Placental expression of hypoxia-induced factors in acute hypoxia and fetal intrauterine growth restriction
Leonova M.D., Bezhenar V.F., Semenova N.Yu., Semenikhin D.V., Frederiks E.V.
Hypoxia-induced factors (HIF-1 and HIF-2) are expressed in the placenta during all trimesters of pregnancy under both normal and hypoxic conditions. However, hypoxia significantly increases placental expression of HIF-1 and HIF-2. The role of HIF-1 expression in chronic placental insufficiency, its significance in preterm birth and preeclampsia, and the relationship between inflammation and hypoxia has been established. Nevertheless, there is a lack of data regarding placental HIF expression at birth in children who experience acute hypoxia during labor.
Objective: To determine the levels of placental HIF-1 and HIF-2 expression in acute hypoxia during labor and fetal intrauterine growth restriction (IUGR).
Materials and methods: An immunohistochemical study was conducted on 44 placentas using primary rabbit antibodies for HIF1a and HIF2a, divided into three groups: group I (n=12), comprising placentas obtained during uncomplicated labor; group II (n=18), consisting of placentas from fetuses that experienced hypoxia during labor (umbilical artery blood pH <7.25); and group III (n=14), including placentas with fetal IUGR.
Results: A statistically significant difference in HIF-1 expression was observed between groups I and III. When assessing the intensity of HIF-1 and HIF-2 staining, significant differences were noted between groups I and II and between groups I and III, while expression in groups II and III did not differ. The highest placental HIF-2 expression was observed in newborn girls.
Conclusion: The results indicate that HIF-1 and HIF-2 expression changes depending on the duration of cell exposure to hypoxia. The maximum placental expression of hypoxia-induced factors is characteristic of fetal IUGR.
Authors' contributions: Bezhenar V.F., Frederiks E.V. – conception and design of the study; Leonova M.D. – collection and processing of material, statistical analysis, writing of the text; Semenikhin D.V., Semenova N.Yu. – pathomorphological and immunohistochemical studies; Bezhenar V.F. – editing of the manuscript. All authors made significant contributions to the study and drafting of the manuscript, and they read and approved the final version before submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Pavlov First St. Petersburg SMU, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Leonova M.D., Bezhenar V.F., Semenova N.Yu., Semenikhin D.V., Frederiks E.V.
Placental expression of hypoxia-induced factors in acute hypoxia and fetal intrauterine growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (6): 106-113 (in Russian)
https://dx.doi.org/10.18565/aig.2025.55
Keywords
The placenta is a unique, temporary organ in the female body that functions exclusively during pregnancy. It operates independently of the nervous system and all processes are regulated by humoral mechanisms [1]. Along with the umbilical cord and fetal membranes, the placenta serves as the sole source of life support for the developing fetus. It is estimated that up to 60% of antenatal fetal deaths can be linked to placental dysfunction [2] and circulatory disorders in the umbilical cord [3].
The human placenta undergoes significant changes throughout development. Morphologically, the syncytiotrophoblast layer decreases in thickness, while the cytotrophoblast layer becomes discontinuous as pregnancy progresses. The number of chorionic villi increases, enhancing the surface area of the tissue that comes in contact with maternal and fetal blood, thereby creating a transport barrier between the two circulations [4].
The placenta is responsible for transporting nutrients, gases, hormones, and immunoglobulins to the fetus, thereby providing passive immunity while removing metabolic waste products. Hormones synthesized by the placenta have both local regulatory effects, essential for organ functioning, and systemic effects on the mother's body, facilitating physiological adaptation to pregnancy [1]. During its functioning, the placenta consumes up to 40–60% of the oxygen and glucose delivered to the uterus. Conditions that alter placental metabolism can indirectly influence nutrient transport to the fetus [5].
Given the placenta's role as the sole conduit between the pregnant woman and the fetus, its functioning undoubtedly affects the health of newborns. However, histological examination of the material alone is insufficient to predict unfavorable outcomes [6].
Hypoxia-induced factors (HIF) [7], discovered in 1995, have enabled the study of the biochemical mechanisms underlying cell adaptation to hypoxia. It has been established that HIF-1 and HIF-2 are expressed by the placenta throughout all trimesters of pregnancy, both in normal conditions and under hypoxia. Notably, hypoxic conditions increase placental expression of HIF-1 and HIF-2 [8]. HIF expression levels in early pregnancy influence trophoblast invasion and maturation and are likely to contribute to the pathogenesis of preeclampsia and intrauterine growth restriction (IUGR) of the fetus [9]. The role of HIF-1 expression in chronic placental insufficiency [10], as well as its significance in premature birth [11] and preeclampsia [12], have been documented, and the relationship between inflammation and hypoxia has been established [13]. However, there are currently no data on the placental expression of HIF in acute hypoxia during labor.
This study aimed to determine the levels of placental HIF-1 and HIF-2 expression in cases of acute hypoxia during labor and fetal IUGR.
Materials and methods
The study material consisted of 44 placentas collected immediately after delivery from full-term pregnancies under cold conditions. The samples were then divided into three groups.
- Group I (Control): This group included placentas obtained from fetuses with umbilical cord blood pH ≥ 7.25 after delivery (n=12).
- Group II: This group included cases of delivery with hypoxia, where the pH of the blood from the fetal umbilical artery was < 7.25 (n=18).
- Group III: This group was isolated separately, regardless of the pH level, and included cases of delivery with intrauterine growth restriction (IUGR) of the fetus n=14).
Inclusion criteria were: age 18–42 years, singleton pregnancy, gestational age at the onset of labor from 37 to 41 6/7 weeks, and signed informed consent to participate in the study. Additional inclusion criteria for Group 2 included signs of metabolic acidosis in the blood from the umbilical artery. Additional criteria for group III included compliance with signs of fetal growth retardation, characterized by estimated fetal weight (EFW) and/or abdominal circumference (AC) growth rates < 10th percentile, in combination with abnormal blood flow according to Doppler ultrasound data, or EFW and/or AC values < 3rd percentile.
Exclusion criteria for all groups included signs of acute bacterial and viral diseases in pregnant women or postpartum women as well as signs of preeclampsia in these patients.
For histological examination of placental tissue, dehydration and paraffin impregnation were performed using a standardized technique in a Donatello automatic histological processor (DiaPath, Italy) with ready-made IsoPREP solution (Biovitrum, Russia) and HISTOMIX paraffin medium (Biovitrum, Russia).
For the immunohistochemical (IHC) study, 2–3 μm thick sections were cut using a rotary microtome HM 325 (Thermo, USA) and mounted on poly L-lysine slides. The sections were incubated with primary rabbit antibodies against HIF1a and HIF2a (polyclonal, Cloud-Clone Corp, China) at a dilution of 1:50 for 30 min at room temperature in a humid chamber.
To assess the expression of the studied markers in the digitized preparations, five fields of view were selected from areas with maximum tissue staining intensity corresponding to a magnification of 200×. The number of positive cells (+ cells) was determined and expressed as the percentage of the total number of cells in the field of view. The intensity of IHC staining was scored from 0 to 3+, and the ratio of cells with different expression intensities was assessed. The mean values were calculated for each histological preparation (Fig. 1).
Blood sampling for pH and blood gas determination was performed from the fetal umbilical artery between the two clamps applied immediately after birth (ABL-800 FLEX, Denmark).
Statistical analysis
Statistical analysis was performed using StatTech v. 4.7.1 (StatTech LLC, Russia). The assumption of normality was assessed using the Shapiro–Wilk test. Given the lack of normal distribution in the groups, quantitative data were described using the median (Me) and lower and upper quartiles [Q1; Q3]. Categorical data are presented as counts and percentages. Comparison of three or more groups for continuous variables that did not meet the normality assumption was performed using the Kruskal–Wallis test, followed by post-hoc comparisons using the Dunn test with Holm's correction. A prognostic model characterizing the dependence of continuous variables on these factors was developed using linear regression. Comparisons of percentages in the analysis of multi-way contingency tables were performed using IBM SPSS Statistics 27 (USA), and the Fisher–Freeman–Halton exact test was conducted. Differences were considered statistically significant at p<0.05.
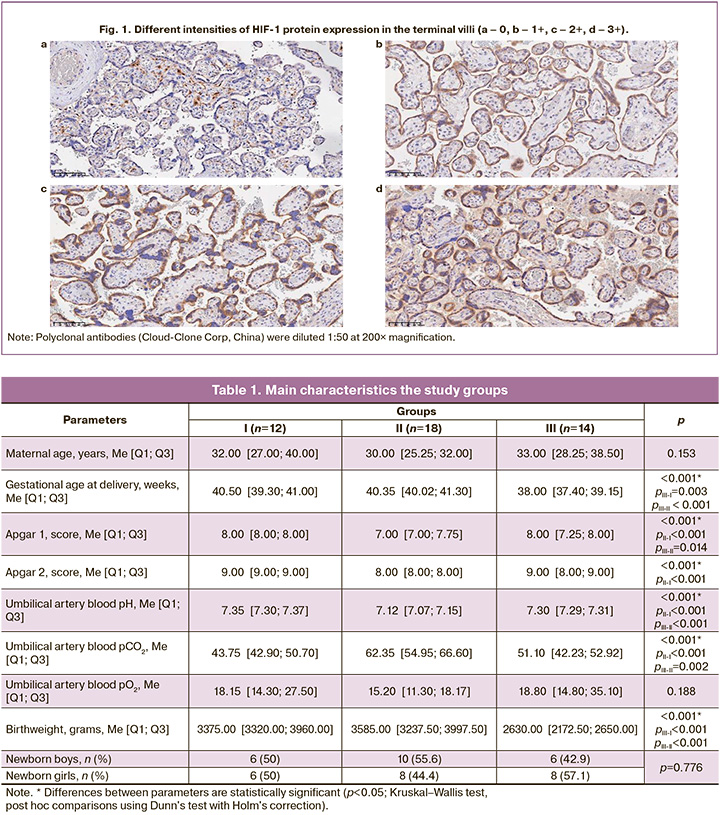
Results
The main characteristics of the study groups are summarized in Table 1.
We conducted a multivariate regression analysis of the dependence of HIF-1 and HIF-2 expression on gestational age at delivery, birth weight, and newborn sex. A significant predictor of increased HIF-1 expression was a decrease in newborn weight (rxy=0.459, p=0.022). Female sex of the newborn was a significant factor in increased HIF-2 expression (rxy=0.583, p<0.001).
The expression of HIF-1%+ cells showed a statistically significant difference: group I, 23.45 [15.50; 31.40], group II: 29.15 [23.54; 42.58], group III – 43.40 [38.67; 49.20] (pIII-I=0.002).
During the analysis, in order to understand the significance of the degree of metabolic acidosis, group II (placentas obtained from newborns who experienced hypoxia during childbirth) was additionally divided into two subgroups by pH level: group I – pH from the umbilical artery from 7.24 to 7.10 – 6 cases, group II – pH from the umbilical artery below 7.09 – 12 cases; group III – IUGR fetus – 14 cases; the control group consisted of cases of birth with normal umbilical cord pH values (>7.25) – 12 cases (Fig. 2).
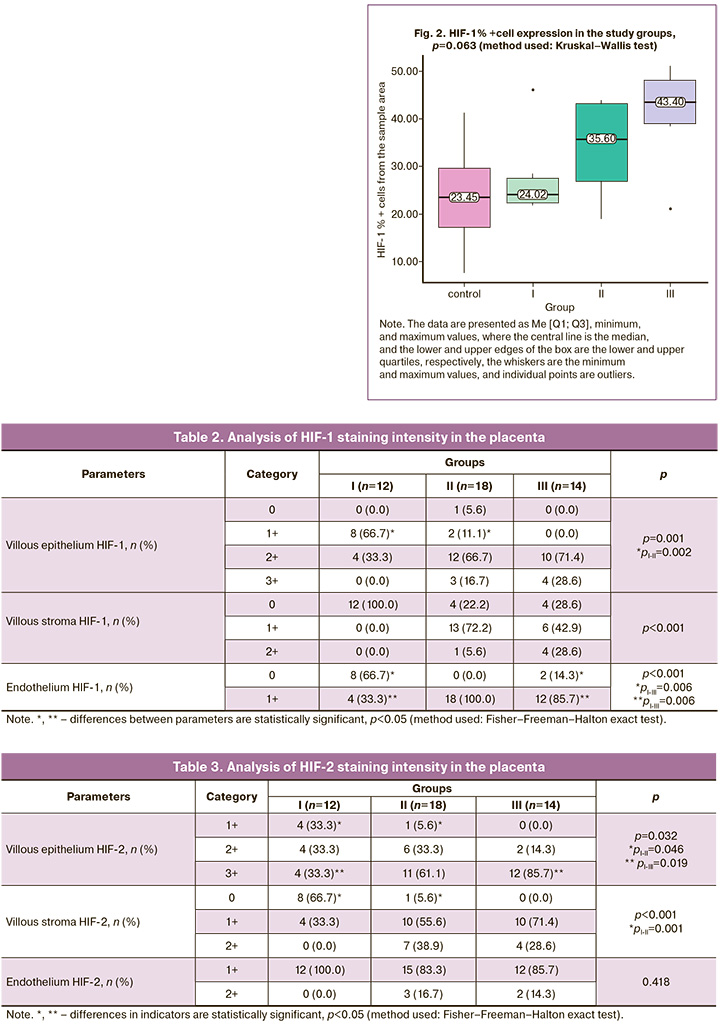
Assessment of HIF-1 cell staining intensity is shown in Table 2.
Analysis of HIF-2% +cell expression did not show statistically significant differences in the study groups: group I – 52.65 [38.50; 64.80], group II – 50.05 [42.88; 64.78], group III – 65.40 [47.68; 67.75] (p=0.163), however, there was a statistically significant difference in the intensity of HIF-2 staining (Table 3).
Of interest was the analysis of HIF-2 expression % + cells depending on the sex of the fetus: male, 46.20 [39.58; 49.10], female: 65.10 [57.10; 67.92] (p=0.002).
We analyzed neonatal outcomes according to the degree of placental expression of HIF-1 and HIF-2 for the following parameters: condition at birth, need for treatment in the intensive care unit (ICU), neonatal neurosonography parameters, and need for transfer to a pediatric hospital. There were no reliable data on the effect of HIF-2 on neonatal outcomes, the effect of HIF-1 on neurosonography parameters, or the need for transfer to a pediatric hospital. Placental HIF-1 expression was 23.05 [16.35; 29.70] in satisfactory neonatal conditions, 39.50 [29.15; 43.45] in moderate conditions, 43.90 [43.90; 43.90] in severe conditions, and 24.00 [23.70; 24.30] in extremely severe conditions (pmoderate condition–satisfactory condition=0.040). In cases where neonatal care in the intensive care unit was necessary, placental expression of HIF-1 was measured at 40.10 [27.07; 43.52]. In contrast, for newborns who stayed with their mothers immediately after birth, the expression was 24.20 [18.05; 33.15] (p=0.018).
Discussion
The observed differences in gestational age at delivery and newborn birth weight are expected and can be attributed to preterm delivery rates among pregnant women with fetal IUGR, consistent with the clinical situation. Group III was specifically identified to illustrate the pathophysiological mechanisms of fetal adaptation to chronic hypoxia.
The pH level of blood from the umbilical artery, measured immediately after birth, served as a marker for identifying group II, as this indicator reflects the presence of acute hypoxia. The diagnosis of fetal hypoxia during labor, determined by the characteristics of the amniotic fluid and cardiotocography indicators, lacks objectivity owing to the low sensitivity and specificity of these methods [14].
Interestingly, we found no relationship between the level of oxygen tension in blood from the umbilical artery and HIF expression. Numerous experiments have shown that an increase in HIF-1 occurs specifically under hypoxic conditions [7]. However, there is evidence that exposure to hypercapnia under conditions of reduced partial pressure of oxygen also leads to an increase in HIF-1 and influences the neuroprotective potential of hypercapnia [15].
Although the Apgar score is not a reliable marker of asphyxia experienced during childbirth [16], we observed differences in this indicator among the study groups. Newborns with metabolic acidosis in the umbilical cord blood had lower Apgar scores at both the 1st and 5th minutes.
When assessing placental expression of HIF-1, we found a statistically significant difference between the control group and group III. Given the absence of low-birth-weight fetuses in the study groups that did not meet the criteria for IUGR, we cannot draw definitive conclusions about whether the high level of HIF-1 expression in group III is associated with low birth weight or chronic hypoxia. The literature presents conflicting data regarding the relationship between HIF-1 levels and fetal malnutrition during normal pregnancy [17] as well as studies indicating no effect of fetal weight on HIF-1 levels [18]. By further dividing group II into subgroups based on the degree of metabolic acidosis, we identified a trend of increasing average HIF-1 values with decreasing pH levels in umbilical artery blood. The highest values of HIF-1 expression were characteristic of group III, which is consistent with previous studies [10]. This trend was likely due to the duration of tissue exposure to reduced oxygen concentrations. HIF-1 levels in cells exposed to hypoxia accumulate non-linearly, depending on the duration of hypoxic exposure, and go through a compensatory phase, where values can reach basal levels [7]. We believe that the time factor determines the elevated levels of placental HIF-1 expression at birth in children with IUGR as well as the intermediate values observed during acute hypoxia in labor.
In assessing the intensity of HIF-1 staining, groups II and III exhibited more intense (2+, 3+) staining of the epithelium, chorionic villus stroma, and vascular endothelium than group I.
Analysis of HIF-2 expression did not reveal statistically significant differences among the studied groups. However, a deeper staining intensity was noted in groups II and III than in group I. Our findings generally align with global perspectives on the role of HIF-2 in chronic processes and are likely associated with the duration of hypoxic conditions [19].
Differences in HIF expression based on the child's sex likely indicate a greater adaptive capacity of females to hypoxia, consistent with contemporary views on gender sensitivity to conditions of reduced partial oxygen pressure [20]. In analyzing neonatal outcomes, a trend of increasing HIF-1 levels was observed as neonatal conditions worsened, along with a sharp decrease in critically ill newborns. Insufficient expression of HIF under hypoxic conditions may reflect failure of the placenta to perform its protective function, resulting in the birth of infants requiring immediate resuscitation.
Limitations. Our study had several limitations. Primarily, the small sample size and uneven distribution across groups limited the statistical power. The absence of low-birth-weight neonates in the study groups who do not meet the criteria for IUGR hinders a clear interpretation of HIF-1 expression levels in group III. Further research with a larger sample size and more in-depth molecular analysis is needed and is currently being conducted to confirm these findings.
Conclusion
The study results illustrate changes in the expression of HIF-1 and HIF-2 in relation to the duration of cellular exposure to hypoxia. Physiologically, normal pregnancy and childbirth are characterized by a specific basal level of placental HIF expression. When hypoxic conditions arise, placental expression of HIF-1 and HIF-2 increases, with levels increasing proportionally as the pH of the fetal umbilical artery blood decreases. The longest-lasting effects of hypoxia was observed in fetal IUGR, with this group showing the highest expression of hypoxia markers. The differences in HIF expression in the placenta based on the sex of the fetus indicate a significantly greater adaptive potential of females to hypoxic conditions, even at the stage of intrauterine development, and raise questions about the priority of hormonal factors.
References
- Khorami-Sarvestani S., Vanaki N., Shojaeian S., Zarnani K., Stensballe A., Jeddi-Tehrani M. et al. Placenta: an old organ with new functions. Front. Immunol. 2024; 15: 1385762. https://dx.doi.org/10.3389/fimmu.2024.1385762
- Травенко Е.Н., Породенко В.А., Меликян М.Г. Антенатальная гибель плода в практике судебно-медицинского эксперта. Тенденции развития науки и образования. 2021; 79(1): 52-5. [Travenko E.N., Porodenko V.A., Melikyan M.G. Antenatal fetal death in the practice of a forensic expert. Trends in the development of science and education. 2021; 79(1): 52-5. (in Russian)]. https://dx.doi.org/10.18411/trnio-11-2021-17
- Юпатов Е.Ю., Курманбаев Т.Е., Галимова И.Р., Хаертдинов А.Т., Мухаметова Р.Р., Миролюбов А.Л., Аблаева Д.Н., Хромова А.М., Тимерзянов М.И., Леонова М.Д. Тромбоз сосудов пуповины: обзор литературы и описание двух клинических наблюдений. Акушерство, гинекология и репродукция. 2022; 16(1): 81-9. [Iupatov E.I., Kurmanbaev T.E., Galimova I.R., Khaertdinov A.T., Mukhametova R.R., Mirolyubov A.L., Ablaeva D.N., Khromova A.M., Timerzyanov M.I., Leonova M.D. Umbilical cord vascular thrombosis: literature review and two clinical cases. Obstetrics, Gynecology and Reproduction. 2022; 16(1): 81-9. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.260
- Reed M.D., Mattison D.R. Treating the placenta: an evolving therapeutic concept. In: Mattison D.R., eds. Clinical pharmacology during pregnancy. Academic Press; 2013: 73-87. https://dx.doi.org/10.1016/B978-0-12-386007-1.00006-4
- Maltepe E., Penn A.A. Development, function, and pathology of the placenta. In: Avery’s Diseases of the Newborn. Elsevier; 2018: 40-60.e8. https://dx.doi.org/10.1016/b978-0-323-40139-5.00005-x
- Kovatis K.Z., Mackley A., Antunes M., Holmes P.J., Daugherty R.J., Paul D. Relationship between placental weight and placental pathology with MRI findings in mild to moderate hypoxic ischemic encephalopathy. Cureus. 2022; 14(5): e24854. https://dx.doi.org/10.7759/cureus.24854
- Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U. S. A. 1995; 92(12): 5510-4. https://dx.doi.org/10.1073/pnas.92.12.5510
- Rajakumar A., Conrad K.P. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol. Reprod. 2000; 63(2): 559-69. https://dx.doi.org/10.1095/biolreprod63.2.559
- Patel J., Landers K., Mortimer R.H., Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010; 31(11): 951-7. https://dx.doi.org/10.1016/j.placenta.2010.08.008
- Беженарь В.Ф., Павлова Н.Г., Большакова М.В., Пастушенков В.Л., Карев В.Е. Экспрессия гипоксия-индуцируемого фактора (HIF-1-α) в плацентах при хронической плацентарной недостаточности в конце беременности. Уральский медицинский журнал. 2020; 05(188): 141-5. [Bezhenar V.F., Pavlova N.G., Bolshakova M.V., Pastushenkov V.L., Karev V.E. Expression of hypoxia-induced factor (HIF-1-α) in placentas with chronic placental insufficiency at the end of pregnancy. Ural Medical Journal. 2020; 05(188): 141-5 (in Russian)]. https://dx.doi.org/10.25694/URMJ.2020.05.29
- Ciampa E.J., Flahardy P., Srinivasan H., Jacobs C., Tsai L., Karumanchi S.A. et al. Hypoxia-inducible factor 1 signaling drives placental aging and can provoke preterm labor. Elife. 2023; 12: RP85597. https://dx.doi.org/10.7554/eLife.85597
- Chen Y., Zhang Y., Xie S., Zhou X., Zhu L., Cao Y. Establishment of a placental lncRNA-mRNA expression network for early-onset preeclampsia. BMC Pregnancy Childbirth. 2024; 24(1): 329. https://dx.doi.org/10.1186/s12884-024-06481-4
- Титова О.Н., Кузубова Н.А., Лебедева Е.С. Роль гипоксийного сигнального пути в адаптации клеток к гипоксии. РМЖ. Медицинское обозрение. 2020; 4(4): 207-13. [Titova O.N., Kuzubova N.A., Lebedeva E.S. The role of the hypoxia signaling pathway in cellular adaptation to hypoxia. Russian Medical Inquiry. 2020; 4(4): 207-13. (in Russian)]. https://dx.doi.org/10.32364/2587-6821-2020-4-4-207-213
- Приходько А.М., Романов А.Ю., Тысячный О.В., Гапаева М.Д., Баев О.Р. Современные принципы кардиотокографии в родах. Медицинский Совет. 2020; (3): 90-7. [Prikhodko A.M., Romanov A.Y., Tysyachnyy O.V., Gapaeva M.D., Baev O.R. Modern principles of cardiotocography in childbirth. Medical Council. 2020; (3): 90-7. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2020-3-90-97
- Tregub P.P., Malinovskaya N.A., Morgun A.V., Osipova E.D., Kulikov V.P., Kuzovkov D.A. et al. Hypercapnia potentiates HIF-1α activation in the brain of rats exposed to intermittent hypoxia. Respir. Physiol. Neurobiol. 2020; 278: 103442. https://dx.doi.org/10.1016/j.resp.2020.103442
- American Academy of Pediatrics Committee on Fetus and Newborn; American College of Obstetricians and Gynecologists Committee on Obstetric Practice. The Apgar Score. Pediatrics. 2015; 136(4): 819-22. https://dx.doi.org/10.1542/peds.2015-2651
- Vrijens K., Tsamou M., Madhloum N., Gyselaers W., Nawrot T.S. Placental hypoxia-regulating network in relation to birth weight and ponderal index: the ENVIRONAGE Birth Cohort Study. J. Transl. Med. 2018; 16(1): 2. https://dx.doi.org/10.1186/s12967-017-1375-5
- Rajakumar A., Jeyabalan A., Markovic N., Ness R., Gilmour C., Conrad K.P. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007; 293(2): R766-74. https://dx.doi.org/10.1152/ajpregu.00097.2007
- Colson A., Depoix C.L., Baldin P., Hubinont C., Sonveaux P., Debiève F. Hypoxia-inducible factor 2 alpha impairs human cytotrophoblast syncytialization: New insights into placental dysfunction and fetal growth restriction. FASEB J. 2020; 34(11): 15222-35. https://dx.doi.org/10.1096/fj.202001681R
- Gargaglioni L.H., Marques D.A., Patrone L.G.A. Sex differences in breathing. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019; 238: 110543. https://dx.doi.org/10.1016/j.cbpa.2019.110543
Received 28.02.2025
Accepted 21.05.2025
About the Authors
Margarita D. Leonova, Obstetrician-Gynecologist, Maternity Hospital No. 13, 191124, Russia, St. Petersburg, Kostromskaya str., 4, +7(812)275-68-70,_margarita_@bk.ru, https://orcid.org/0000-0002-3813-2995
Vitaly F. Bezhenar, Dr. Med. Sci., Professor, Head of the Departments of Obstetrics, Gynecology and Neonatology/Reproductology, Head of the Clinic of Obstetrics and Gynecology, Pavlov First State Medical University, Ministry of Health of Russia, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8; Main Supernumerary Specialist Obstetrician-Gynecologist of the Health Committee of St. Petersburg, +7(812)338-78-66, bez-vitaly@yandex.ru, https://orcid.org/0000-0002-7807-4929
Natalia Yu. Semenova, PhD, Head of Scientific Department, Morfolab, St. Petersburg, Russia, https://orcid.org/0000-0003-4069-0678
Dmitry V. Semenikhin, Pathologist, Chief Physician, Morfolab, Russia, St. Petersburg, https://orcid.org/0000-0002-0636-5241
Elena V. Frederiks, PhD, Chief Physician, Maternity Hospital No. 13, 191124, Russia, St. Petersburg, Kostromskaya str. 4; Teaching Assistant at the Department of Obstetrics, Gynecology and Neonatology, Pavlov First State Medical University, Ministry of Health of Russia, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8,
https://orcid.org/0000-0002-2513-6209



