Efficiency of graduated compression for varicose veins during pregnancy
Objective: To investigate the clinical efficiency of graduated elastic compression stockings in pregnant women with varicose veins. Materials and methods: Sixty pregnant women were enrolled in the study, thirty of whom used compression stockings and formed a study arm; another 30 patients comprised the control group. The clinical grade of varicose disease was assessed according to the CEAP classification. The severity of varicose disease was assessed using the Venous Clinical Severity Score (VCSS). Quality of life (QOL) was calculated using the Chronic Venous Insufficiency Questionnaire (CIVIQ-20). The diameters of the great and small saphenous veins were measured using Doppler ultrasonography. The tibia circumference was measured by measuring tape in an upright position. Results: At the end of the study there was a significant reduction of VCSS index (p<0,001) in the study group, whereas in the control group varicose disease severity increased (p<0,001). The control group showed negative clinical changes according to CEAP classification: the ratio of patients with varicose disease shifted to the C3 class from 3/30 (10%) to 15/30 (50%). At the final follow-up, the overall QOL score improved in the study group (p=0.099), while the control group showed a statistically significant decrease of QOL according to CIVIQ-20 (p<0.001). The use of compression stockings was associated with a statistically significant reduction in the diameters of the great and small saphenous veins at all measurement points and a bilateral decrease in the circumference of the lower supramalleolar region (p<0,001). Conclusion: Graduated elastic compression is an effective method for the prevention and treatment of varicose vein disease during pregnancy.Khryshchanovich V.Ya., Rogovoy N.A., Skobeleva N.Ya., Krasko O.V.
Keywords
Varicose veins are among the most common chronic conditions currently observed in populations, affecting approximately half of women and one-third of men [1, 2]. Epidemiological studies indicate a predominance of patients with chronic vein disease in Europe and North America, whereas varicose veins are less common in the Mediterranean basin, India, South America and Africa [3, 4]. The true causes of varicose disease remain poorly understood to date; whereas, the 4 main risk factors - heredity, female sex hormones, gravitational force, and muscle dysfunction - are confirmed triggers of varicose vein transformation [5, 6].
Researchers are particularly interested in varicose disease in pregnant women, which is characterized by early and sudden occurrence of varicose veins of the lower limbs, severity of venous symptoms and equally rapid reduction (often incomplete) of clinical manifestations postpartum [7, 8]. Varicose syndrome occurs in 20–50% of pregnant women, but taking into account telangiectasia and reticular veins, its prevalence can reach 70% [8].
Studies have shown that a physiological increase in progesterone and estrogen levels has a degenerative effect on the vein wall [9, 10]. Progesterone inhibits smooth muscle contraction, whereas the estrogen effect is associated with vasodilatation. Both mechanisms can lead to valve failure due to increased vascular capacity and diameter of the superficial veins. In addition, with the growth of the uterus and fetus, there is a complication of venous outflow and increased hydrostatic pressure in the veins of the lower extremities [8, 10].
Varicose veins are commonly associated with the symptoms of heavy legs, feeling of swelling, fatigue, sensation of tension in the lower extremities, night cramps, skin itching, paresthesia, and edema [11]. These manifestations of varicose disease can complicate daily activities and affect the quality of life (QOL) of patients. Therefore, there is no doubt that it is necessary to apply therapeutic measures, the scope of which ranges from lifestyle modification and compression therapy to phlebotonics and surgical treatment using traditional stripping or endovenous obliteration [12]. Intermittent external pneumatic compression can reduce paramalleolar edema, while immersion of the lower extremities in water at 32° C for 50 min is associated with stimulation of diuresis and blood pressure reduction [13]. However, it is not possible to judge the advantages of these mentioned active and passive nonpharmacological therapies because they do not have any evidence base.
Therapeutic interventions during pregnancy are usually noninvasive and include the wearing of medical compression stockings and elevation of the lower extremities (lying on the back) [14]. However, none of these methods has been reliably validated in comparative clinical trials or randomized controlled trials [14, 15].
This study aimed to investigate the clinical efficiency of graduated elastic compression stockings in pregnant women with varicose veins.
Materials and methods
This was a study with two groups of pregnant women. The study population was under observation at the Minsk Regional Clinical Maternity Hospital and the N.E. Savchenko City Clinical Hospital No. 4 in Minsk. The study was reviewed and approved by the Research Ethics Committee of the N.E. Savchenko City Clinical Hospital No. 4 of Minsk (protocol No. 14 dated December 24, 2021) and was conducted for 2 months.
Patients aged 18–40 years who provided informed consent according to the WMA Declaration of Helsinki – Ethical Principles for Medical Research involving Humans, 2013, revised by the Secretariat of the World Medical Association on 5 May 5, 2015, were included in the study. Inclusion criteria for the study were gestational age more than 12 and less than 25 weeks, C1–C3 grades and symptoms of primary varicose disease according to CEAP classification [16].
Exclusion criteria were deep vein thrombosis, thrombophilia associated with high risk of deep vein thrombosis and postthrombotic syndrome, postoperative recurrence of varicose disease, history of pulmonary embolism, use of elastic compression 7 days before study inclusion, C4-C6 clinical classes of varicose disease according to CEAP classification, lower extremity peripheral arterial disease and lymphoedema, absolute contraindications to exercise as recommended by the American College of Obstetricians and Gynecologists (ACOG) [17].
Basic therapeutic measures in both groups included 10-minute periods of rest in a horizontal position (on the left side) after each hour of standing or sitting motionless, elevation of the lower extremities during nighttime sleep, safe physical exercise (walking, cycling, swimming, yoga, light gymnastics, Pilates, jogging) of 30 minutes at least 5 times a week, foot baths for 30 minutes (at a water temperature of +26ºC…+30ºC) and a 15 minute rising contrast shower in the morning and evening with a temperature difference from +35ºC to +20ºC) [18, 19].
Class 2 compression stockings of RAL-GZ 387, tested according to Oeko-Tex Standard 100, ISO, CE, were worn for 8 hours daily for at least 2 months in the study group. The size of the stockings was determined by taking the circumference of the ankle, calf, thigh 5 cm below the gluteal fold and the length from heel to thigh. Patients received verbal and written instructions about the rules of medical stockings use, and care. Regular compression stocking wear was monitored weekly (by telephone) throughout follow-up.
Obstetric and clinical characteristics of the patients, including gestational age, number of pregnancies and births, occupation, age, weight, height, BMI, duration, and family history of varicose veins were recorded electronically. Visual examination, physical examination, and photography of the lower extremities were performed upright in a well-lit room within two visits and gestational age (between 12–24 and 25–34 weeks' gestation). Unified assessment of visible changes (clinical class of varicose disease) was performed according to the CEAP classification for chronic venous disorders [16]. The examination revealed objective signs of chronic venous diseases - telangiectasia and reticular varicosity (C1), varicose veins (C2), venous edema (C3), hyperpigmentation and lipodermatosclerosis (C4), open or healed trophic ulcers (C5–C6).
The severity of clinical manifestations of venous disease was determined using a validated Venous Clinical Severity Score (VCSS) scale, which presented 10 objective and subjective indicators of varicose disease with corresponding scoring [20]. A 10-point visual analogue scale was used to determine the intensity of the pain syndrome at the time intervals indicated above. The value of this scale at the level "0" corresponded to the complete absence of pain, and at the level "10" – to the maximum intensity of pain. A score of 1–3 was considered mild pain, 4–6 was considered to be moderate, and 7-10 was considered to be an intense pain response. The cumulative QOL score was determined using the Chronic Venous Insufficiency Questionnaire (CIVIQ-20) using a modified formula: (S-20)×1.25, where the S value corresponded to the sum of the scores obtained by answering each of the 20 questions [21]. At the same time, 1 point indicated the absence of symptoms or subjective sensations, while 5 points corresponded to their maximum expression.
The circumference of the tibia was measured at room temperature (~23°C) with a measuring tape at the same time (between 13:00 and 15:00) in orthostasis at two points: 3 cm above the medial ankle (A) and 10 cm below the tibial tuberosity (B).
Ultrasonography (USG) was performed using Mindray M7 color duplex ultrasound machines (Shenzhen Mindray Bio-Medical Electronics Co. Ltd., PRC) and Samsung Medison SonoAce R7 (Samsung Medison Co. Ltd., DPRK) in different modes with a 5-10 MHz linear transducer. The imaging protocol involved dynamic USG of the deep and superficial systems of 120 lower extremities in 60 pregnant women with assessment of great and small saphenous veins (GSV/SSV) diameters at 7 predetermined points. The GSV mapping was carried out at five points: 5 cm below the saphenofemoral junction (A), in the middle (B) and lower (C) thigh, and in the upper (D) and middle (E) thirds of the tibia. A small saphenous vein was mapped at two points: 2 cm below the sapheno-popliteal artery (F) and in the middle third (G) of the tibia. Valve status was determined using the Valsalva technique and compression test [22]. Reflux was considered pathological when its duration was more than 0.5 s for superficial veins.
The final phase of the study (after wearing compression stockings for 2 months) included verbal surveys of the study group patients to explain possible problems and adverse events that might have resulted from the use of compression stockings.
The primary endpoints were pain intensity, severity of clinical manifestations of varicose veins, and QOL. Secondary end points were the 7-point diameters of the GSV and SSV of both lower limbs by USG, the tibial circumference, changes in the pathological venous reflux and the CEAP clinical classes of varicose disease before and after the study.
Statistical analysis
Quantitative measures
The distribution of continuous variables was tested for normality using the Shapiro-Wilk test. Parametric and non-parametric methods of descriptive and analytical statistics were used depending on the correspondence/discrepancy between the distribution types of the analyzed characteristics and the normal distribution law. Quantitative variables that showed normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, medians (Me) with interquartile range (Q1; Q3) were reported. Variables that did not meet normality assumptions were compared with a nonparametric Wilcoxon–Mann–Whitney test. Normally distributed continuous variables were compared between two groups with a Student’s and Welch-t test (unequal variance t-test). The Wilcoxon test and Student’s t-test for paired samples were used to compare before-after or matched subjects.
In the QOL study, differences between the initial value and the final value were calculated, which were considered the effect size. The effect variable was processed for each group and compared between groups. Location differences (Hodges-Lehman median difference score) were also assessed, with construction of a 95% confidence interval (CI). To interpret the size of the shear effect, a rank-biserial correlation (rpb) score was calculated, which was interpreted as 0.10 to <0.3 (small effect) 0.30 to <0.5 (medium effect), and ≥0.5 (large effect) [23, 24].
When investigating the effect of vein diameter change, the difference between the initial and final diameter in each subject was calculated. Thus, a positive value indicated a decrease in diameter at the end of the study compared to the initial value. The overall effect was assessed using analysis of variance with repeated measurements, taking into account patient variability, measurement point, and side of measurement. Effect size differences were estimated based on marginal means and standardized Cohen's d.
Interpretation of the effect size estimate of differences was performed using threshold values defined: |d| <0.2 – "insignificant", |d|<0.5 – "small", |d|<0.8 – "medium", otherwise "large", where | | is the absolute value, module of the value [24].
The circumference of the patients' tibia was examined separately by 2 points. The values of the points on the left and right were averaged, since there were no differences between the right and left positions of the measurement point. The difference between the initial and final averaged values in each subject was calculated when examining the effect of changing the circumference of the tibia. Therefore, a positive value indicated a decrease in the average diameter at the end of the study compared to the initial value. The intergroup effect for each point was assessed using the Wilcoxon–Mann–Whitney test, and the difference in location parameters (Hodges–Lehman median difference score) was assessed by construction of a 95% CI. A rank-biserial correlation (rpb) score was calculated to interpret the effect size of the shift, which was interpreted as described above.
Qualitative measures.
Categorical variables were reported by counts and percentages as n (%). The chi-square test (χ2) was used for 2×2 contingency tables; the Fisher–Freeman–Halton exact test was used if the assumptions underlying the χ2 test were violated.
For reflux studies, the McNimar test was used to compare to compare before-after subjects in each group. The effect size of the changes in each group was calculated taking into account repeated measures of the binominal variable. Differences between groups between effects were calculated as the difference in the proportions of effects between groups with 95% CI constructed.
All calculations were performed using the statistical package R, version 4.2. [R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/]. The first-order error level α was assumed to be 0.05 for statistical inference.
Results
Baseline demographic and clinical characteristics of the patients are shown in Table 1. There were no statistically significant differences in mean age, body weight, height, and BMI, as well as gestational age, number of previous pregnancies, births, and duration of varicose disease in the compared cohorts. Family history (predominantly maternal) was present in 20/30 (66%) and 28/30 (93%) pregnant women from the treatment and control groups, respectively. Occupational activities involving heavy physical labor or prolonged (>5 h per day) work in an orthostatic position were present in 20/30 (66%) and 21/30 (70%) women in the study and control groups, respectively. The duration of follow-up to the endpoint in the study and control groups was 63 [61; 63] and 63 [56; 65] days, respectively (p=0.892).
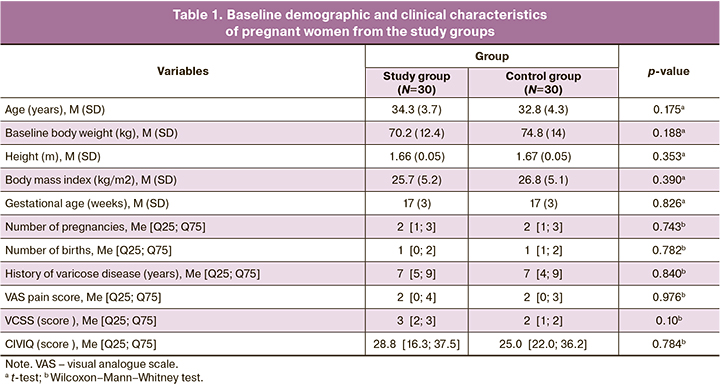
At baseline, the intensity of pain in both groups was comparable and had no differences (Table 1). At the final visit, the tendency was positive with a decrease in pain level in the compression group, while the control group showed a slight moderate increase (Table 2). The effect size of the differences between the baseline and endpoints for the main group was 0 [0; 1.75] (p=0.082), for the control group – -1.5 [-3.0; 0] (p<0.001). The estimate of the median difference in effects was 2 (95% CI 1–3) points, which we interpreted as a large effect (p<0.001).
Before the study, the cumulative VCSS venous disease in the treatment and control groups was 3 [2; 3] and 4 [2; 5], respectively (p>0.05; Table 1). At the final follow-up in the study group, there was a significant decrease in the VCSS score (p<0,001; Table 2), while in the control group there was an increase in the severity of varicose disease severity (p<0,001); the effect of differences was 3 (95% CI 2–4) points and was interpreted as high (p<0,001). At final follow-up, the cumulative QOL score on the CIVIQ-20 questionnaire in the study group showed a trend toward improvement (p=0.099; Table 2), whereas, the control group showed a statistically significant decrease in QOL (p<0.001, paired Wilcoxon test). Differences in effects were conclusively estimated at 10 (95% CI 5–16) points.
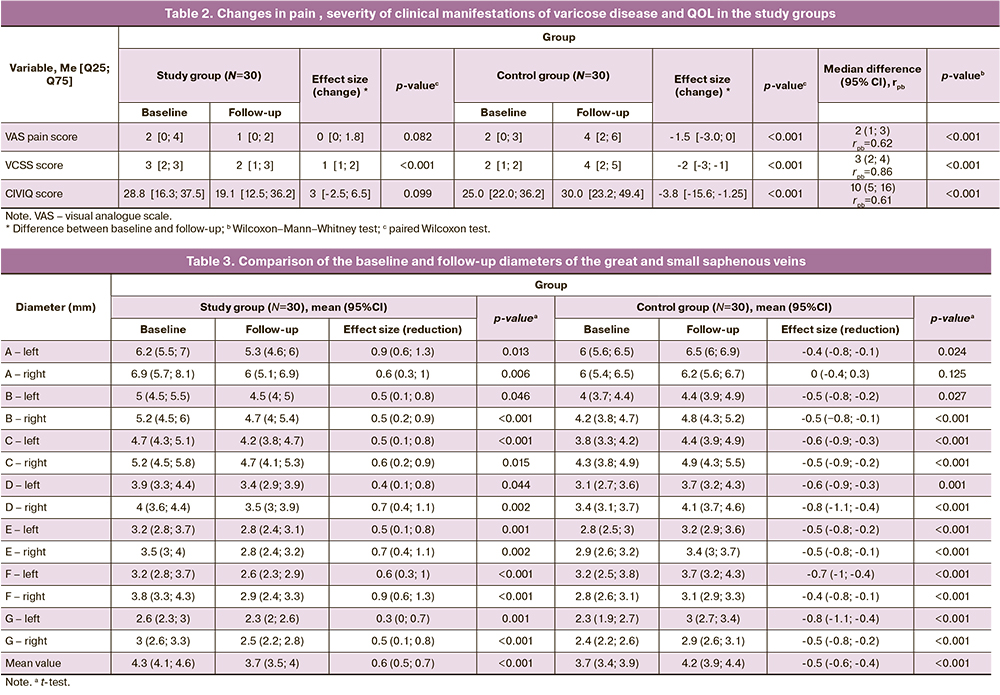
According to the baseline clinical grade, the majority (>80%) of patients in both groups were classified as C1 and C2. The distribution of pregnant women according to CEAP classification before and after the study is shown in Table 3. A decrease or maintenance of the CEAP grade was seen in 90% of the study population, while the control group showed the same trend in only 47% of cases, with a difference in therapeutic efficacy between the compared groups of 43 (95% CI 19–67)%.
At baseline, the number of pregnant women in the study and control groups with reflux duration >0.5 s in the GSV/SSV was 28/30 (93%) and 14/30 (47%), respectively. The number of pregnant women with pathological reflux in the treatment group halved (p<0.001) after the study ended, while the control group showed an increase to 20/30 (67%) with pathological reflux (p=0.077). Thus, as a result of the negative trend of the index in the control group and the improvement of venous hemodynamics in the main group, the effect size of the differences was 67 (95% CI 42–91)%.
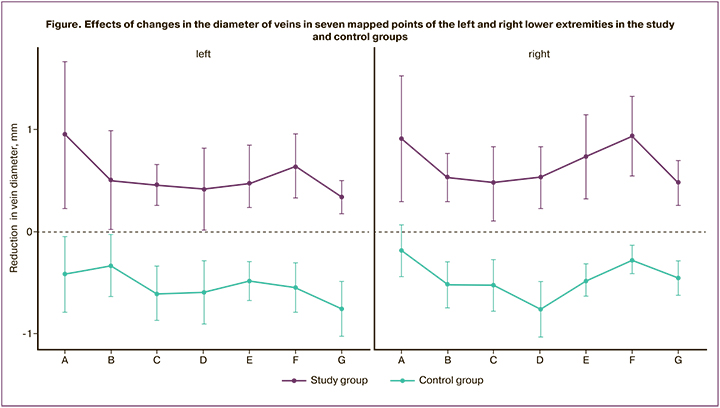
At the beginning of the study, the mean vein diameter was 0.7 (95% CI 0.3–1.0) mm smaller in the control group than in the study group (p<0.001; Table 3). After 2 months, there was a change in vein diameter at almost all measurement points: the control group showed a statistically significant 0.5 mm increase in mean diameter of GSV/SSV, while the study group showed a 0.6 mm reduction in the same parameter compared to baseline values (p<0.001). In summary, the overall effect size of Cohen's d was 4.58, with differences reaching 1.1 (95% CI 0.9–1.2) mm in absolute values (p<0.001). The figure illustrates the effects of changes in vein diameter at seven USG-mapped points of the left and right lower extremities in the study and control groups.
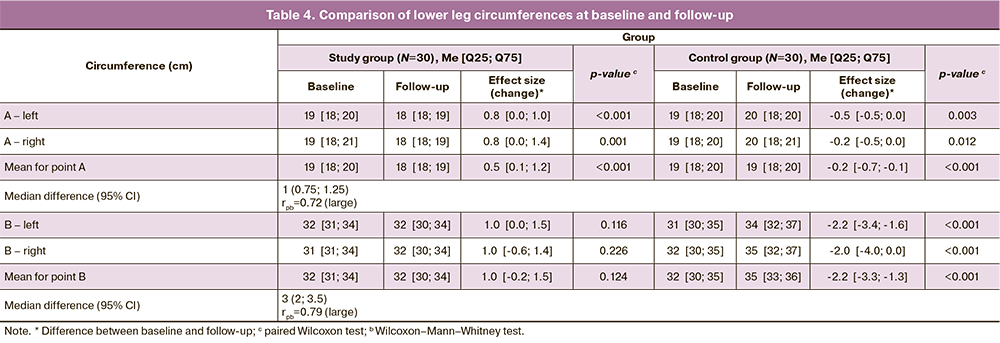
At the beginning of the study, right and left tibial circumferences in both measurement points and their mean values were not statistically significant different in the study groups (Table 4). Women wearing compression stockings showed a bilateral decrease of the ankle circumference by 0.5 cm (p<0.001), compared with the baseline, whereas the control group showed a statistically significant increase of 0.2 and 2.2 cm in the A and B points, respectively (p<0.001). The median difference between groups in effect size for points A and B was large and was 1 (0.75; 1.25) and 3 (2; 3.5) cm, respectively.
Most of the interviewed women did not have any difficulties in using stockings (63.3%) and did not need outside help when putting them on (80%), while 27/30 (90%) of the respondents noted an acceptable or significant reduction in the severity of varicose disease symptoms.
Discussion
This quasiexperimental comparative study aimed to determine the effectiveness of graded elastic compression in pregnant women with C1–C3 varicose veins. Its distinctive feature was the combination of clinical, anthropometric, and imaging techniques for the dynamic assessment of venous insufficiency in the lower extremities. Repeated USG showed a larger diameter of the GSV and MBV in the group of patients not wearing compression stockings compared to the study group. Similarly, studies by other authors reported a bilateral increase of the diameter of the BMV during pregnancy, with only a partial reduction of the varices postpartum [25, 26].
On the other hand, against the background of compression therapy, the diameter of veins decreased throughout the entire length of the GSV/MSV, which was accompanied by the relief of valvular dysfunction and pathological venous reflux in 14/30 (47%) cases. As the analysis of USG data and histological analysis showed, dilatation of the saphenous veins occurred mainly distal to the established valves and often preceded the occurrence of their insufficiency [27]. Such a sequence of lower limb vein remodeling during pregnancy is inconsistent with the current ideas about the pathogenesis of varicose disease and indicates a multifactorial etiology of gestational varicosity.
Based on the assessment of visible changes in the lower extremities, the majority of pregnant women were diagnosed with class C1 and C2 varicose disease at the beginning of the study. However, in the final stage of the study, the control group showed negative dynamics in more than half of the cases, with varicose disease progressing to class C3–C4. On the contrary, such changes were absent in the main group – the initial varicose disease status either regressed or remained the same in 27/30 (90%) patients.
Pain is known to be one of the common symptoms of varicose veins of the lower limbs. In the group of pregnant women who did not use elastic compression, a significant increase in pain syndrome was observed with increasing gestational age, while in the study group the intensity of pain response on the visual analogue scale decreased. As a result, there was a large effect of differences between the effects of the groups according to the specified indicator.
Edema of the lower extremities is observed in 70–80% of pregnant women (more often in the last trimester); and it was not related to manifestations of gestational hypertension or preeclampsia [8]. In this study, compression treatment was associated with a reduction of venous edema (0.5 cm at the ankles), whereas the control group showed a significant increase of the circumference of the lower leg. The effectiveness of graded elastic compression was additionally confirmed by a significant decrease of the VCSS. In contrast, an increase in the severity of varicose vein syndrome (VCSS) was observed in the absence of compression, probably due to a simultaneous decrease in quality of life. It should be noted that the difference in QOL after the end of the study was significant, both due to a decrease in QOL in the control group and an increase in QOL in the study group.
Weekly telephone follow-up seems to have increased women's adherence to regularly wearing medical stockings. Furthermore, the positive response of most women to the effective management of symptoms and signs of varicose disease was an additional argument for their systematic use as a preventive and curative measure. This finding is in agreement with that of Allegra et al. who confirmed the importance of a standard compression regimen to relieve symptoms of varicose veins and improve QOL during pregnancy [28].
The etiopathogenetic factors of varicose veins during pregnancy is still a subject of debate. However, according to the majority of specialists, one of the important triggers of degenerative dystrophic changes of the vein wall is hormonal rearrangement during pregnancy [29]. At the same time, compression therapy is aimed at improving venous outflow, which is impaired by hormonal imbalance and vein compression by the pregnant uterus. The choice of the degree of compression is therefore essential to achieve the desired clinical effect, in particular a reduction of the volume of the lower extremities. In this study, stockings of class 2 (23–32 mmHg) graded according to RAL-GZ387 were prescribed, which resulted in a reduced number of patients with reflux and edema (C3 clinical grade) in the study group. Thaler et al. demonstrated similar results in pregnant women with gestational varicose disease and reflux at the sapheno-femoral junction [30]. Another randomized controlled trial based on computed tomography data showed a 70% reduction in GSV diameter in the supine position with elastic compression at 36 mmHg. [31]. The reduction in GSV and SSV diameters in this study confirmed the efficacy of the approved treatment protocol for pregnant women with varicose disease. The graded pressure (maximal pressure at the ankles) generated by the stockings ensured the venous blood return to the heart closest to the physiological one [13]. One of the hypotheses explaining the decrease in the diameter of the vein above the area of compression is the acceleration of blood flow and the relief of venostasis in the proximal venous segment.
However, our study has some limitations regarding patient selection, small sample size, relatively short follow-up, and the absence of a predetermined manufacturer and model of compression stockings. On the other hand, the compared groups had a balanced number of pregnancies and births, while the majority of the studies of this type reported only primiparous women.
The reliability of the study results was improved by bilateral measurement of the corresponding lower extremity parameters. As follows from Tables 5 and 6, the mean values of baseline and final GSV/SSV diameters and tibial circumferences were not absolutely identical for the right and left lower extremities. Bilateral USG and anthropometric evaluation of venous hemodynamics and edema seems warranted.
Conclusion
The use of compression stockings in pregnant women with varicose disease in the second and third trimesters was associated with a decrease in saphenous vein diameter, relief of vein-specific symptoms and signs, and an improvement in quality of life. Dynamic assessment of clinical classes of CEAP indicated a slight improvement in the study group and a significant progression of venous insufficiency in the control group. This comparative clinical study demonstrated good tolerability and efficacy of graduated elastic compression in pregnant women with varicose disease.
References
- Yetkin E., Ileri M. Dilating venous disease: pathophysiology and a systematic aspect to different vascular territories. Med. Hypotheses. 2016; 91: 73-6.https://dx.doi.org/10.1016/j.mehy.2016.04.016.
- Янушко В.А., Турлюк Д.В., Климчук И.П., Красько О.В., Шестак Н.Г. Эпидемиология хронических заболеваний вен в Республике Беларусь. Медицинские новости. 2016; 6: 78-82. [Yanushko V.A., Turlyuk D.V., Klimchuk I.P., Kras'ko O.V., Shestak N.G. Epidemiology of chronic venous disease in the Republic of Belarus. Medicinskie novosti/Medical News. 2016; 6:78-82. (in Russian)].
- Bihari I., Tornoci L., Bihari P. Epidemiological study on varicose veins in Budapest. Phlebology. 2012; 27(2): 77-81. https://dx.doi.org/10.1258/phleb.2011.010063.
- Beebe-Dimmer J.L., Pfeifer J.R., Engle J.S., Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005; 15(3): 175-84. https://dx.doi.org/10.1016/j.annepidem.2004.05.015.
- Sahoo M.R., Misra L., Deshpande S., Mohanty S.K., Mohanty S.K. Subfascial endoscopic perforator surgery: a safe and novel minimal invasive procedure in treating varicose veins in 2nd trimester of pregnancy for below knee perforator incompetence. J. Minim. Access Surg. 2018; 14(3): 208-12.https://dx.doi.org/10.4103/jmas.JMAS_107_17.
- Ismail L., Normahani P., Standfield N.J., Jaffer U. A systematic review and meta-analysis of the risk for development of varicose veins in women with a history of pregnancy. J. Vasc. Surg. Venous Lymphat. Disord. 2016; 4(4): 518-24.https://dx.doi.org/10.1016/j.jvsv.2016.06.003.
- Asbeutah A.M., Al-Azemi M., Al-Sarhan S., Almajran A., Asfar S.K. Changes in the diameter and valve closure time of leg veins in primigravida women during pregnancy. J. Vasc. Surg. Venous Lymphat. Disord. 2015; 3(2): 147-53.https://dx.doi.org/10.1016/j.jvsv.2014.09.005.
- Хрыщанович В.Я., Скобелева Н.Я. Медицинская профилактика и лечение беременных с варикозной болезнью. Российский вестник акушера-гинеколога. 2021; 21(4): 27-34. [Khryshchanovich V.Ya., Skobeleva N.Ya. Medical prevention and management of varicose vein disease during pregnancy. Russian Bulletin of Obstetrician-Gynecologist. 2021; 21(4): 27-34. (in Russian)].https://dx.doi.org/10.17116/rosakush20212104127.
- Ropacka-Lesiak M., Kasperczak J., Breborowicz G.H. Risk factors for the development of venous insufficiency of the lower limbs during pregnancy – part 1. Ginekol. Pol. 2012; 83(12): 939-42.
- Barros-Junior N., Perez M.D.C.J., Amorim J.E., Miranda F. Pregnancy and lower limb varicose veins: prevalence and risk factors. J. Vasc. Bras. 2010; 9(2): 29-35.
- Heller J.A., Evans N.S. Varicose veins. Vasc. Med. 2015; 20(1): 88-90. https://dx.doi.org/10.1177/1358863X14566224.
- Nicolaides A., Kakkos S., Baekgaard N., Comerota A., de Maeseneer M., Eklof B. et al. Management of chronic venous disorders of the lower limbs. Guidelines According to Scientific Evidence. Part I. Int. Angiol. 2018; 37(3): 181-254. https://dx.doi.org/10.23736/S0392-9590.18.03999-8.
- Smyth R.M., Aflaifel N., Bamigboye A.A. Interventions for varicose veins and leg oedema in pregnancy. Cochrane Database Syst. Rev. 2015; 2015(10): CD001066. https://dx.doi.org/10.1002/14651858.CD001066.pub3.
- Ochalek K., Pacyga K., Curyło M., Frydrych-Szymonik A., Szygula Z. Risk factors related to lower limb edema, compression, and physical activity during pregnancy: a retrospective study. Lymphat. Res. Biol. 2017; 15(2): 166-71. https://dx.doi.org/10.1089/lrb.2016.0038.
- Saliba O.A.J., Rollo H.A., Saliba O., Sobreira M.L. Compression stocking prevents increased venous retrograde flow time in the lower limbs of pregnant women. Phlebology. 2020; 35(10): 784-91.https://dx.doi.org/10.1177/0268355520939371.
- Eklof B., Rutherford R.B., Bergan J.J., Carpentier P.H., Gloviczki P., Kistner R.L. et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J. Vasc. Surg. 2004; 40(6): 1248-52.https://dx.doi.org/10.1016/j.jvs.2004.09.027.
- ACOG Committee Opinion No. 650. Physical activity and exercise during pregnancy and the postpartum period. Obstet. Gynecol. 2015; 126: e135-42.
- Tsakiridis I., Bakaloudi D.R., Oikonomidou A.C., Dagklis T., Chourdakis M. Exercise during pregnancy: a comparative review of guidelines. J. Perinat. Med. 2020; 48(6): 519-25. https://dx.doi.org/10.1515/jpm-2019-0419.
- Rodríguez-Blanque R., Aguilar-Cordero M.J., Marín-Jiménez A.E., Menor-Rodríguez M.J., Montiel-Troya M., Sánchez-García J.C. Water exercise and quality of life in pregnancy: a randomised clinical trial. Int. J. Environ. Res. Public Health. 2020; 17(4): 1288. https://dx.doi.org/10.3390/ijerph17041288.
- Passman M.A., McLafferty R.B., Lentz M.F., Nagre S.B., Iafrati M.D., Bohannon W.T. et al. Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum, National Venous Screening Program. J. Vasc. Surg. 2011; 54(6, Suppl.): 2S-9S. https://dx.doi.org/10.1016/j.jvs.2011.05.117.
- Launois R. A quality of life tool kit in chronic venous disorders. Phlebolymphology. 2014; 21(3): 152-60.
- Coleridge-Smith P., Labropoulos N., Partsch H., Myers K., Nicolaides A., Cavezzi A. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs--UIP consensus document. Part I. Basic principles. Eur. J. Vasc. Endovasc. Surg. 2006; 31(1): 83-92. https://dx.doi.org/10.1016/j.ejvs.2005.07.019.
- Ellis P. The essential guide to effect sizes. Statistical power, meta-analysis, and the interpretation of research results. Cambridge University Press; 2010.https://dx.doi.org/10.1017/CBO9780511761676.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Routledge; 1988. https://dx.doi.org/10.4324/9780203771587.
- Gardenghi L.A., Dezotti N.R.A., Dalio M.B., Martins W.P., Joviliano E.E., Piccinato C.E. Lower limb venous diameters and haemodynamics during pregnancy and postpartum period in healthy primigravidae. Phlebology. 2017; 32(10): 670-8. https://dx.doi.org/10.1177/0268355516671586.
- Pemble L. Reversibility of pregnancy-induced changes in the superficial veins of the lower extremities. Phlebology. 2007; 22(2): 60-4.https://dx.doi.org/10.1258/026835507780346196.
- Lim C.S., Davies A.H. Pathogenesis of primary varicose veins. Br. J. Surg. 2009; 96(11): 1231-42. https://dx.doi.org/10.1002/bjs.6798.
- Allegra C., Antignani P.L., Will K., Allaert F. Acceptance, compliance and effects of compression stockings on venous functional symptoms and quality of life of Italian pregnant women. Int. Angiol. 2014; 33(4):357-64.
- Lenković M., Cabrijan L., Gruber F., Batinac T., Manestar-Blazić T., Stanić Zgombić Z. et al. Effect of progesterone and pregnancy on the development of varicose veins. Acta Dermatovenerol Croat. 2009; 17(4): 263-7.
- Thaler E., Huch R., Huch A., Zimmermann R. Compression stockings prophylaxis of emergent varicose veins in pregnancy: a prospective randomised controlled study. Swiss. Med. Wkly. 2001; 131(45-46): 659-62.
- Uhl J.F. 3D multislice CT to demonstrate the effects of compression therapy. Int. Angiol. 2010; 29(5): 411-5.
Received 11.05.2022
Accepted 20.06.2022
About the Authors
Vladimir Ya. Khryshchanovich, Dr. Med. Sci., Professor, Professor at the Department of General Surgery, Belarussian State Medical University, +375 29 624 55 78, vladimirkh77@mail.ru, https://orcid.org/0000-0001-5353-205X, 83 Dzerzhinsky Ave., Minsk 220116, Republic of Belarus.Nicolay A. Rogovoy, PhD, Associate Professor at the Department of General Surgery, Belarussian State Medical University, +375 29 385 27 53, kolia_med@mail.ru,
83 Dzerzhinsky Ave., Minsk 220116, Republic of Belarus.
Natalia Ya. Skobeleva, MD, Postgraduate Student, Department of Obstetrics and Gynecology, Belarussian State Medical University; Obstetrician-Gynecologist,
Head of the Obstetric Department of Pregnancy Pathology, Clinical Maternity Hospital of Minsk Region, +375 29 309 67 05, nataliaskobeleva@yandex.by,
https://orcid.org/0000-0003-2267-579X, 16 Frantsisk Skorina str., Minsk 220114, Republic of Belarus.
Olga V. Krasko, PhD, Associate Professor, Senior Researcher at the Bioinformatics Laboratory, Joint Institute for Informatics Problems of the National Academy of Sciences
of Belarus, +375 29 707 88 03, krasko@newman.bas-net.by, https://orcid.org/0000-0002-4150-282X, 6 Surganova str., Minsk 220012, Republic of Belarus.
Corresponding author: Vladimir Ya. Khryshchanovich, vladimirkh77@mail.ru
Authors' contributions: Khryshchanovich V.Ya. – conception and design of the study, manuscript editing; Rogovoy N.A. – data collection and analysis; Krasko O.V. – statistical analysis; Skobeleva N.Ya. – data collection, manuscript drafting.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the N.E. Savchenko City Clinical Hospital No. 4 of Minsk (protocol No. 14 dated December 24, 2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Khryshchanovich V.Ya., Rogovoy N.A., Skobeleva N.Ya., Krasko O.V.
Efficiency of graduated compression for varicose veins during pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 7: 50-59 (in Russian)
https://dx.doi.org/10.18565/aig.2022.7.50-59



