Phenotypic characterization of peripheral blood innate immune cell subpopulations in women with endometriosis before and after surgery
Objective: To investigate phenotypic characteristics of innate immune cell subpopulations in women with endometriosis before and after combination therapy, including surgery. Materials and methods: The study included women with stage I–II (n=12) and III–IV (n=28) endometriosis, who underwent clinical and immunological investigations before and after combination therapy, including surgery. Peritoneal fluid samples obtained intraoperatively and blood samples drawn from the study subjects before and six months after surgery were phenotyped for mononuclear cells using flow cytometry (n=15). Results: In the peritoneal fluid of women with endometriosis, the proportions of TCRγδ cells (p<0.001) and T regulatory cells with the CD4+CD25+CD127low/- phenotype (Treg cells) (p=0.028) were lower than in controls. Compared with peripheral blood, there was a decrease in the proportion of cytotoxic СD3-CD56+CD16+-NK cells (p<0.001), an increase in the proportion of regulatory CD56brightCD16dim-NK cells (p=0.005). Compared to the control, the proportion of Treg cells in peripheral blood was also reduced (p=0.028). Besides, the neutrophil-lymphocyte index (p=0.022) was increased. There was a reduction in the proportion of CD14highCD16low -classical monocytes (p=0.017), while the proportion of non-classical was increased (p= 0.009). Six months after surgery, the total leukocyte count, neutrophil-lymphocyte index, and the proportion of Treg cells in the peripheral blood significantly differ from the baseline values (p=0.008, p=0.017, and p=0.001, respectively), not differing from the control values. Conclusion: The imbalance between regulatory and cytotoxic subpopulations is more pronounced at the local than at the systemic level. Nevertheless, after combination therapy, including surgery, there was a noticeable tendency towards normalization of the altered peripheral blood parameters against the background of an improvement in clinical symptoms, particularly in relieving the pain.Korotkova T.D., Krechetova L.V., Inviyaeva E.V., Vtorushina V.V., Vanko L.V., Adamyan L.V.
Keywords
One of the most challenging problems of modern gynecology is endometriosis, a pathology characterized by the growth of endometrium-like tissue in aberrant locations outside of the uterus. Endometriosis causes significant chronic pelvic pain, dysmenorrhea, and infertility and affects 7–10% of all women interfering with their quality of life.
The mechanisms underlying the onset of endometriosis include overproduction of estradiol, progesterone resistance, increased production of proinflammatory cytokines, and insufficient ability of immune cells to suppress the inflammatory response [1]. Currently, the most accurate method for diagnosing genital endometriosis is a pelvic laparoscopy. Management of endometriosis is complex with the use of surgical or hormonal therapy. Many issues related to the pathogenesis and the management of endometriosis remain unexplored, although they have been extensively studied [2–4].
The development of endometriosis results from interactions between various hormonal, immunological, genetic, and other factors. Systemic and local changes in immune responses that include impaired function of different populations of immune cells have been frequently reported as contributing factors in endometriosis pathogenesis [5–9]. The cells and humoral factors of innate immunity, which are aimed at maintaining and maintaining homeostasis, perform effector and regulatory functions and have a role in adaptive immunity.
The functional orientation of cells is determined by their phenotype. Subpopulations of NK cells, depending on the intensity of expression of one or another receptor, exhibit predominantly regulatory capacity (CD56bright CD16dim\--NK cells) or cytotoxic activity (CD56dim\-CD16bright-NK cells) [10–12]. The regulatory capacity is possessed by expressing the T-cell receptor (TCR), mainly αβTCR, NKT cells (CD3+CD56+16+-NK cells) and a subpopulation expressing gdTCR (CD56gdTCR-NK cells).
Blood and peritoneal fluid monocytes are precursors of macrophages, which play an important role in inflammation, trauma, and the development of neoplastic processes [13–16]. Depending on the intensity of expression of CD14 and CD16 molecules, two subpopulations were identified: CD14highCD16low (or CD14++CD16-, classical) and CD14+/lowCD16high (or CD14+CD16++, nonclassical), as well as an intermediate subpopulation (CD14high CD16+, or CD14++ CD16+) [17, 18].
Studies investigating the role of innate immune cells such as NK cells and monocytes/macrophages in endometriosis have been mainly focused on their functional properties. They reported impaired phagocytosis of peritoneal macrophages, a decrease in the cytotoxic activity of NK cells, which positively correlated with the severity of the disease [7, 19]. However, in women with endometriosis, the phenotypic characteristics and the ratio of subpopulations of these cells after surgical treatment of endometriosis have not been sufficiently characterized.
The present study aimed to investigate phenotypic characteristics of innate immune cell subpopulations in women with endometriosis before and after combination therapy, including surgery.
Materials and methods
This prospective study included patients managed at the gynecological department of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The study group consisted of 40 patients with extragenital endometriosis (EGE), confirmed by ultrasound and/or MRI data, and subsequently by histological examination of endometriotic lesions to verify the diagnosis. Women with endometriosis were divided into two groups according to the extent of the invasion. Groups EM-1and EM-2 included women with stage I–II (n=12) and III–IV (n=28) endometriosis. The Control group consisted of 15 patients, including seven healthy fertile women and eight women with an incomplete intrauterine septum, without inflammatory and proliferative gynecological diseases, with the absence of genital endometriosis, which was confirmed laparoscopically.
Inclusion criteria for study patients were the age of 18–45 years, confirmed diagnosis of EGE, and signed informed consent to participate in the study. Exclusion criteria were malignant neoplasms, acute inflammatory diseases of the pelvic organs, and severe comorbidities.
Peritoneal fluid samples obtained intraoperatively, and blood samples drawn from the study subjects before surgical removal of endometriotic lesions were subjected to immunological examination. Six months after surgery, blood samples were phenotyped again in 15 patients (4 women from the EM-1 group and 11 women from the EM-2 group).
Fasting blood samples were drawn from the antecubital vein on days 13–24 of the cycle before surgery and six months later. Peritoneal fluid was collected in the peritoneal cavity after insertion of the laparoscope. Lymphocyte phenotyping was performed by flow cytometry using monoclonal antibodies (mAb) (Becton Dickinson and eBioscience, USA) labeled with FITC, PE, APC. The lymphocyte gate, which allows excluding other blood cells from the analysis, was detected using anti-CD45 mAb (Dako, Denmark). We analyzed subpopulations of NK cells (CD56+CD16+) and monocytes (CD14+CD16+). Regulatory T cells were defined as cells expressing the γδ T-cell receptor (TCRγδ, or TCRγδ), as well as Treg with the CD4+CD25+CD127low/- phenotype. The analysis was performed on a Navios flow cytometer (Beckman Coulter, USA) using the Kaluza software.
Statistical analysis
Statistical analysis was performed using the Microsoft Office Excel 2010 and MedCalc (version 16.8) software. The normality of the distribution was tested by the Shapiro–Wilk and Kolmogorov–Smirnov tests. Data are presented as arithmetic mean (M) and standard deviation (SD). To assess the significance of differences in the case of a normal distribution of signs, the Student's t-test was used. Differences were considered significant at p˂0.05. Data with non-normal distribution were reported as the median (Me) and interquartile range (Q1; Q3) and compared using Kruskal–Wallis test followed by post-hoc pairwise comparisons by the Mann–Whitney U-test or the Wilcoxon test with Bonferroni correction for multiple comparisons. Categorical variables were reported as counts and proportions (%) and compared using Fisher’s exact test. Differences were considered significant at p<0.05.
Results
Patients’ characteristics regarding medical history, complaints, gynecological diseases, comorbidities, previous surgical interventions, and the presence of familial and hereditary disorders are presented in tables 1 and 2.
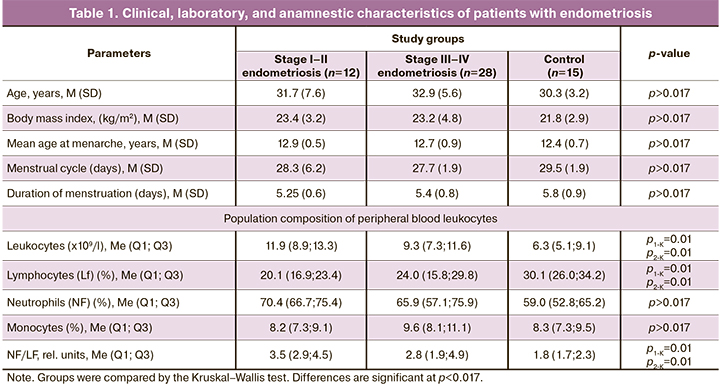
There were no statistically significant differences in age, body mass index, age at menarche, and time of menstruation between patients with endometriosis of varying stages and the control group. Complete blood count indicated a significant increase in leukocyte counts, a decrease in lymphocyte proportions, and an increase in the neutrophil to lymphocyte ratio in patients with endometriosis compared with the control group.
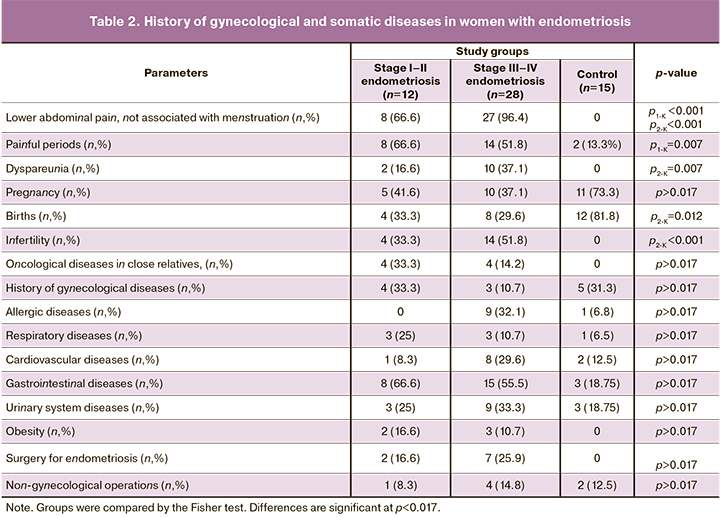
Women with endometriosis in both groups often complained of lower abdominal pain, not associated with menstruation, in the absence of such complaints in the control group. Painful menstruation occurred significantly more often in the EM-1 group; dyspareunia was observed more frequently among the patients in the EM-2 group than in the control group (Table 2).
There were no significant differences between the study groups regarding the history of past pregnancies. The number of previous births was lower in both groups than in the control group but was significantly different only for the EM-2 group. Patients in the EM-2 group were also more likely to have infertility.
There were no significant differences in the incidence of obesity and allergic diseases between the groups. Oncological diseases in close relatives were observed in patients with endometriosis of both groups in the absence of the control group, but the difference was insignificant. There were no significant differences in respiratory, cardiovascular, urinary, gastrointestinal diseases between the groups. Previous surgery for endometriosis was reported in both groups of patients with endometriosis without significant differences.
During a gynecological examination before surgery, adnexal tenderness to palpation was more common in patients with grade III–IV than grade I–II EMs [28/28 (100%) vs. 5/12 (41, 6%), respectively, p=0.001]. Tenderness to palpation over the sacro-uterine ligaments was more common in women in group EM-2 [24/28 (81.5%) vs. 3/12 (25%), p<0.001].
All women with endometriosis underwent surgery. Indications for surgical treatment were lower abdominal pain of various types and locations, painful menstruation, pain during sexual intercourse, and the absence of pregnancy for more than one year of regular sexual activity without contraception. In 100% of cases, the operation was performed by laparoscopic access.
During surgery, superficial lesions were most often found on the peritoneum [(in 8/12 (66.6%) and 28/28 (100%) patients] in groups EM-1 and EM-2, respectively, p=0.005) and on the sacro-uterine ligaments [(group EM-1 in 4/12 (33%) cases, in group EM-2 in 17/28 (61.1%) cases, p>0.05)], on the intestine and in the retrocervical region in group EM-1 [(in 5/12 (41.6%) and in group EM-2 in 11/28 (40.7%) cases (p>0.05)]. In group EM-1, 3/12 (25%) of women had endometrioid cysts in only one ovary. In group EM-2, 8/28 (29.6%) women had endometrioid cysts in one and 12/28 (44%) on two ovaries (p<0.05).
Concurrently with surgical removal of endometriotic lesions, patients received complex therapy, including medication to normalize the balance of hormones and suppress inflammation. Mean hospital stay was 5.8 (1.2) days, depending on the extent of surgery. Postoperatively, patients were administered antibacterial, symptomatic, and, if necessary, antianemic therapy. As part of postoperative rehabilitation, physiotherapy was also used (low-frequency impulse currents, magnetic and electromagnetic fields of low frequency in the mode of a magnetic field with a frequency of 50 Hz and inductance from 35 mT, 1–3 times a day, up to 20 per course). Patients received balneotherapy at the next rehabilitation stage, including general radon and iodine-bromine baths (mean radon concentration from 40 to 200 nCi/l (1.5–7.5 kBq/l), iodine concentration at least 10 mg/dm3 and bromine at least 25 mg/dm3).
After surgery, depending on its extent, age, and reproductive plans, 55.5% of patients underwent hormonal therapy: in 33.3% of cases in the EM-1 group and in 66.6% in the EM-2 group. The drugs of choice were gonadotropin-releasing hormone (GnRH) agonists 3.6 mg every 28 days; dienogest at a dose of 2 mg. Depending on the stage of the disease and the presence of comorbidities, GnRH-ages were administered for 3–6 months and dienogest for 6–12 months.
Outcomes of surgical, hormonal, and anti-inflammatory treatment were assessed six months after elective surgery. Nine women in the EM-1 group and 18 in the EM-2 group underwent clinical examination (bimanual gynecological examination, pelvic ultrasound). Small pelvis adhesions were diagnosed in 2/9 (22.2%) patients in the EM-1 group and 6/18 (33.3%) in the EM-2 group (p>0.05).
Recurrence of the disease was observed in 3 (11.1%) of 27 women. Patients complained of recurrent lower abdominal pain and painful menstruation. Ultrasound examination revealed IGE lesions.
After the treatment, 6 (22.2%) women became pregnant: 1/9 (11.1%) in group EM-1 and 5/18 (27.7%) in group EM-2. In 16.6% of cases, pregnancies were spontaneous, whereas ART was needed in 83.3%.
Long-term results of organ-sparing surgery for EGE were followed for 6 to 24 months in 39 patients. After the treatment, pregnancy occurred in 10/39 (26%) women, in 2 (16.6%), they were spontaneous, and 8/39 (20%) underwent ART.
An analysis of baseline content of peripheral blood and peritoneal fluid lymphocyte subpopulations (NK cells, TCRgd and Treg) and monocytes (classical, nonclassical, and intermediate) in women in the control group (n=15) and patients with endometriosis (n=15). Six months after the operation, they underwent another immunological study of cells of the same phenotype.
Mean baseline peripheral blood leukocyte counts and ratios of neutrophils to lymphocytes (NF/LF) in the study and general groups were higher than in the control group [(9.7 (6.9; 12.4) vs. 6.3 (5.1; 9.1), p=0.029, and 3.4 (2.07; 5.01) vs. 1.9 (1.68; 2.55), p=0.022, Mann–Whitney test]. At the same time, there was a decrease in the proportion of lymphocytes and no change in the content of monocytes. The peripheral blood NF/LF ratio in patients with endometriosis was almost 1.5-fold higher than in the control group.
Comparisons of baseline peripheral blood and peritoneal fluid content of NK cell subpopulations, TCRgd and Treg in women with endometriosis and control subjects presented in Figure 1.
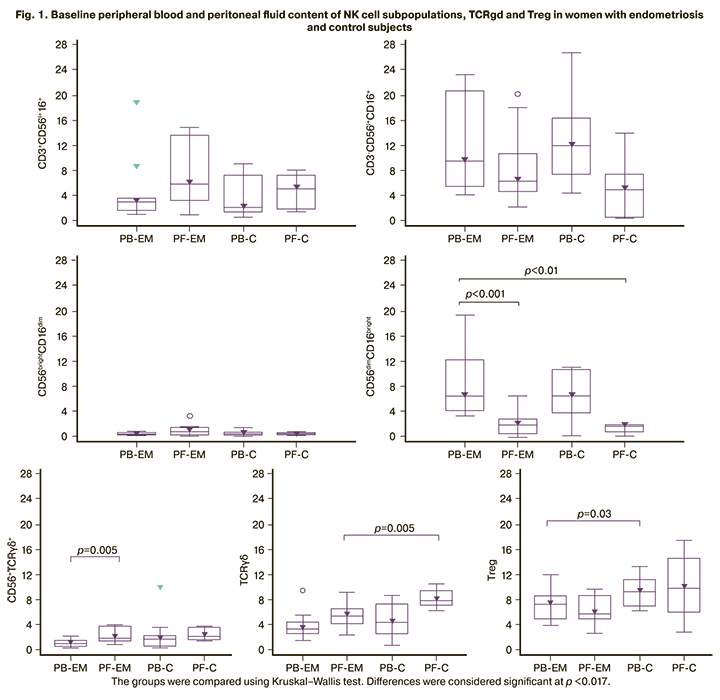
There were significant differences between peripheral blood and peritoneal fluid NK cell subpopulations: cytotoxic CD56dimCD16bright- and regulatory CD56TCRgd cells. In the peritoneal fluid, the proportion of CD56dimCD16bright- cells was lower than in the peripheral blood both in women with endometriosis (p<0.001) and in the control group (p<0.01). The proportion of CD56TCRgd cells in the peritoneal fluid of women with endometriosis were higher than in the peripheral blood (p=0.005). There were no differences in the content of NK cell subpopulations in the peripheral blood of women with endometriosis compared with the control group.
No significant differences were found between the content of a subpopulation of TCRgd cells in the peripheral blood and peritoneal fluid in women with endometriosis, but their proportion was lower in the peritoneal fluid than in the controls (p=0.005). There were also no differences between the content of T-regulatory cells with the CD4+CD25+CD127low/- phenotype in the peripheral blood and peritoneal fluid of women with endometriosis. Still, there was a decrease in the content in the peripheral blood compared to the control (p=0.028).
Figure 2 shows the peripheral blood and peritoneal fluid monocyte subpopulations in women with endometriosis and the control subjects before surgery.
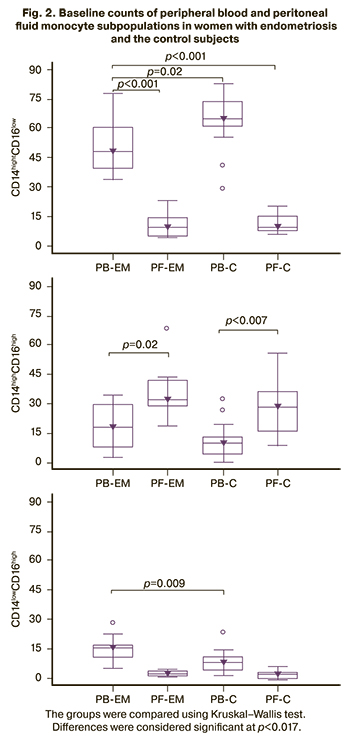
There was a predominance of classical monocyte subpopulations in peripheral blood in both groups of women (p<0.001 in endometriosis and p=0.0001 in control) while in the peritoneal fluid of intermediate monocyte subpopulations were prevalent (p=0.002 in endometriosis and p<0.007 in control). At the same time, in the peripheral blood of women with endometriosis, there was a decrease in the content of subpopulations of classical (p=0.017) and an increase in nonclassical (p=0.009) monocytes, compared with the control group. There were no significant differences in the subpopulation composition of monocytes in the peritoneal fluid between the study and control groups.
Six months after surgery, the content of leukocyte populations, NK cell subpopulations, regulatory lymphocytes, and peripheral blood monocytes was examined and compared with the baseline findings in women operated on for endometriosis. The results are presented in Table 3.
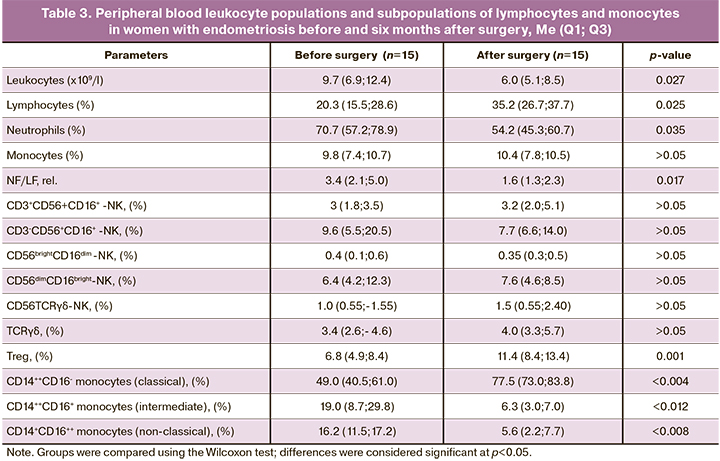
The presented data indicate a significant decrease in peripheral blood leukocyte counts, proportions of neutrophils, and an increase in the proportion of lymphocytes compared with baseline in the operated patients.
The analysis shows that complex therapy, including surgery, resulted in the normalization of the leukocyte count and the ratio of neutrophils to lymphocytes since there were no differences with the data of the control group [(6.0 (5.1; 8.5) and 6.3 (5.1; 9.1), p=0.743; 1.6 (1.3; 2.3) and 1.8 (1.7; 2.3), p=0.303, respectively, data from tables 1 and 3].
There were no significant differences in the content of the studied subpopulations of NK cells and the subpopulation of regulatory TCRgd cells after surgery compared to the baseline data. However, an increase in the subpopulation of Treg cells and a change in the ratio of subpopulations of monocytes was observed since the proportion of classical monocytes increased with a decrease in the proportion of intermediate and nonclassical monocytes, which may indicate a reduction in the intensity of the inflammatory process.
Discussion
Women with endometriosis had the following clinical and anamnestic characteristics: pelvic pain not associated with menstruation, painful menstruation, dyspareunia, a decrease in the number of pregnancies and childbirth, infertility in almost every second patient, adnexal and sacro-uterine ligament pain to palpation. There were no significant differences between the groups with less and more severe stages of the disease in the incidence of most of these symptoms. This confirms the well-established opinion that the accuracy of the diagnosis depends on the surgical intervention and morphological examination to establish the endometriotic structure of the lesions. A constant search for non-invasive diagnostic and prognostic markers is associated with the desire to avoid surgery to diagnose endometriosis.
Examination of patients six months after the surgical removal of endometriotic lesions located in the abdominal cavity and postoperative treatment with the use of hormonal, antibacterial, symptomatic therapy and, if necessary, physiotherapeutic procedures revealed a significant decrease in the rates of recurrence of lower abdominal pain, painful menstruation, irregular menstrual cycles, and dyspareunia. After the treatment, 22.2% of women became pregnant. However, 11% of patients showed symptoms of relapse.
In the peripheral blood of women in the study group, there was initially a clear tendency to an increase in the total leukocyte count compared to the control (p=0.058) and a significant increase in the neutrophil-lymphocyte index (p=0.022), which may indicate the presence of an inflammatory process in endometriosis. In the literature, there is ambiguous information about the results of assessing the populations of leukocytes and different opinions about the possibility of using an increased index of the ratio of neutrophils to lymphocytes as a diagnostic marker in endometriosis. There are data both on the absence of changes in the content of leukocyte populations and their ratio in endometriosis [20], and comparable with our results data on the increase in the number of neutrophils and their ratio with lymphocytes [21]. According to the authors, these data make it possible to recommend using the neutrophil-lymphocyte index as an additional diagnostic indicator for stages III–IV of endometriosis.
Six months after the complex treatment, there was a decrease in peripheral blood leukocyte count compared with baseline values (p<0.01, Table 2). The normalization of the leukocyte count and the neutrophil-lymphocyte index occurs mainly due to a decrease in neutrophil counts, indicating a reduction in the intensity of the inflammatory process in endometriosis.
Analysis of NK cell subpopulations showed that women with endometriosis and control subjects had lower counts of cytotoxic CD56dimCD16bright cells and more CD56TCRgd cells with regulatory activity in the peritoneal fluid than in the peripheral blood (Fig. 1). There were no differences in the relative content of NK cell subpopulations in the peripheral blood of women with endometriosis compared to the control group before surgery. After complex treatment, no changes were found either in comparison with the control or with the baseline values. The contribution of NK cells to the pathogenesis of endometriosis is explained to a greater extent by impaired functional activity and not by a change in their number or subpopulation composition. It is assumed that the dysfunction of these cells, particularly cytotoxic, facilitates the survival, implantation, and proliferation of endometrial cells in the abdominal cavity [7].
The content of subpopulations of TCRgd cells and T regulatory cells with the CD4+CD25+CD127low/- phenotype in women's peripheral blood and peritoneal fluid did not differ significantly. When compared with the control group, a decrease in the proportion of TCRgd cells in the peritoneal fluid were observed, and the proportion of CD4+CD25+CD127low/- Тreg tended to decrease in the peripheral blood, which may indicate a decrease in their regulatory function (p=0.028). Our results suggest an essential role in the pathogenesis of endometriosis of the immune response dysregulation associated with Treg cells. After complex treatment, there were no significant changes in the content of the TCRgd cell subpopulation in the peripheral blood, while the proportion of Treg cells increased, reaching the control level (Table 3).
Our findings suggest that the abnormal ratio of regulatory and cytotoxic subpopulations is more pronounced at the local than at the systemic level (Fig. 1). The peritoneal fluid is represented by a complex of soluble factors and cellular components exerting immunosuppressive and stimulating effects on endometriotic lesions. Abdominal endometriosis increases the number of cells producing factors that determine intercellular interactions, regulation of apoptosis, and proliferation of all types of cells [10, 22]. The increase in the content of Treg cells after surgical treatment may reflect an increase in the mechanisms of self-limiting inflammation.
Women with endometriosis had a similar relative number of monocytes in the peripheral blood and peritoneal fluid; changes in the ratio of monocyte subpopulations were most pronounced in the peripheral blood (Fig. 2).
There was a predominance of classical monocytes subpopulation in the peripheral blood, while intermediate and non-classical monocytes were more prevalent in the peritoneal fluid. The reduction in classical, an increase in non-classical and a tendency to a rise in the proportion of intermediate monocytes in the peripheral blood of women with endometriosis, compared with the control group, is associated with the presence of an inflammatory process or tumor growth [13, 15, 17, 23, 24]. The latter is of interest in connection with the data on a significant increase in these cells in the peripheral blood of women with ovarian cancer [18].
A decrease in classical monocyte counts occurs when they migrate to tissues to become macrophages or differentiate into dendritic cells, which is essential for endometriotic lesions' growth, development, and vascularization. Macrophages, perceiving the accumulation of ectopic endometrial cells as tissue damage, which results in the increased survival of ectopic endometrial cells culminating in endometriosis [25]. The systemic inflammatory process is associated with a redistribution of monocyte subpopulations in the blood. The proportion of classical monocytes decreases, and the number of intermediate and non-classical monocytes increases proportionally. Changes in the ratio of monocyte subpopulations make it possible to judge the severity of the inflammatory process. The change in the ratio of subpopulations in endometriosis is associated with a chronic inflammatory process, the indicators of which are normalized due to complex treatment observed six months after the surgical removal of endometriotic lesions.
Conclusion
The study findings suggest that women with endometriosis have an increase in peripheral blood total leukocyte count, the proportion of neutrophils and neutrophil-lymphocyte index, a decrease in the proportion of T-regulatory cells and classical monocytes. Changes in the content of regulatory and cytotoxic subpopulations of natural killer cells are manifested to a greater extent at the local than at the systemic level. In the peritoneal fluid, the content of regulatory subpopulations is higher and cytotoxic subpopulations are lower, and vice versa in the peripheral blood. A comparative analysis of the content and ratio of lymphocytes and monocytes subpopulations in peripheral blood of women with endometriosis showed that after combination therapy, including surgery, there was a noticeable tendency towards normalization of the altered peripheral blood parameters against the background of an improvement in clinical symptoms, particularly in relieving the pain.
References
- Rafique S., DeCherney A.H. Medical management of endometriosis. Clin. Obstet. Gynecol. 2017; 60(3): 485-96. https://dx.doi.org/10.1097/GRF.0000000000000292.
- Адамян Л.В., Андреева Е.Н. Роль современной гормономодулирующей терапии в комплексном лечении генитального эндометриоза. Проблемы репродукции. 2011; 17(6): 66-77. [Adamyan L.V., Andreeva E.N. The role of modern hormone-modulating therapy in the complex treatment of genital endometriosis. Problems of Reproduction. 2011; 6: 66-77 (in Russian)].
- Burney R.O., Giudice L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012; 98(3): 511-9. https://dx.doi.org/10.1016/j.fertnstert.2012.06.029.
- Laganà A.S., Garzon S., Götte M., Viganò P., Franchi M., Ghezzi F. et al. The pathogenesis of endometriosis: molecular and cell biology insights. Int. J. Mol. Sci. 2019; 20(22): 5615. https://dx.doi.org/10.3390/ijms20225615.
- Анциферова Ю.С., Сотникова Н.Ю., Назаров С.Б. Иммунные механизмы развития генитального эндометриоза. Иваново; 2007. 312с. [Antsiferova Yu.S., Sotnikova N.Yu., Posiseeva L.V., Nazarov S.B. Immune mechanisms of development of genital endometriosis. Ivanovo; 2007. 314 p. (in Russian)].
- Ярмолинская М.И. Цитокиновый профиль перитонеальной жидкости и периферической крови больных с наружным генитальным эндометриозом. Журнал акушерства и женских болезней. 2008; 57(3): 30-4. [Yarmolinskaya, M.I. Cytokine profile of peritoneal fluid and peripheral blood in patients with external genital endometriosis. Journal of Obstetrics and Women's Diseases. 2008; 57(3): 30-4. (in Russian)].
- Jeung I., Cheon K., Kim M.R. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed. Res. Int. 2016; 2016: 2916070. https://dx.doi.org/10.1155/2016/2916070.
- Xu H., Zhao J., Lu J., Sun X. Ovarian endometrioma infiltrating neutrophils orchestrate immunosuppressive microenvironment. J. Ovarian Res. 2020; 13(1): 44. https://dx.doi.org/10.1186/s13048-020-00642-7.
- Kang Y.-J., Jeung I.C., Park A., Park Y.J., Jung H., Kim T.D. et al. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014; 29(10): 2176-89. https://dx.doi.org/10.1093/humrep/deu172.
- Michel T., Poli A., Cuapio A., Briquemont B., Iserentant G., Ollert M. et al. Human CD56bright NK cells: an update. J. Immunol. 2016; 196(7): 2923-31. https://dx.doi.org/10.4049/jimmunol.1502570.
- Fu B., Tian Z., Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014; 141(4): 483-9. https://dx.doi.org/10. 1111/imm.12224.
- Ming B., Wu T., Cai S., Hu P., Tang J., Zheng F. et al. The increased ratio of blood CD56 bright NK to CD56 dim NK is a distinguishing feature of primary Sjögren's syndrome. J. Immunol. Res. 2020; 2020: 7523914. https://dx.doi.org/10.1155/2020/7523914.
- Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014; 14(6): 392-404. https://dx.doi.org/10.1038/nri3671.
- Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327(5966): 656-61. https://dx.doi.org/10.1126/science.1178331.
- Eljaszewicz A., Wiese M., Helmin-Basa A., Jankowski M., Gackowska L., Kubiszewska I. et al. Collaborating with the enemy: function of macrophages in the development of neoplastic disease. Mediators Inflamm. 2013; 2013: 831387. https://dx.doi.org/10.1155/2013/831387.
- Hogg С., Horne A.W., Erin C. Endometriosis-associated macrophages: origin, phenotype, and function. Front. Endocrinol. (Lausanne). 2020; 11: 7. https://dx.doi.org/10.3389/fendo.2020.00007.
- Wong K.L., Yeap W.H., Tai J.J., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: implications for health and disease. Immunol. Res. 2012; 53(1-3): 41-57. https://dx.doi.org/10.1007/s12026-012-8297-3.
- Prat M., Naour A.L., Coulson K., Lemée F., Leray H., Jacquemin G. et al. Circulating CD14high CD16low intermediate blood monocytes as a biomarker of ascites immune status and ovarian cancer progression. J. Immunother. Cancer. 2020; 8(1): e000472. https://dx.doi.org/10.1136/jitc-2019-000472.
- Gogacz M., Winkler I., Bojarska-Junak A., Tabarkiewicz J., Semczuk A., Rechberger T. et al. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J. Reprod. Immunol. 2016; 117: 39-44. https://dx.doi.org/10.1016/j.jri.2016.04.289.
- Kim S.K., Park J.Y., Jee B.C., Suh C.S., Kim S.H. Association of the neutrophil-to-lymphocyte ratio and CA 125 with the endometriosis score. Clin. Exp. Reprod. Med. 2014; 41(4): 151. https://dx.doi.org/10.5653/cerm.2014.41.4.151.
- Yang H., Lang J., Zhu L., Wang S., Sha G., Zhang Y. Diagnostic value of the neutrophil-to-lymphocyte ratio and the combination of serum CA-125 for stages III and IV endometriosis. Chin. Med. J. (Engl). 2013; 126(11): 2011-4.
- Сельков С.А., Ярмолинская М.И. Эндометриоз как патология регуляторных механизмов. Журнал акушерства и женских болезней. 2017; 66(2): 9-13. [Selkov S.A., Yarmolinskaya M.I. Endometriosis as a pathology of regulatory mechanisms. Journal of Obstetrics and Women's Diseases. 2017; 66(2): 9-13. (in Russian)]. https://dx.doi.org/10.17816/JOWD6629-13.
- Калашникова А.А., Ворошилова Т.М., Чиненова Л.В., Давыдова Н.И., Калинина Н.М. Субпопуляции моноцитов у здоровых лиц и у пациентов с сепсисом. Медицинская иммунология. 2018; 20(6): 815-24. [Kalashnikova A.A., Voroshilova T.M., Chinenova L.V., Davydova N.I., Kalinina N.M. Subpopulations of monocytes in healthy individuals and in patients with sepsis. Medical immunology. 2018; 20(6): 815-24. (in Russian)]. https://doi.org/10.15789/1563-0625-2018-6-815-824.
- Инвияева Е.В., Короткова Т.Д., Вторушина В.В., Кречетова Л.В., Ванько Л.В., Адамян Л.В. Эффекторные и регуляторные субпопуляции клеток врожденного иммунитета в периферической крови и перитонеальной жидкости женщин с эндометриозом. Акушерство и гинекология. 2021; 6: 96-104. [Inviyaeva E.V., Korotkova T.D., Vtorushina V.V., Krechetova L.V., Vanko L.V., Adamyan L.V. Blood and peritoneal fluid effector and regulatory subpopulations of innate immunity cells in women with endometriosis. Akusherstvo I Ginekologiya/Obstetrics and Gynecology. 2021; 6: 96-104 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.6.96-104.
- Capobianco А., Rovere-Querini Р. Endometriosis, a disease of the macrophage. Front. Immunol. 2013; 4: 9. https://dx.doi.org/10.3389/fimmu.2013.00009.
Received 02.06.2021
Accepted 23.07.2021
About the Authors
Tatiana D. Korotkova, Obstetrician-Gynecologist, Ph.D. Student at the Department of Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,t_korotkova@oparina4.ru, https://orcid.org/0000-0002-3367-6, 117997, Russia, Moscow, Oparina str., 4.
Lubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-11-83, l_krechetova@oparina4.ru, https://orcid.org/0000-0001-5023-3476, 117997, Russia, Moscow, Oparina str., 4.
Eugenia V. Inviyaeva, Ph.D. (bio.sci.), Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-11-83, e_inviyaeva@oparina4.ru, https://orcid.org/0000-0001-9878-3637, 117997, Russia, Moscow, Oparina str., 4.
Valentina V. Vtorushina, Ph.D., Immunologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(495)438-11-83, v_vtorushina@oparina4.ru, https://orcid.org/0000-0002-8406-3206, 117997, Russia, Moscow, Oparina str., 4.
Ludmila V. Vanko, Dr. Med. Sci., Professor, Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-11-83, LVanko@oparina4.ru, https://orcid.org/0000-0003-1139-3797, 117997, Russia, Moscow, Oparina str., 4.
Leyla V. Adamyan, Dr. Med. Sci., Academician of the RAS, Professor, Head of the Department of Gynecology, Deputy Director for Science, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Adamyanleila@gmail.com, https://orcid.org/0000-0002-3253-4512, 117997, Russia, Moscow, Oparina str., 4.
Authors' contributions: Adamyan L.V., Krechetova L.V., Vanko L.V. – conception and design of the study; Korotkova T.D., Inviyaeva E.V., Vtorushina V.V. – data collection and analysis; Korotkova T.D., Krechetova L.V., Vanko L.V. – statistical analysis; Korotkova T.D., Inviyaeva V.V. – manuscript preparation; Adamyan L.V., Krechetova L.V., Vanko L.V. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the state assignment 2021 8-A21.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Korotkova T.D., Krechetova L.V., Inviyaeva E.V., Vtorushina V.V., Vanko L.V., Adamyan L.V. Phenotypic characterization of peripheral blood innate immune cell subpopulations in women with endometriosis before and after surgery.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 10: 93-102 (in Russian)
https://dx.doi.org/10.18565/aig.2021.10.93-102



