Организм человека населен сложными сообществами бактерий, грибов, простейших, которые являются индивидуальными для конкретных биологических ниш каждого человека (желудочно-кишечный, респираторный, урогенитальный тракты, кожа и др.). В 2012 г. стало известно, что геном человека состоит из 20 000–25 000 генов, в то время как геном всего микробного сообщества отдельного человека в несколько сотен раз больше и состоит из миллионов генов [1]. Большой вклад в осознание истинной роли микробиоты внес проект «Микробиом человека» (Human Microbiome Project), который был разделен на две фазы. В рамках 1-й фазы изучалась характеристика микробных сообществ различных локализаций человеческого организма. Показано, что изучения одного лишь таксономического состава микробиоты недостаточно. Это послужило основой для разработки 2-й фазы проекта, которая, помимо изучения качественно-количественного состава микробиоты, продемонстрировала большую роль нейроэндокринного, иммунного, метаболического состояния самого макроорганизма в реализации свойств микроорганизмов [1].
Результаты проекта в целом показали, что каждый человек несет свой собственный, в основном индивидуальный набор микробных штаммов, который приобретается сразу после рождения [2, 3] и имеет расовые и средовые различия [4], может претерпевать быстрые качественно-количественные изменения [5] или же оставаться относительно неизменным [6].
Одной из самых динамичных экосистем в организме женщины является микробиота влагалища. Ее нарушение играет ключевую роль в развитии ряда урогенитальных заболеваний в акушерско-гинекологической практике, таких как бактериальный вагиноз, аэробный вагинит, инфекции, передаваемые половым путем, инфекции мочевыводящих путей, воспалительные заболевания органов малого таза, неопластические процессы шейки матки, бесплодие, невынашивание беременности, хориоамнионит, преэклампсия, плацентарная недостаточность, послеродовый эндометрит, субинволюция матки и др. [7–9].
По мнению большинства ученых, ключевую роль в гомеостазе микробиоты влагалища играют бактерии, продуцирующие молочную кислоту, в основном Lactobacillus spp., которые являются представителями резидентной вагинальной микрофлоры у женщин репродуктивного возраста и составляют 80–90% всего пула микроорганизмов влагалища. На долю условно-патогенных микроорганизмов (УПМ) остается всего 10–20% [10]. По мере созревания клетки многослойного плоского эпителия накапливают гликоген, который ферментируется Lactobacillus spp. в процессе их жизнедеятельности до молочной кислоты, способствуя установлению pH влагалищного содержимого <4,5, что создает неблагоприятные условия для роста условных и абсолютных патогенов [10].
Наиболее ярким примером нарушения микробиоты влагалища является БВ (полимикробный невоспалительный синдром, возникающий в результате замены нормальной микробиоты влагалища (различных видов Lactobacillus spp., продуцирующих молочную кислоту и перекись водорода) на повышенную генерацию многочисленных видов облигатных и факультативных анаэробных микроорганизмов (Gardnerella vaginalis, Atopobium vaginae, Bacteroides spp., Prevotella spp., Mobiluncus spp., Mycoplasma hominis и др.) [10]. Патологические вагинальные выделения, характерные для БВ, – частая причина обращения женщины к врачу.
В поддержании нормобиоценоза, помимо качественно-количественного состава микробиоты, большую роль также играет иммунный, нейроэндокринный и метаболический фон самого макроорганизма. Предполагается, что именно иммунная система определяет исход заболевания, следовательно, внимание необходимо уделять не только состоянию микробиоценоза влагалища, но и оценке местного иммунного статуса [1].
Важным компонентом местной иммунной защиты влагалища являются цитокины. Они продуцируются различными клетками, присутствуют в вагинальном секрете в виде провоспалительных (ФНО-α, IL-1β, IL-6, IL-8, IL-12) и противовоспалительных (IL-4, IL-10, TGFβ) цитокинов [11] и являются не только эндогенными регуляторами иммунных реакций, но и ключевыми факторами, инициирующими развитие дисбиотических процессов различной локализации. При сбалансированной работе факторов врожденного иммунитета происходит активация адаптивного иммунитета, в результате чего достигается нормоценоз. Недостаточность факторов врожденного иммунитета может привести к хронизации и персистированию инфекции [12].
На сегодняшний день, в соответствии с методическими рекомендациями Центра по контролю и профилактике инфекций, передаваемых половым путем (ИППП), США (СDC, 2021), рекомендациями европейского руководства (IUSTI, 2018), Российскими рекомендациями по диагностике и лечению заболеваний, сопровождающихся патологическими выделениями из половых путей (РОАГ, 2019), лечение показано только женщинам с симптомным течением БВ [13]. По всей видимости, это является оправданным, поскольку существуют здоровые женщины с типом бактериального сообщества, который характеризуется преобладанием анаэробов, что обусловлено генетическими особенностями иммунной системы макроорганизма [14].
Лечение бессимптомных пациенток с результатами лабораторных методов обследования, соответствующих БВ, показано пациенткам, планирующим хирургические операции на влагалище, шейке матки, матке, ее придатках, и беременным женщинам с преждевременными родами или поздним выкидышем в анамнезе [15].
Основным терапевтическим направлением при БВ является, с одной стороны, снижение числа и разнообразия строгих и факультативных анаэробных УПМ, с другой – восстановление доминирования Lactobacillus spp. (пробиотики, иммунокоррекция, восстановление pH влагалища). Имевшееся в прошлые десятилетия стремление к «полной санации» слизистых может привести к последующему инфицированию прежними или более вирулентными микроорганизмами [16].
Во всем мире врачи стремятся использовать антибактериальные препараты согласно актуальным гайдлайнам и принципам глобальной компании ВОЗ по борьбе с антибиотикорезистентностью. В соответствии с рекомендациями по лечению ИППП (CDC, 2021) лечение БВ проводится одноэтапно метронидазолом перорально/интравагинально или клиндамицином интравагинально. Альтернативные схемы лечения БВ включают несколько режимов применения тинидазола и клиндамицина перорально, а также деквалиния хлорида интравагинально [13, 15, 17]. Однако антимикробная терапия при БВ часто не обеспечивает стойкого эффекта, и перед учеными и врачами стоит проблема высокого процента его рецидивирования [18]. Так, по данным различных авторов, процент рецидивирования БВ сразу после лечения составляет 15–30%, достигая 58–80% спустя год после терапии [19]. К сожалению, этиология и патогенез рецидивирующих вагинальных инфекций изучены далеко не полностью. Интерес представляют факторы риска рецидивирования БВ, опубликованные в американском обзоре научной литературы по данной теме (2020) [20]. К ним относятся: наличие или отсутствие постоянного полового партнера, персистирование БВ-ассоциированных бактерий в биопленке, колонизация de novo, наличие резервуаров БВ-ассоциированных бактерий вне влагалища, несоблюдение схем терапии и формирование патологических биоценозов вследствие необоснованного лечения несуществующих заболеваний (фактически неправильной трактовки результатов лабораторных исследований). Кроме того, к факторам риска можно отнести отсутствие восполнения количества Lactobacillus spp., т.е. невозвращение после лечения к оптимальному составу микробиома, в котором доминируют лактобациллы, и неполноценность местной иммунной защиты.
В связи с этим были разработаны препараты, главной целью которых явилось восстановление доминирования лактобактерий. Многими мировыми экспертами был признан «русский» метод двухэтапной терапии лечения БВ с применением пробиотиков с целью предотвращения развития рецидивов [15, 21].
Пробиотики – это живые бактерии, которые формируют здоровую микрофлору. Используются при лечении иммунных заболеваний, авитаминоза, дисбактериоза. К ним относятся, в том числе, и лактобактерии. Пробиотики конкурируют за питательные вещества с патогенами, приводят к изменению местного рН, что создает неблагоприятную среду для патогенов, уничтожают супероксидные радикалы, стимулируют эпителиальную продукцию муцина, усиливают барьерную функцию, конкурируют с патогенами за адгезию, модифицируют токсины патогенных микроорганизмов.
Накоплен большой опыт применения пробиотиков. В ряде исследований [22] показана их эффективность. Недавний систематический обзор и метаанализ показали, что применение пробиотиков в комплексе с основной терапией обеспечивает снижение разнообразия вагинальной микрофлоры и рост популяции Lactobacillus spp., нарушение структуры патогенных биопленок, что приводит к повышению эффективности лечения [23]. Кроме этого, в литературе имеются данные о содружественном влиянии Lactobacillus spp. и факторов местного иммунитета на слизистую влагалища: Lactobacillus spp. могут регулировать уровень иммунного ответа, выступая в роли его естественного стимулятора. Это происходит за счет активации локальных макрофагов, B-клеточного иммунитета, выработки клетками эпителия влагалища комплемента, лизоцима, секреторного IgA, модуляции цитокинового профиля [24].
Однако вопрос применения пробиотиков при лечении БВ постоянно обсуждается. Опубликованные исследования разнятся по качеству выполнения. Все рекомендации по пробиотикам имеют слабую силу. К тому же нет достаточных доказательств противорецидивной эффективности препаратов, содержащих пробиотики и факторы местного иммунитета [25]. Более того, в литературе недостаточно таких исследований. Поэтому для подкрепления имеющихся данных требуются новые высококачественные исследования, основанные на применении пробиотиков у большого количества пациенток при лечении БВ.
Цель исследования: оценить клинико-лабораторную эффективность, приемлемость и безопасность противорецидивной терапии кремом, содержащим в своем составе ацидофильные бактерии (Lactobacillus acidophilus), витаминно-минеральный белково-пептидный комплекс (Суперлимфлайф) у пациенток с БВ.
Исследование было одобрено Этическим комитетом ФГБУ «НМИЦ АГП им. В.И. Кулакова» Минздрава России.
Материалы и методы
На базе научно-поликлинического отделения ФГБУ «НМИЦ АГП им. В.И. Кулакова» Минздрава России были обследованы 116 женщин в возрасте от 18 до 49 лет (средний возраст обследуемых составил 31,4±5,95 года). Пациентки обратились с жалобами на обильные выделения из половых путей, в некоторых случаях сопровождающиеся неприятным «рыбным» запахом, дискомфортом во влагалище, зудом. Всем пациенткам, согласно критериям Амселя, поставлен диагноз «бактериальный вагиноз». У всех пациенток отмечались обострения БВ (4 и более эпизода) в течение последнего года.
Было проведено комплексное клинико-лабораторное обследование, включающее сбор и анализ анамнестических данных, гинекологическое обследование, определение pH влагалищной среды, тест с KOH, микроскопическое исследование мазков отделяемого влагалища, комплексное исследование микрофлоры урогенитального тракта методом полимеразной цепной реакции (ПЦР) в режиме реального времени (количественная оценка состояния биоценоза), оценку состояния локального иммунитета по уровню мРНК ключевых генов иммунного ответа. Методом ПЦР в режиме реального времени определяли наличие абсолютных патогенов (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis, вирус простого герпеса (ВПГ) 1, 2 типов, цитомегаловирус (ЦМВ)) у всех пациенток, включенных в исследование. Наличие патогенов являлось основанием для исключения пациентки из исследования.
Критериями включения в исследование явились: возраст пациенток от 18 до 49 лет (репродуктивный возраст), подтвержденный диагноз БВ на основании критериев Амселя, отсутствие ИППП, желание и способность пациентки выполнять рекомендации врача, подписание информированного согласия пациенткой на участие в исследовании.
Критерии невключения: применение антибактериальной, иммуномодулирующей и гормональной терапии в течение 30 дней до исследования, наличие аутоиммунных заболеваний, в т.ч. сахарного диабета, соматических заболеваний в стадии декомпенсации, онкологических заболеваний, ожирения, эндокринных нарушений; активного туберкулеза, вирусных гепатитов, ВИЧ-инфекции, дисплазии шейки матки тяжелой степени, рака шейки матки in situ, беременности и лактации в настоящее время.
Диагноз устанавливался на основании наличия 3 из 4 критериев Амселя, перечисленных ниже:
1) наличие гомогенных беловато-серых выделений, равномерно распределяющихся по стенкам влагалища;
2) повышение уровня влагалищного рН >4,5 (pH-метрия – обязательный метод диагностики);
3) положительный тест с 10% раствором KOH (появление или усиление специфического запаха несвежей, гнилой рыбы) – определение летучих аминов;
4) обнаружение в нативных препаратах или мазках, окрашенных по Граму, «ключевых клеток».
При гинекологическом осмотре пациенток на всех этапах исследования оценивались такие клинические признаки, как наличие/отсутствие гиперемии слизистой оболочки стенок влагалища и вульвы, отечность, характер выделений (цвет, консистенция, количество, запах).
рН-метрию содержимого заднего свода влагалища проводили с помощью индикаторных полосок Ури-рН-1 («Биосенсор АН», Россия) – от 3,5 до 7,5, с шагом деления 0,5. Аминный тест считался положительным при появлении или усилении неприятного запаха несвежей рыбы при смешивании 10% раствора KOH и влагалищного отделяемого.
При микроскопическом исследовании нативного и/или окрашенного по Граму влагалищного отделяемого, полученного с верхней трети боковых сводов влагалища, оценивались количество лейкоцитов, эпителиальных клеток, количество и морфологический тип бактерий, наличие/отсутствие «ключевых клеток», представляющих собой эпителиоциты влагалища с плотно прикрепленными (адгезированными) по их поверхности грамвариабельными микроорганизмами, а также наличие/отсутствие ряда специфических возбудителей, таких как грибы рода Candida, трихомонады и гонококки.
Для выделения ДНК микроорганизмов при комплексном исследовании микрофлоры урогенитального тракта методом ПЦР в режиме реального времени (количественная оценка состояния биоценоза) использовались наборы «Проба ГС» (ООО «НПО ДНК-Технология», Россия). Метод основан на сорбции ДНК на носителе (сорбенте), отмывке примесей с последующей элюцией нуклеиновых кислот с сорбента. Амплификация осуществлялась на приборе «ДТ-964» («ДНК-Технология», Россия) с измерением уровня флуоресценции на каждом цикле амплификации по каналам FAM, HEX, ROX. При количественной оценке биоценоза влагалища учитывали: общее количество бактерий, количество Lactobacillus spp. и 14 основных групп микроорганизмов, представляющих условно-патогенную флору, включая факультативно-анаэробные (Enterobacterium spp., Streptococcus spp., Staphylococcus spp.), облигатно-анаэробные микроорганизмы (Gardnerella vaginalis, Prevotella spp., Porphyromonas spp., Eubacterium spp., Sneathia spp., Leptotriсhia spp., Fusobacterium spp., Megasphaera spp., Veillonella spp., Dialister spp., Lachnobacterium spp., Clostridium spp., Mobiluncus spp., Corynebacterium spp., Peptostreptococcus spp., Atopobium vaginae), а также Candida spp., Ureaplasma spp., Mycoplasma hominis, Mycoplasma genitalium. При оценке состава условно-патогенной флоры учитывались не только ее присутствие, но и количество по отношению к общей бактериальной массе (ОБМ). Также определялось количество лактобактерий по отношению к ОБМ.
Как абсолютный нормоценоз рассматривался вариант биоценоза, при котором доля нормофлоры (Lactobacillus spp.) составляла более 80% по отношению к общему количеству бактерий. Анаэробный дисбиоз расценивался как дисбаланс микробиоты, обусловленный одним или несколькими облигатно-анаэробными УПМ в количестве более 20% по отношению к общему количеству бактерий и долей нормофлоры менее 80%.
У всех исследуемых пациенток исключалось наличие патогенов Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis методом ПЦР с помощью комплекта реагентов «TNC Комплекс»; вирусов: ВПГ 1, 2 типов, ЦМВ (с помощью комплекта реагентов «ГерпесКомплекс»).
Для оценки состояния локального (мукозального) иммунитета по уровню мРНК ключевых генов иммунного ответа использовался метод экспрессионного профилирования. Для определения уровня экспрессии мРНК генов иммунной системы взятие материала осуществляли с боковых стенок влагалища. Для выделения нуклеиновых кислот использовали наборы «Проба НК». В образце эпителиальных клеток учитывался уровень экспрессии 8 генов: IL-1β, IL-10, IL-18, TNF-α, TLR4, GATA3, CD68, B2M (ИммуноКвантэкс). Полученный экспрессионный профиль сравнивался с профилем, характерным для локального воспаления. Заключение о наличии локальной воспалительной реакции проводилось на основании расчета значения индекса воспаления (ИВ), проводимого программным обеспечением детектирующего амплификатора в автоматическом режиме. Пороговое значение вероятности воспаления составило 60% (р=60%). При расчете показателя p у 16 пациенток значение оказалось более 60% и было расценено как маркер вагинита, в связи с чем данные пациентки были исключены из исследования.
Таким образом, после оценки критериев включения и невключения в исследование вошли 100 женщин. Рандомизация пациенток осуществлялась при помощи таблицы случайных чисел. Пациентки были разделены на 2 группы: в 1-ю группу вошли женщины с рецидивирующим БВ (n=50), которым была назначена одноэтапная терапия, пациенткам 2-й группы с рецидивирующим БВ (n=50) была назначена двухэтапная терапия.
Пациентки 1-й группы получали одноэтапную терапию комплексным препаратом местного действия, содержащим метронидазол 500 мг и миконазола нитрат 100 мг, – 1 свеча 2 раза в день в течение 7 дней. Выбор в пользу этого препарата был сделан на основании имеющихся данных о большей частоте развития вульвовагинального кандидоза (ВВК) у пациенток, применяющих метронидазол [26], а добавление миконазола нитрата к метронидазолу приводит к снижению риска развития ВВК.
Пациенткам 2-й группы в качестве противорецидивной терапии после окончания первого этапа лечения был назначен крем, содержащий в своем составе ацидофильные бактерии (Lactobacillus acidophilus) и витаминно-минеральный белково-пептидный комплекс Суперлимфлайф (Ацилакт DUO), оказывающий стимулирующее действие на функциональную активность клеток фагоцитарного ряда (моноциты и нейтрофилы), а именно выработку цитокинов (ИЛ-1, ФНО), индукцию противоопухолевой цитотоксичности макрофагов, регуляцию миграции клеток в очаг воспаления, увеличение активности естественных киллеров. Кроме того, препарат обладает пролиферирующим действием на фибробласты, что обуславливает регенерацию и эпителизацию раневых дефектов, обладает антиоксидантной активностью, снижает развитие воспалительных реакций.
Крем использовался интравагинально 1 раз в сутки в течение 10 дней согласно рекомендованной схеме, указанной в инструкции по медицинскому применению.
Клиническое и лабораторное обследование пациенток проводилось четырехкратно.
1-й визит включал сбор анамнеза, жалоб пациентки, гинекологический осмотр, измерение pH влагалищного содержимого, оценку теста с 10% раствором КОН, забор материала с последующим проведением лабораторных методов исследования. По результатам обследования, после постановки предварительного и окончательного диагноза БВ всем пациенткам назначался первый этап терапии.
2-й визит осуществлялся через 7–10 дней после окончания 1-го этапа терапии у пациенток обеих групп с оценкой его эффективности (клинической и лабораторной), переносимости, наличия или отсутствия нежелательных явлений. Пациенткам 2-й группы назначался 2-й этап терапии.
3-й визит проводился через 1 месяц после окончания одноэтапной терапии у пациенток 1-й группы и через 1 месяц после окончания двухэтапной терапии у пациенток 2-й группы и включал клинико-лабораторное обследование пациенток с целью оценки эффективности терапии, наличия или отсутствия рецидивов в обеих группах.
4-й визит предусматривал клинико-лабораторное обследование пациенток через 3 месяца после окончания лечения с оценкой эффективности терапии и частоты рецидивирования заболевания в обеих группах.
Регистрировались нежелательные явления, имеющие место в обеих группах на всех этапах наблюдения.
Статистический анализ
Статистический анализ фактического материала был выполнен с применением программ Excel Microsoft 8.0, Statistica for Windows 6.0 по стандартным методикам вычислений показателей описательной статистики. Проверка выборок на нормальность распределения осуществлялась при помощи теста Колмогорова–Смирнова. Сравнения производились с применением t-критерия Стьюдента и критерия Уилкоксона для зависимых выборок. Для анализа различия частот признаков в независимых группах использовался критерий χ2 с поправкой Йейтса.
Результаты
Согласно результатам, большинство обследованных женщин (n=75; 75%) находились в раннем репродуктивном возрасте. Средний возраст пациенток составил 33,6 года (95% ДИ 32,67–34,57; min – 19; max – 48; Me – 32,9). Среди сопутствующих нозологий у женщин 1-й группы были указаны хронический цистит (15,3%), хронический пиелонефрит (16,7%), хронический тонзиллит (17,7%), дискинезия желчевыводящих путей (18%), синдром раздраженного кишечника (10%), апоплексия яичника (7,9%), эндометриоз (5,3%). Во 2-й группе: хронический цистит (16,1%), хронический пиелонефрит (15,1%), хронический тонзиллит (16,3%), дискинезия желчевыводящих путей (17,3%), синдром раздраженного кишечника (9,5%), апоплексия яичника (8,1%), эндометриоз (4,9%). Количество беременностей в 1-й группе пациенток варьировало от 0 до 6 и в среднем составило 3,7±0,6; количество родов – 2,5±0,4; количество абортов – 3,9±0,7. У пациенток 2-й группы количество беременностей составило от 0 до 7, в среднем – 4,1±0,5; количество родов – 2,8±0,5; количество абортов – 4,2±0,6. К периоду наблюдения все обследуемые женщины вели активную половую жизнь. Число половых партнеров у обследованных женщин варьировало от 1 до 10 за всю жизнь и в среднем составило 5,6±0,6; количество партнеров за последний месяц в среднем составило 1,2±0,4 (от 1 до 3). Частота половых актов за неделю составляла от 1 до 8, в среднем – 2,4±1,2. Анализируя семейное положение, отмечено, что в браке состояли 87 пациенток (87%): 42 (42%) в 1-й группе; 45 (45%) во 2-й группе; 13 (13%) пациенток были не замужем: 8 (8%) в 1-й группе и 5 (5%) – во 2-й. Таким образом, при изучении всех анамнестических данных не было выявлено статистической разницы между пациентками 1-й и 2-й групп (р>0,05).
Результаты оценки жалоб на момент первичного приема продемонстрировали, что большинство пациенток – 91 (91%) отмечали выделения из половых путей: 35 (35%) пациенток – умеренные, 56 (56%) – обильные. Неприятный «рыбный» запах отмечался у 53 (53%) женщин: у 25 (25%) при применении пробы с 10% раствором КOH запах появлялся, у 28 (28%) – усиливался. Жалобы на дискомфорт во влагалище отмечали 64 (64%) пациентки, зуд в области наружных половых органов и во влагалище – 15 (15%). Анализ клинических проявлений БВ при включении в исследование статистической разницы между группами не выявил.
У всех (100%) пациенток уровень pH влагалищного отделяемого составил более 4,5: средний уровень pH – 5,6, min – 4,8, max – 7,0, Me – 5,3. При анализе результатов микроскопического исследования вагинальных мазков было выявлено, что у пациенток обеих групп в мазках количество лейкоцитов не превышало 10 в п/зр., преобладали мелкие грамвариабельные и грамотрицательные кокки, изогнутые грамвариабельные палочки. «Ключевые» клетки были обнаружены у 85 (85%) пациенток, включенных в исследование. Таким образом, при постановке диагноза БВ 4 критерия Амселя были обнаружены у 89 (89%) пациенток, в то время как у 11 (11%) женщин наблюдалось только 3 из 4 критериев. Чаще всего встречался критерий повышения уровня влагалищного рН>4,5 (в 100% случаев); наличие гомогенных беловато-серых выделений определялось у 91 (91%) пациентки, ключевые клетки при микроскопическом исследовании мазка влагалищного отделяемого обнаруживались у 85 (85%) женщин, у 53 (53%) пациенток отмечался положительный тест с 10% раствором КОН.
Оценка динамики жалоб была проведена внутри каждой группы до назначения терапии, через 7 дней после окончания первого этапа терапии, через 1 месяц и через 3 месяца после окончания терапии. Динамика клинических данных по группам представлена в таблицах 1 и 2.
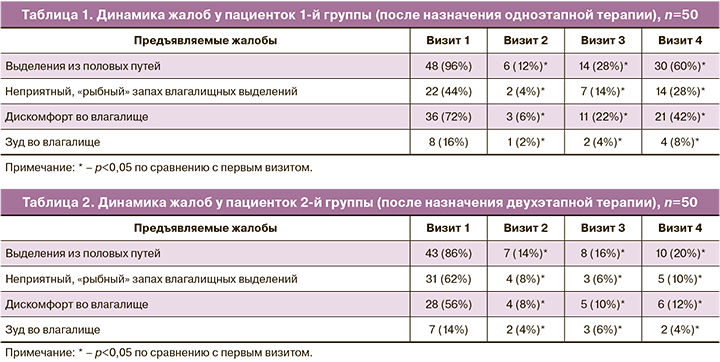
Согласно полученным данным, у пациенток, получавших двухэтапную терапию, к 4-му визиту количество жалоб уменьшалось, в то время как после проведения одноэтапной терапии отмечалось их возвращение.
При анализе уровня pH влагалищного отделяемого среди пациенток 1-й группы было выявлено, что на 1-м визите уровень pH >4,5 наблюдался у 50 (100%) пациенток, на 2-м – у 8 (16%), на 3-м – у 12 (24%); к 4-му визиту количество пациенток с уровнем pH>4,5 составило 20 (40%). Во 2-й группе пациенток на 1-м визите уровень pH>4,5 определялся у 50 (100%) пациенток, на 2-м и 3-м визитах – у 7 (14%), на 4-м визите – у 8 (16%). Имеющиеся различия между группами были статистически значимыми (р<0,05). Согласно полученным данным, во 2-й группе к 4-му визиту количество женщин с уровнем pH>4,5 было ниже, чем в 1-й группе (р<0,05).
Проведен сравнительный анализ параметров микроскопического исследования вагинальных мазков пациенток обеих групп. У всех пациенток уровень лейкоцитов был в пределах нормы. В 1-й группе преобладание грамположительных палочек над грамвариабельными палочками и кокками встречалось у 15 (30%) пациенток, отсутствие «ключевых клеток» – у 24 (48%). Во 2-й группе к 4-му визиту преобладание грамположительных палочек над грамвариабельными палочками и кокками было выявлено у 45 (90%) пациенток, отсутствие «ключевых клеток» – у 48 (96%). Таким образом, по данным микроскопического исследования вагинальных мазков, к 4-му визиту преобладание бактерий морфотипа Lactobacillus spp. и отсутствие «ключевых клеток» встречались чаще среди пациенток, получавших противорецидивную терапию, по сравнению с пациентками, получавшими одноэтапную терапию (р<0,05).
При сравнительной оценке результатов комплексного исследования микробиоты урогенитального тракта методом ПЦР в режиме реального времени среди пациенток 1-й и 2-й групп при 1-м обращении наблюдалось нарушение микробиоты за счет преобладания облигатно-анаэробных УПМ и снижения количества Lactobacillus spp. Среди пациенток 1-й группы на 1-м визите БВ был выявлен у 50 (100%) женщин, на 2-м – у 10 (20%), на 3-м – у 15 (30%), на 4-м – у 28 (56%). Среди пациенток 2-й группы на 1-м визите преобладание облигатно-анаэробных УПМ над Lactobacillus spp. наблюдалось у 50 (100%) женщин, на 2-м – у 12 (24%), на 3-м – у 6 (12%); на 4-м – у 5 (10%).
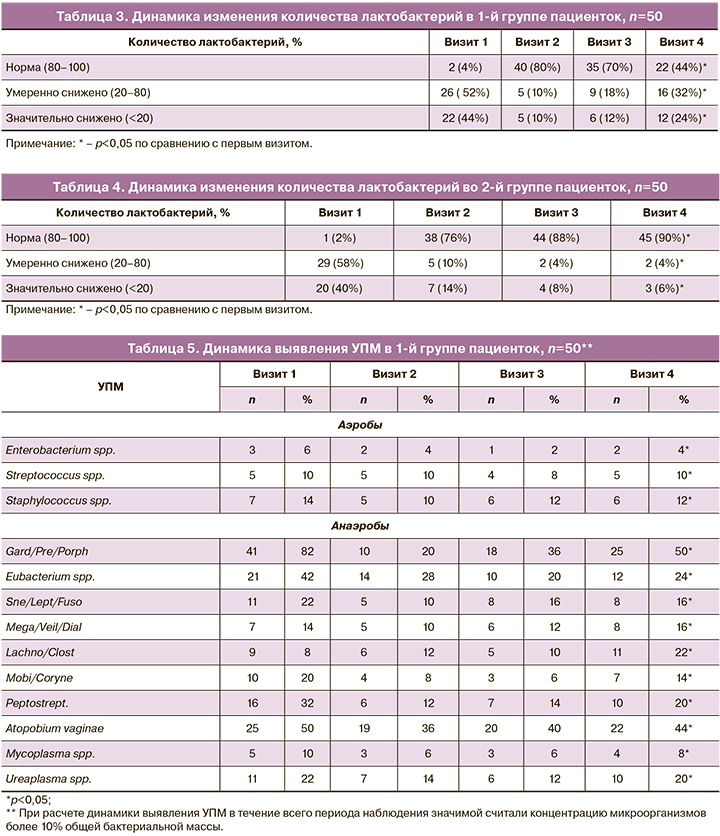
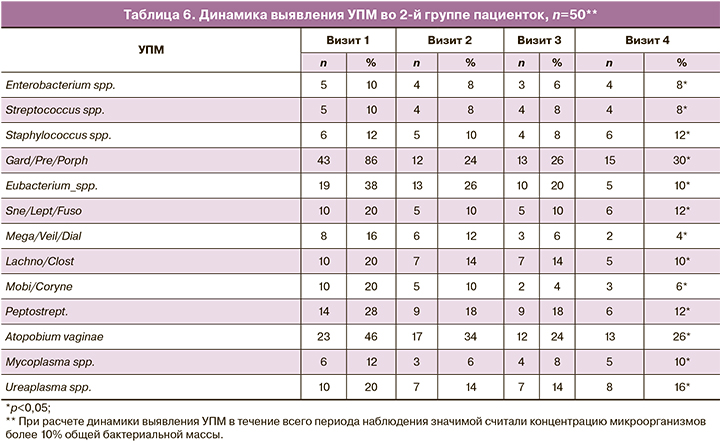
Оценка динамики изменения количества лактобактерий внутри каждой группы представлена в таблицах 3 и 4. Подробный анализ динамики выявления УПМ в группах исследования представлен в таблицах 5 и 6. Значимой считали концентрацию микроорганизмов более 10% ОБМ.
Согласно полученным данным, можно предположить, что применение двухэтапной терапии способствует более устойчивому восстановлению нормальных показателей лактофлоры. Так, во 2-й группе женщин, получавших двухэтапную терапию, к 4-му визиту у 45 (90%) пациенток количество лактобактерий соответствовало норме, умеренное снижение лактобактерий было установлено у 2 (4%), значительное снижение – у 3 (6%), в то время как в 1-й группе к 4-му визиту нормальное количество лактобактерий наблюдалось у 22 (44%) женщин, умеренное снижение – у 16 (32%); значительное снижение – у 12 (24%).
В ходе исследования были изучены транскрипционные профили генов иммунного ответа во влагалищных мазках женщин 1-й и 2-й групп на всех визитах. Анализ уровня мРНК генов провоспалительных (IL-8, TLR2, TLR4, IL-10, TNF-α) и противовоспалительных маркеров (IL-18, GATA3) не выявил статистической разницы между пациентками 1-й и 2-й групп на 1-м визите. При изучении полученных данных у пациенток 1-й группы было выявлено на 1-м визите увеличение уровня экспрессии провоспалительных маркеров TLR4, TNF-α, IL-8, IL-10 и TLR2 соответственно в 1,4; 1,18; 3,31; 2,1 и 3,07 раза и снижение уровня экспрессии IL-18, GATA3 соответственно в 0,22 и 0,24 раза; во 2-й группе увеличение уровня экспрессии провоспалительных маркеров TLR4, TNF-α, IL-8, IL-10 и TLR2 в 1,5; 1,2; 3,45; 2,3; 3,1 раза соответственно и снижение уровня экспрессии IL-18, GATA3 соответственно в 0,21 и 0,2 раза.
К 4-му визиту в 1-й группе было выявлено некоторое увеличение уровней IL-8, TLR2, TLR4, IL-10, TNF-α – в 3,01; 2,2; 1,6; 1,5; 1,3 раза по сравнению с референтными значениями и снижение экспрессии IL-18, GATA3 в 0,22 и 0,2 раза соответственно; во 2-й группе экспрессия TLR4, TNF-α, IL-8, IL-10 и TLR2 была повышена в 1,02; 1,01; 1,4; 1,3; 1,8 раза; экспрессия IL18, GATA3 была снижена в 0,5 и 0,42 раза соответственно.
При анализе динамики изменения уровня экспрессии провоспалительных и противовоспалительных цитокинов в каждой из групп на протяжении 4 визитов было выявлено, что в группе женщин, получавших двухэтапную терапию, наблюдалась тенденция к снижению провоспалительных маркеров и повышению противовоспалительных маркеров (р<0,05). В группе женщин, получавших одноэтапную терапию, наоборот, наблюдалось снижение уровня экспрессии противовоспалительных маркеров и повышение TLR4 и TNF-α по сравнению с этапом скрининга, в то время как уровни экспрессии IL-8, IL-10 и TLR2 снижались (р<0,05).
На основании полученных данных был произведен расчет ИВ. При анализе динамики изменения ИВ было выявлено, что в группе женщин, получавших двухэтапную терапию, к 4-му визиту наблюдалась тенденция к снижению ИВ, в то время как в 1-й группе данный показатель, наоборот, несколько возрастал по сравнению с этапом скрининга, но не превышал нормативных значений. Так, средний показатель ИВ во 2-й группе к 4-му визиту достоверно снизился и составил 41,74±6,25; в 1-й – увеличился и составил в среднем 55,65±7,35.
Таким образом, в группе женщин, получавших одноэтапную терапию, ИВ к 4-му визиту возрос на 6,58%, в то время как в группе женщин, получавших двухэтапную терапию, снизился на 13,02%.
При оценке эффективности терапии на 2-м визите было выявлено, что 1-й этап терапии оказался эффективным у 42 (84%) пациенток 1-й группы и 43 (86%) пациенток 2-й группы.
При отслеживании рецидивирования процесса было установлено, что в 1-й группе на 3-м визите случаи рецидива наблюдались у 20 (40%) пациенток, на 4-м визите – у 35 (70%); во 2-й группе на 3-м визите – у 11 (22 %) пациенток, на 4-м – у 15 (30%).
Заключение
Таким образом, учитывая клинические данные, результаты микроскопического исследования влагалищных мазков, комплексной оценки микробиоты влагалища методом ПЦР в режиме реального времени, динамики изменения уровня экспрессии провоспалительных и противовоспалительных маркеров, ИВ, можно предположить, что двухэтапная терапия с включением крема Ацилакт DUO повышает эффективность антибактериальной терапии при сочетанном применении за счет нормализации местного иммунного ответа, выработки определенных цитокинов (IL-1, ФНО-α), способствует более устойчивому восстановлению нормального соотношения представителей микробиоты влагалища, снижению частоты рецидивирования процесса.



