля овариального резерва и зависит в основном от личного опыта специалиста. Для фоллитропина дельта, в отличие от его аналогов, разработан алгоритм индивидуализированного подбора стартовой дозы с учетом его фармакокинетических и фармакодинамических характеристик. Персонализированный подход к определению исходной дозы гонадотропина позволяет обеспечить высокую эффективность программ ВРТ, не снижая при этом их безопасность. В двух рандомизированных контролируемых исследованиях было показано, что индивидуальный режим дозирования фоллитропина дельта позволяет снизить риск cиндрома гиперстимуляции яичников (СГЯ) по сравнению с другими препаратами рФСГ и достичь при этом сопоставимых показателей живорождения [8, 9]. Немаловажным плюсом индивидуального дозирования является удобство назначения для врача в соответствии с таблицей назначения препарата, и гарантия высокой комплаентности пациенток. Для подбора стартовой дозы фоллитропина дельта используются индивидуальные характеристики пациенток. Учитывается уровень антимюллерова гормона (АМГ), являющийся одним из наиболее чувствительных маркеров овариального резерва и наиболее точных прогностических показателей ответа яичников на стимуляцию суперовуляции [10, 11]. Также важным критерием подбора дозы препарата является масса тела пациентки, которая оказывает значимое влияние на распределение препарата и его депонирование в жировой ткани [12]. Одним из плюсов персонализированного подбора дозы является отсутствие необходимости в ее последующей корректировке в процессе стимуляции.
Цель работы: оценить эффективность использования в программах ЭКО фоллитропина дельта по сравнению с фоллитропином альфа по критериям количества полученных ооцитов, ооцитов МII, частоты оплодотворения, выхода бластоцист высокого качества, доли эуплоидных эмбрионов по данным преимплантационного генетического тестирования на анеуплоидии (ПГТ-А), а также частоты наступления беременности. Оценить безопасность, удобство использования и комплаентность пациенток к применению фоллитропина дельта.
Материалы и методы
В соответствии с поставленной целью были обследованы 35 пациенток в возрасте от 22 до 36 лет включительно, с показаниями к достижению беременности методами ВРТ.
В исследование были включены пациентки с трубно-перитонеальным фактором и не тяжелым мужским фактором бесплодия. Основными критериями включения являлись: индекс массы тела от 18,5 до 24,9, сохранный овариальный резерв (АМГ не ниже 1,2 нг/мл, ФСГ менее 10 мМЕд/мл), первая предстоящая программа ЭКО, планируемый перенос в полость матки одного эмбриона или проведение ПГТ-А (табл. 1). При желании пациентки проводить генетическую диагностику эмбрионов, предварительно оценивался ее кариотип. Основными критериями исключения были: сопутствующие экстрагенитальные и генитальные заболевания, сниженный овариальный резерв (АМГ менее 1,2 нг/мл, ФСГ более 10 мМЕд/мл), индекс массы тела более 25 кг/м2, попытки ЭКО в анамнезе, поздний репродуктивный возраст, тяжелый мужской фактор бесплодия.
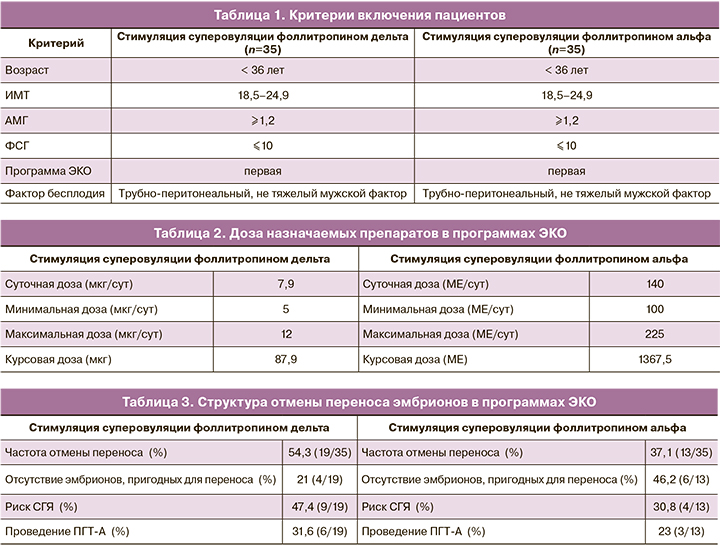
В группу фоллитропина альфа были включены 35 пациенток с сопоставимыми исходными данными. У всех пациенток планировалась первая программа ЭКО (табл. 1).
Программы ЭКО проводились по протоколам с антагонистами ГнРГ. В группе фоллитропина дельта стимуляция суперовуляции проводилась согласно индивидуально подобранным дозировкам. Стартовая доза подбиралась в соответствии с таблицей в зависимости от уровня антимюллерова гормона и массы тела пациенток на момент вступления в программу ЭКО. В среднем доза препарата составила 7,9 мкг/сут и варьировала от 5 до 12 мкг/сут (табл. 2). Доза была фиксированной на протяжении всего периода стимуляции и не требовала корректировки на протяжении цикла. В группе фоллитропина альфа стартовая доза подбиралась эмпирическим путем, варьировала от 100 до 225 МЕ/сут, в среднем доза препарата составила 140 МЕ в сутки (табл. 2). Следует отметить, что у 4/35 пациенток (11,4%) в ходе программы ЭКО потребовалась корректировка дозы фоллитропина альфа в сторону ее повышения. Для предотвращения преждевременного выброса лютеинизирующего гормона вводился антагонист гонадотропин-рилизинг-гормона (ГнРГ) (Цетрореликс) в суточной дозе 0,25 мг при достижении размера растущих фолликулов 13 мм и более. При достижении у 3 и более фолликулов диаметра ≥17 мм, вводился триггер финального созревания фолликулов – рекомбинантный хорионический гонадотропин 250 мкг (Овитрель).
Через 35–36 ч после введения триггера финального созревания ооцитов производилась трансвагинальная пункция фолликулов с последующим оплодотворением методом ЭКО или ИКСИ. Частота проведения ЭКО и ИКСИ были сопоставимы. При наличии условий на 5-е сутки культивирования производился перенос 1 эмбриона в полость матки. Остальные эмбрионы высокого качества подвергались криоконсервации.
При наблюдении гиперергического ответа яичников (≥20 фолликулов) и клинических признаках синдрома гиперстимуляции яичников производилась замена триггера финального созревания фолликулов. Перенос эмбрионов в нативном цикле не проводился и все полученные эмбрионы высокого качества подвергались криоконсервации с целью минимизации риска развития СГЯ.
Так же перенос эмбрионов в цикле стимуляции не производился в случае отсутствия эмбрионов высокого качества или при планировании проведения ПГТ-А (табл. 3). Генетическое тестирование эмбрионов, учитывая молодой репродуктивный возраст пациенток, проводилось по желанию женщины.
Параметры эффективности программ ЭКО включали количество полученных ооцитов, долю ооцитов МII, процент оплодотворения, количество эмбрионов высокого качества на 5-е сутки культивирования и наличие эмбрионов для последующей криоконсервации. Эффективность программ оценивалась также по частоте наступления беременности, доле эуплоидных эмбрионов по результатам проведенного ПГТ-А (табл. 4).
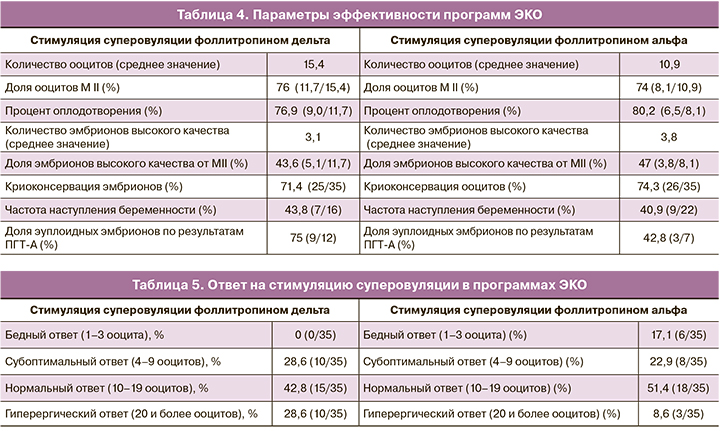
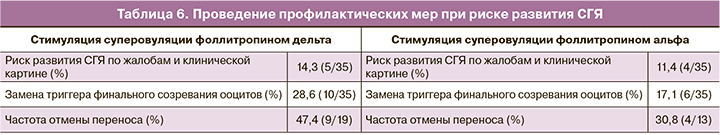
Ответ яичников на стимуляцию суперовуляции оценивался как нормальный при получении от 10 до 19 ооцитов, бедный и субоптимальный – при получении 1–3 и 4–9 ооцитов соответственногиперергический – при получении 20 и более ооцитов (табл. 5). Критерии безопасности применения фоллитропина дельта оценивались по риску развития СГЯ и необходимости проведения профилактических мер в этом случае, что включало замену триггера финального созревания ооцитов и/или отмену переноса эмбриона в цикле стимуляции (табл. 6).
Результаты
Результаты исследования показали, что при проведении стимуляции суперовуляции фоллитропином дельта у 35 пациенток средняя суточная доза препарата составила 7,9 мкг/сут, что приблизительно соответствует дозе фоллитропина альфа 118,5 МЕ. Минимальная доза составила 5 мкг/сут, максимальная – 12 мкг/сут и была сопоставима с 75 МЕ и 180 МЕ фоллитропина альфа соответственно. Средняя курсовая доза у пациентов в группе фоллитропина дельта составила – 87,9 мкг на весь период стимуляции, что аналогично 1318,5 МЕ фоллитропина альфа (табл. 2). На протяжении всего периода стимуляции стартовая доза препарата не требовала корректировки. Стоит отметить, что у 4/35 (11,4%) пациенток в ходе стимуляции суперовуляции фоллитропином альфа требовалась корректировка дозы препарата в сторону ее увеличения. В группе фоллитропина альфа средняя суточная доза составила 140 МЕ (минимальная доза составила 100 МЕ, максимальная – 225 МЕ). Средняя курсовая доза при использовании фоллитропина альфа была выше и составила 1367,5 МЕ (табл. 2). Учитывая приблизительное соответствие 10 мкг фоллитропина дельта 150 МЕ фоллитропина альфа, можно сделать вывод об экономической эффективности применения фоллитропина дельта в результате снижения стартовой и курсовой дозы препарата. Так средняя стартовая доза фоллитропина дельта составила 7,9 мкг/сут, что соответствует дозе фоллитропина альфа 118,5 МЕ, в сравнении со средней стартовой дозой 140 МЕ в группе фоллитропина альфа. Данная особенность может быть связана с более высоким содержанием сиаловых кислот в фоллитропине дельта, снижением клиренса, что требует меньших затрат препарата и говорит о его экономической выгодности.
Если говорить о количестве дней стимуляции, то оно было сопоставимо в обеих группах. Стимуляция суперовуляции длилась 11±3 дня в группе фоллитропина дельта и 10±3 дня в группе фоллитропина альфа. При оценке количества полученных ооцитов и ответа яичников нами было отмечено следующее. Количество полученных ооцитов в группе фоллитропина дельта составило 15,4 среди всех стимулированных пациенток, в группе фоллитропина альфа – 10,9, что говорит о более высокой эффективности стимуляции суперовуляции с применением фоллитропина дельта (табл. 4). При этом в группе фоллитропина дельта доля нормального ответа яичников (от 10 до 19 ооцитов) составила 42,8% (15/35) случаев, не было отмечено ни одного случая бедного ответа (1–3 ооцита, р=0,01), доля субоптимального ответа (4–9 ооцитов) составила 28,6% (10/35), гиперергического (20 и более ооцитов, р=0,03) – 28,6% (10/35) случаев. В группе фоллитропина альфа нормальный ответ яичников (10–19 ооцитов) наблюдался в 51,4% (18/35) случаев, бедный и субоптимальный (1–3, р=0,01 и 4–9 ооцитов соответственно) – у 17,1% (6/35) и 22,9% (8/35) пациенток соответственно и гиперергический (20 и более ооцитов) – в 8,6% (3/35, р=0,03) случаев (табл. 5). Несмотря на более высокую частоту гиперергического ответа в группе фоллитропина дельта, риск развития СГЯ наблюдался лишь в 14,3% (5/35) случаев. Стоит отметить, что в группе фоллитропина альфа низкая доля гиперергического ответа связана с тем, что в данной группе пациенток наблюдался бедный ответ на стимуляцию суперовуляции и требовалась коррекция дозировки препарата в 11,4% (4/35) программ ЭКО. Несмотря на низкую долю гиперергического ответа в данной группе, частота риска СГЯ была сопоставима с группой фоллитропина дельта. Из всех полученных ооцитов доля ооцитов качества МII составила 76% (11,7/15,4) в группе фоллитропина дельта и 74% (8,1/10,9) в группе фоллитропина альфа. Процент оплодотворения составил в группах фоллитропина дельта и альфа 76,9% (9,0/11,7) и 80,2% (6,5/8,1) соответственно. При этом количество эмбрионов высокого качества составило 43,6% (5,1/11,7) от числа ооцитов МII в группе фоллитропина дельта и 47% (3,8/8,1) в группе фоллитропина альфа. Частота наступления беременности рассчитывалась на перенос эмбриона в нативном цикле и была равна 43,8% (7/16) в группе фоллитропина дельта, 40,9% (9/22) – в группе фоллитропина альфа. Все беременности были подтверждены клинически, репродуктивных потерь на период проведения исследования зарегистрировано не было. Данных о живорождении на момент окончания исследования еще не получено. Криоконсервация эмбрионов при наличии эмбрионов высокого качества была произведена в 71,4% (25/35) программ ЭКО с использованием фоллитропина дельта и в 74,3% (26/35) программ на фоне стимуляции фоллитропином альфа (табл. 4). В 54,3% (19/35) случаев была произведена отмена переноса эмбриона в цикле стимуляции фоллитропином дельта и в 37,1% (13/35) программ на фоллитропине альфа. В группе фоллитропина дельта отмена переноса была связана со следующими причинами: у 47,4% (9/19) пациенток с высоким риском развития СГЯ, у 31,6% (6/19) с проведением ПГТ-А полученных эмбрионов и у 21% (4/19) с отсутствием эмбрионов, пригодных для переноса в полость матки. В группе фоллитропина альфа перенос эмбрионов в полость матки был отменен в виду отсутствия эмбрионов, пригодных для переноса, – в 46,2% (6/13) случаев, в 30,8% (4/13) случаев – в связи с риском СГЯ и в 23% (3/13) по причине проведения ПГТ-А эмбрионов (табл. 3).
По результатам проведенного ПГТ-А 75% (9/12) эмбрионов в группе фоллитропина дельта были пригодны для переноса. В группе фоллитропина альфа мы получили 42,8% (3/7) эуплоидных эмбрионов, что безусловно является плюсом, но требует сбора большего количества данных.
С целью оценки профиля безопасности фоллитропина дельта был изучен риск развития СГЯ. Повышенный риск наблюдался у 14,3% (5/35) пациенток. Во всех случаях наблюдался СГЯ легкой степени, проявляющийся жалобами на дискомфорт, вздутие в области живота, тошноту и диарею в отдельных случаях. При этом ни у одной пациентки не было выявлено признаков СГЯ средней или тяжелой степени, к которым относятся признаки асцита или гидроторакса по данным УЗ-исследования, а также значимые отклонения по результатам клинического, биохимического анализов крови и коагулограммы. Так же ни одной пациентке с проявлением СГЯ не потребовалась госпитализация в стационар. В группе фоллитропина альфа повышенный риск СГЯ наблюдался в 11,4% (4/35) случаев, что сопоставимо с результатами, полученными при стимуляции суперовуляции фоллитропином дельта. СГЯ так же протекал только в легкой степени. В исследовании мы оценивали также необходимость проведения профилактических мер при повышенном риске развития СГЯ, которые включали замену триггера финального созревания ооцитов и сегментацию цикла. Количество пациенток, у которых потребовалась замена триггера овуляции, составило – 28,6% (10/35) в группе фоллитропина дельта и 17,1% (6/35) в группе фоллитропина альфа (табл. 6).
Также стоит отметить, что фоллитропин дельта был удобен для применения и нами была отмечена высокая комплаентность пациенток в ходе проведения исследования.
Обсуждение
Настоящее исследование показало эффективность и безопасность применения рекомбинантного ФСГ – фоллитропина дельта в сравнении с аналогами (фоллитропин альфа) у пациенток молодого репродуктивного возраста с сохранным овариальным резервом и трубно-перитонеальным фактором бесплодия. В нашем исследовании была продемонстрирована положительная взаимосвязь между индивидуально назначенными дозами фоллитропина дельта согласно таблице подбора дозировки препарата и ответом яичников на стимуляцию суперовуляции, включая такие параметры как количество полученных ооцитов, процент полученных при трансвагинальной пункции ооцитов МII, процент оплодотворения, количество эмбрионов высокого качества, доля эуплоидных эмбрионов по результатам ПГТ-А и частота наступления беременности на перенос эмбриона.
Говоря о применяемых в циклах стимуляции суперовуляции дозах гонадотропинов, хочется отметить явную экономическую эффективность применения фоллитропина дельта в сравнении с фоллитропином альфа. В исследовании, проведенном Qiao J. et al., общее использование гонадотропина было значительно (P<0,001) снижено в среднем со 109,9±32,9 мкг (1498±448 МЕ) фоллитропина альфа до 77,5±24,4 мкг фоллитропина дельта, что свидетельствует о не меньшей эффективности фоллитропина дельта в его индивидуальном режиме дозирования по сравнению с фоллитропином альфа [13]. По данным исследования, проведенного Nyboe Andersen A. et al., суммарная дозировка гонадотропина при использовании фоллитропина дельта была ниже, чем при назначении фоллитропина альфа (90,0±25,3 по сравнению с 103,7±33,6 мг) [8]. Нами так же была отмечена экономическая выгодность назначения фоллитропина дельта. Так средняя стартовая доза фоллитропина дельта составила 7,9 мкг/сут, что соответствует дозе фоллитропина альфа 118,5 МЕ, в сравнении с 140 МЕ фоллитропина альфа в контрольной группе. Средняя курсовая доза у пациентов группы фоллитропина дельта составила 87,9 мкг на весь период стимуляции, что аналогично 1318,5 МЕ фоллитропина альфа. В группе фоллитропина альфа средняя курсовая доза составила 1367,5 МЕ. Данная особенность может быть связана с более высоким содержанием сиаловых кислот в фоллитропине дельта по сравнению с аналогами, снижением клиренса действующего вещества, что позволяет снизить затрату препарата и говорит о его экономической выгодности.
По данным исследования, проведенного Nyboe Andersen A. et al., индивидуализированный режим дозирования фоллитропина дельта по сравнению с фоллитропином альфа обеспечил достижение целевого ответа на стимуляцию суперовуляции у большего количества женщин (43,3% по сравнению с 38,4%), меньшую частоту бедного ответа (11,8% по сравнению с 17,9%), меньшую частоту гиперергического ответа (27,9% по сравнению с 35,1% и 10,1% по сравнению с 15,6% соответственно). При этом в основном наблюдался нормоэргический ответ яичников на стимуляцию (от 10 до 19 ооцитов) – в 42,8% случаев [8]. По нашим наблюдениям бедный ответ (1–3 ооцита) не был отмечен ни у одной пациентки на фоне стимуляции суперовуляции фоллитропином дельта, что, по всей видимости, является следствием более высокого профиля гликозилирования фоллитропина дельта и более выраженным ответом яичников на стимуляцию сопоставимыми дозами фоллитропина альфа. В группе фоллитропина альфа бедный ответ был получен у 17,1% (6/35) пациенток, что, вероятно, также связано с подбором дозы эмпирическим путем, в отличие от возможности персонализированного подбора дозы соответственно таблице применения фоллитропина дельта. На субоптимальный ответ, который по ряду исследований так же можно отнести к оптимальному уровню ответа яичников (4–9 ооцитов) пришлось 28,6% (10/35). Несмотря на более высокую частоту гиперергического ответа в группе фоллитропина дельта (28,6% – 10/35), риск развития СГЯ был ниже и наблюдался лишь в 14,3% (5/35) случаев. В группе фоллитропина альфа более низкая доля гиперергического ответа (8,6% – 3/35) связана с тем, что в данной группе пациенток отмечались случаи бедного ответа яичников на стимуляцию суперовуляции и в ряде программ ЭКО требовалась коррекция дозировки препарата – 11,4% (4/35) случаев. Несмотря на низкую долю гиперергического ответа в группе фоллитропина альфа, частота риска СГЯ была сопоставима с группой фоллитропина дельта (11,4 и 14,3% соответственно). Более высокая доля гиперергического ответа яичников в группе фоллитропина дельта может говорить о более высокой чувствительности ткани яичников к препарату, в следствие чего мы можем назначать более низкие дозы препарата, ожидая сопоставимого по эффективности результата при применении более высоких доз других групп гонадотропинов, что однозначно выгодно с экономической точки зрения.
Согласно данным, полученным Ishihara O. et al., было установлено, что на фоне использования фоллитропина дельта количество ооцитов, полученных при трансвагинальной пункции яичников, сопоставимо числу ооцитов, полученных при назначении фоллитропина бета (9,3 против 10,5) [9]. В исследовании Nyboe Andersen A. также было отмечено аналогичное количество полученных ооцитов (10,0±5,6 по сравнению с 10,4±6,5) и сопоставимое количество бластоцист (3,3±2,8 по сравнению с 3,5±3,2) [8]. Согласно нашим данным, среднее количество полученных ооцитов было несколько больше в группе фоллитропина дельта – 15,4, чем в группе фоллитропина альфа – 10,9 ооцитов. Из всех полученных ооцитов 76% (11,7/15,4) составили ооциты МII, что несколько выше результатов, полученных в группе фоллитропина альфа – 74% (8,1/10,9), и может играть решающую роль при выборе препарата для стимуляции суперовуляции у пациенток молодого репродуктивного возраста, планирующих проведение витрификации ооцитов. Процент оплодотворения в группе фоллитропина дельта составил 76,9% (9,0/11,7), в группе фоллитропина альфа – 80,2% (6,5/8,1). При этом количество эмбрионов высокого качества от числа ооцитов МII составило 43,6% (5,1/11,7) и 47% (3,8/8,1) соответственно. Мы получили сопоставимую частоту наступления беременности в обеих группах, что аналогично данным ряда других исследований. На фоне стимуляции суперовуляции фоллитропином дельта данный показатель составил 43,8% (7/16) и 40,9% (9/22) при применении фоллитропина альфа.
Одним из немаловажных критериев применения гонадотропинов в циклах стимуляции суперовуляции является профиль безопасности их использования, в том числе риск развития СГЯ. В ряде исследований сообщается, что возникновение СГЯ наблюдается практически в 2 раза реже при индивидуальном дозировании фоллитропина дельта по сравнению с использованием фоллитропина бета – 11,2 и 19,8% соответственно (СГЯ любой степени) и 7,1 и 14,1% соответственно (умеренная/тяжелая форма СГЯ) [9]. В одном из исследований частота СГЯ составила 20% для всех доз фоллитропина дельта и 22% в контрольной группе, включающей стимуляцию суперовуляции на фоне применения фоллитропина бета [14]. Согласно результатам работы Fernández-Sánchez M. et al., индивидуальный режим дозирования фоллитропина дельта был связан с 50% снижением частоты СГЯ средней/тяжелой степени по сравнению с традиционным подходом дозирования фоллитропина альфа [15]. Согласно нашим данным, не смотря на более высокую долю гиперергического ответа при стимуляции суперовуляции фоллитропином дельта, риск СГЯ был сопоставим в группах фоллитропина дельта и альфа. СГЯ развился в 14,3% (5/35) случаев на фоне стимуляции фоллитропином дельта и у 11,4% (4/35) пациенток группы фоллитропина альфа. В работе, проведенной Yacoub S. et al., ни у одной из пациенток, стимулированных фоллитропином дельта не было зафиксировано СГЯ средней или тяжелой степени, несмотря на то, что максимальное число извлеченных ооцитов достигло 37 [16]. Стоит отметить, что в ходе нашего исследования также во всех наблюдаемых случаях СГЯ проявил себя в легкой степени, что может быть связано с индивидуализированным подбором дозы фоллитропина дельта. В литературе имеются сведения о значительном снижении частоты профилактических вмешательств по поводу раннего СГЯ с 9,6% в группе использования фоллитропина альфа до 5,0% в группе индивидуально подобранного фоллитропина дельта [13]. По результатам нашей работы проведение профилактических мер при повышенном риске развития СГЯ выражалось в замене триггера финального созревания ооцитов, а также в тенденции к секвенированию цикла ЭКО с криоконсервацией эмбрионов. Триггер финального созревания ооцитов был заменен у 28,6% (10/35) пациентов группы фоллитропина дельта и в 17,1% (6/35) случаев в группе фоллитропина альфа (табл. 6). На отмену переноса эмбриона в связи с риском развития СГЯ пришлось 47,4% (9/19) среди всех отмен переносов в группе фоллитропина дельта и 30,8% (4/13) в группе фоллитропина альфа (табл. 3). Стоит отметить, что у части пациенток отмена переноса была связана с планированием проведения ПГТ-А эмбрионов, по результатам которого нами было получено 75% (9/12) эмбрионов пригодных для переноса в группе фоллитропина дельта, в сравнении с 42,8% (3/7) эуплоидных эмбрионов в группе фоллитропина альфа. Данный показатель безусловно говорит в пользу применения фоллитропина дельта в программах ЭКО с планированием генетической диагностики эмбрионов, но требует сбора данных на более крупной когорте пациенток. Хотелось бы упомянуть, что в группе фоллитропина альфа частота отмены переноса, связанная с отсутствием эмбрионов высокого качества была выше – 46,2% (6/13), в сравнении с 21% (4/19) при применении фоллитропина дельта, что может быть связано как с более высоким профилем гликозилирования фоллитропина дельта и, как следствие, более высоким сродством к организму человека, так и необходимостью эмпирического подбора дозы фоллитропина альфа и говорит о безусловном преимуществе применения фоллитропина дельта в программах ЭКО (табл. 3).
По данным проведенного исследования фоллитропин дельта не уступает в результативности фоллитропину альфа и по некоторым параметрам оценки эффективности имеет существенные плюсы. В исследовании у пациенток группы фоллитропина дельта не отмечалось ни одного случая бедного ответа на стимуляцию суперовуляции, в отличие от пациенток группы фоллитропина альфа, частота субоптимального и нормоэргического ответа была сопоставима в обеих группах. У пациенток группы фоллитропина дельта реже отмечалась отмена переноса по причине получения эмбрионов низкого качества. У женщин, которым была проведена стимуляция суперовуляции препаратом фоллитропина дельта, было отмечено получение несколько большей доли ооцитов МII, в связи с чем фоллитропин дельта может быть рекомендован к применению в программах ЭКО с витрификацией ооцитов. В группе фоллитропина дельта причинами отмены переноса эмбриона являлись риск СГЯ или планируемое проведение ПГТ-А, при этом отмечалась большая доля пригодных к переносу эмбрионов по сравнению с группой фоллитропина альфа, что является следствием получения ооцитов более высокого качества. При анализе результатов программ ЭКО, завершенных переносом эмбрионов в полость матки, частота наступления беременности на перенос эмбриона оказалась сопоставимой в обеих группах.
Таким образом, полученные данные позволяют сделать вывод, что фоллитропин дельта может занять особое место в арсенале препаратов, используемых для стимуляции суперовуляции, по эффективности сопоставим с аналогами, а в некоторых аспектах имеет преимущества и может являться препаратом выбора в программах ВРТ. Фоллитропин дельта безопасен, позволяет получать прогнозируемый ответ яичников, без риска получения бедного ответа на стимуляцию суперовуляции. Препарат удобен для введения, что обеспечивает его высокую комплаентность.
Заключение
В современных реалиях репродуктивной медицины, не смотря на широкий выбор препаратов для стимуляции суперовуляции, все еще ведутся поиски препарата, способного обеспечить большую эффективность программ ВРТ при максимальной безопасности для пациентки. Возможность индивидуального подбора дозы гонадотропинов при использовании фоллитропина дельта на основе исходных характеристик пациенток, позволяет обеспечить оптимизацию ответа яичников, сохраняя высокую эффективность проводимых программ ЭКО при сопоставимом риске развития СГЯ.



