Differentiated approach to the embryological stage in frozenthawed embryo transfer
Objective. To evaluate the effectiveness of additional embryological techniques in frozen-thawed embryo transfer programs.Petrosyan Ya.A., Syrkasheva A.G., Romanov A.Yu., Makarova N.P., Kalinina E.A.
Materials and methods. The study included 288 couples treated for infertility using assisted reproductive technologies (ART) with frozen-thawed embryo transfer. The couples were stratified into two groups depending on the onset of pregnancy: group 1 consisted of couples who achieved pregnancy (pregnancy +, n=92), group 2 included the couples who did not achieve pregnancy (pregnancy -, n=196). The influence of the embryological stage on the effectiveness of ART programs was evaluated.
Results. The use of pronase hatching increases the ART effectiveness in patients under 33 years by 3.3 times and the use of pronase hatching reduces the pregnancy rate in patients aged 34 years and older, while laser hatching increases the pregnancy rate by 4.8 times. The use of a culture medium enriched with hyaluronic acid (Embryoglue) increases the pregnancy rate in patients aged 38 years and older and in patients with previous history of three or more ineffective embryo transfer cycles. Prolonged embryo cultivation after thawing and before embryo transfer to the uterine cavity does not influence the effectiveness of frozen-thawed embryo transfer.
Conclusion. None of the additional embryological techniques has shown its effectiveness in its regular use. Further research should be aimed at identifying groups of patients who need to be recommended certain modern embryological techniques, as well as their combinations.
Keywords
The number of embryological techniques used in the assisted reproductive technologies (ART) programs is steadily increasing [1–10], however, embryo cryopreservation remains the most studied and widely implemented in clinical practice [11–14]. At present, cryopreservation is a widespread, safe, cost-effective method of increasing the cumulative pregnancy rate [15, 16]. In addition, the successful cryopreservation of embryos makes it possible the elective single embryo transfer in order to reduce the risks associated with the development of multiple pregnancy [17, 18].
Despite this, today there is no unified tactics for carrying out thawed embryo transfer (ET). The main criterion for the efficacy of cryopreservation and embryo thawing is morphological intactness of embryos and their ability for cleavage. However, the necessary morphological parameters to solve the problem associated with embryo vitrification or thawed ET to the uterine cavity are not clearly defined. The data on the optimal time interval between embryo thawing and transfer to the uterine cavity are also controversial. [19]. The indications for the use of different assisted hatching (AH) and other embryological techniques in cryo-thawed embryo transfer programs are not clearly defined [20–26].
Purpose of the study: to assess the efficacy of advanced embryological techniques in cryo-thawed embryo transfer programs.
Мaterials and methods
The prospective study included 288 married couples, who for infertility treatment to Prof. B.V. Leonov Department of Assisted Technologies in Infertility Treatment of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation (E.A. Kalinina – Head of the Department) in the period from 2017 to 2019. They had no contraindication for ART and signed the informed consent to participate in the study. The inclusion criteria were: normal karyotype in both marital partners, the absence of pronounced pathozoospermia (100% teratozoospermia, absolute asthenozoospermia, all types of azoosperima), the presence of vitrified embryos. The exclusion criteria were: the use of donor gametes or surrogacy, as well as cancellation of thawed ET in this cycle for any reason.
All married couples participating in the study were examined according to the Order № 107n dated 30.08.2012 of the Ministry of Health of Russia «About the use of assisted reproductive technologies; contraindications and limitations on the use».
Oocytes insemination was carried out using classical in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), physiological intracytoplasmic sperm injection (PICSI). Commercial culture media were used for embryo vitrification/thawing. Embryo thawing was performed 2–3 hours before embryo transfer (short-time cultivation) or in the evening before the day of embryo transfer (10–12 hours before embryo transfer, prolonged cultivation). The embryologist used light microscopy to assess the embryo quality according to the Gardner classification system [4]. Assisted hatching was performed using laser micromanipulation or total removal of the zona pellucida (pronase hatching). In some of the cycles, embryo thawed ET transfer was performed with media containing hyaluronic acid (HA).
Endometrial preparation for cryo-thawed embryo transfer was carried out using cyclic hormonal therapy (estrogens + gestagens) or in a spontaneous ovulatory menstrual cycle. Monitoring of the state of the endometrium and folliculogenesis was implemented by dynamic ultrasound. Embryo transfer was on day 7 after the peak of endogenous LH in a spontaneous cycle or on day 5–6 of taking progesterone preparations in the cyclical hormone therapy.
Management of patients in the period following embryo transfer was conducted in accordance with the protocols in clinical practice. 14 days after embryo transfer, the concentration of chorionic gonadotropin be β-subunit in the patient's serum was determined. 5 weeks after embryo transfer, the embryo's heartbeat was visualized, and the clinical pregnancy was stated. Then the patients were stratified into groups and the pregnancy rate was determined.
Statistical analysis
The statistical software package Statistica 10 (USA) was used for analysis. The Shapiro–Wilk test was used for the normal distribution. Normally distributed data were presented as the mean (standard deviation).
The impact of different embryological techniques on clinical pregnancy rates was determined. For evaluation of data collection the operator protocol was not taken into account. Also, the manufacturer of preparation was not selected and the clinical parameters of the ovarian stimulation cycle were not stratified.
The statistical analysis was performed using χ2-test to compare the categorical variables. The measure of association for binary comparisons was odds ratio (OR) with a 95% confidence interval (95% CI). The differences between the statistical values were considered statistically significant at the level р<0,05. The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of the Healthcare of the Russian Federation.
Results
The study included 288 women, who underwent cryo-thawed embryo transfer in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of the Russian Federation in the period January, 2017–March, 2019. The mean age of patients was 33,5 (4,6) years. The clinical pregnancy rate was 31/9% (n=92).
The routine use of different AH techniques, HA-enriched culture medium, as well as variation in the timing of embryo cultivation after thawing had no impact on pregnancy rates. The obtained data are presented in Tables 1,2.
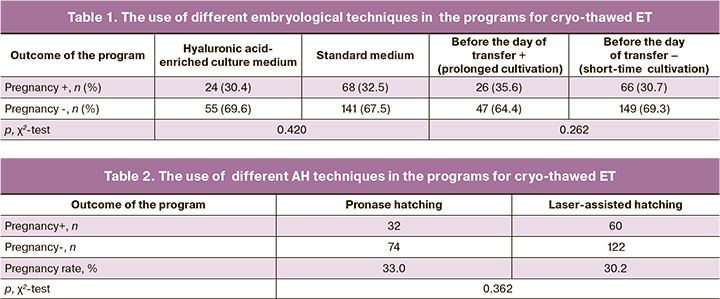
Further data analysis included the search for the subgroups of patients, in whom these techniques increase pregnancy rates. The following indicators were analyzed:
- Age of patients;
- Body mass index (BMI), presence or absence of obesity;
- ART failures in anamnesis;
The presence and the type of pathozoospermia, the method of oocyte insemination.
The first step involved assessment of the efficacy of different AH techniques in patients in different age groups. Initially, AH was assessed in patients of different age groups. It was found that total removal of the zona pellucida was more effective in younger patients, while, on the contrary, partial dissection of the zona pellucida was more effective in older patients. ROC analysis found that the threshold level of pronase hatching efficacy was 33 years. Pregnancy rate due to the use of AH in patients in different age groups is presented in Table 3.
Thus, in the subgroup of patients under 33 years of age, total removal of the zona pellucida increased the likelihood of conceiving by 3.3 times, and in the subgroup of patients aged 34 years and older, partial dissection of the zona pellucida increased the likelihood of conceiving by 4.8 times.
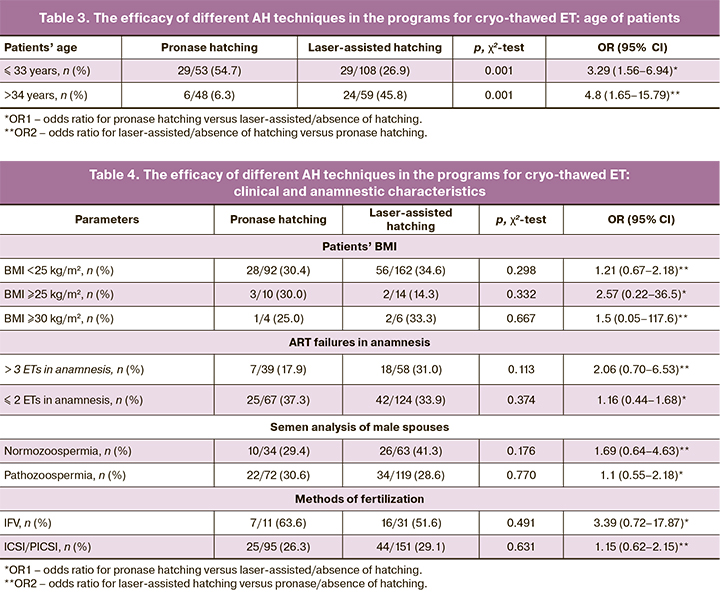
Next, the efficacy of different AH techniques depending on the patients’ BMI, ART failures in anamnesis, the method of oocyte insemination was assessed (Table 4).
In patients stratified into groups depending on BMI, infertility treatment in history, the presence or absence of pathozoospermia, as well as the method of fertilization, there were no significant differences in the efficacy of different AH techniques (Table 4).
The second step was the analysis of the efficacy of HA-enriched culture medium in the ART programs for cryo-thawed ET. The use of HA-enriched culture medium did not increase the efficacy of ART treatment in patients of early and late reproductive age, as well as in patients with normal BMI and in overweight patients (Table 5). However, in the subgroup of patients, who had 3 failed embryo transfers in anamnesis, the use of HA-enriched medium increased the pregnancy rate: 47.1% compared to 11.4%. OR of conception due to the use of HA-enriched medium in the presence of 3 and more ET in anamnesis was 6.89 (95% CI 1.62; 30.47) (Table 5).
The method of oocyte insemination and the presence of pathozoospermia in male spouses had no impact on the efficacy of HA-enriched medium (Table 6).
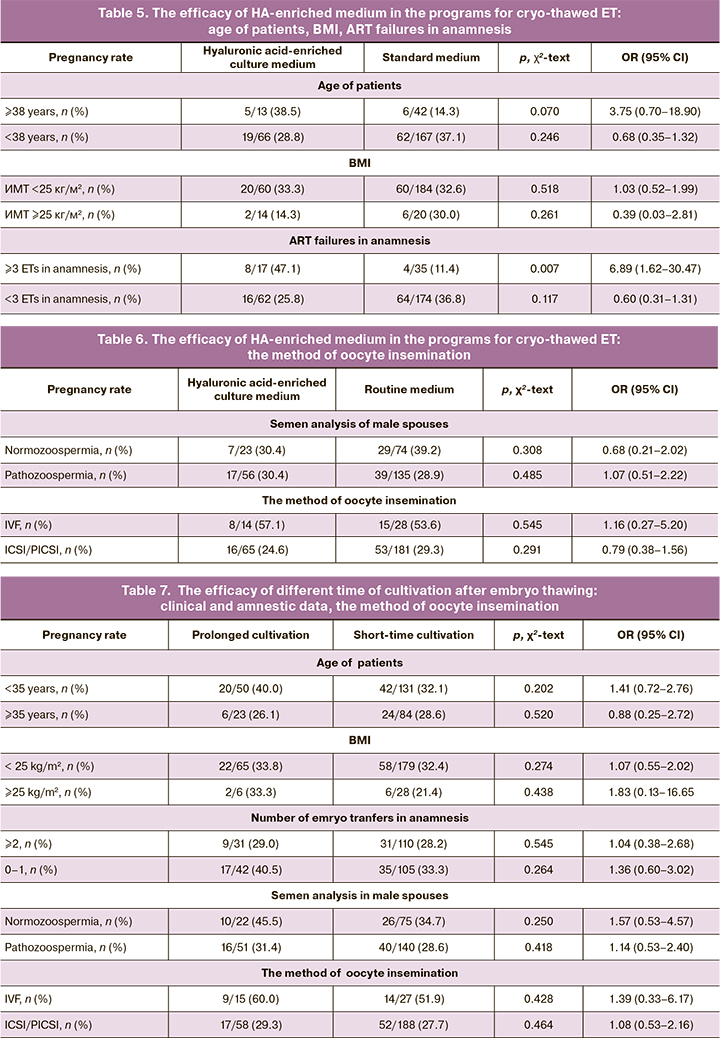
The third step was the analysis of the effectiveness of different time of cultivation after embryo thawing and before the transfer: embryo thawing 10–12 hours before embryo transfer (prolonged cultivation) and embryo thawing 3–4 hours before embryo transfer (short-time cultivation) (table 7).
The rate of pregnancy due to the use of different methods of embryo cultivation after thawing did not differ in general, as well as in different subgroups of patients. Similar data were obtained when analyzing the effectiveness of different methods of embryo cultivation, depending on the quality of semen sample used at the stage of fertilization and the method of fertilization. (Table 7).
Discussion
According to the obtained data, the use of different AH techniques, HA-enriched culture medium, as well as the change in duration of embryo cultivation after thawing had no effect on pregnancy rate. Thus, none of the studied embryological techniques should be routinely used for all groups of patients or indicated upon patients request, and this is consistent with the most published research data [7–10].
Further analysis included the search for the subgroups of patients, in whom these methods increase pregnancy rates. According to previously obtained data, the efficacy of pronaze and laser-assisted hatching may differ [24, 25]. In view of the above, the effecacy of different AH techniques in different subgroups of patients was analyzed.
In the group of patients under 33 years old (threshold age, inherent data), the use of pronase hatching increased the efficacy of ART and was more expedient than laser hatching. On the contrary, in patients aged 34 years, the use of pronase hatching reduced the pregnancy rate, and laser-assisted hatching was more expedient, since it increased the pregnancy rate by 4.8 times. With age, the quality of gametes and embryos decreases, and the degree and severity of genetic abnormalities and other embryonic malformations increases [27–30]. Thus, if AH (especially pronase hatching) is completely safe for a blastocyst of excellent quality, then being performed on a blastocyst with reduced compensatory capabilities, it may lead to a decrease in its implantation potential. The fundamental difference between pronaze hatching and laser-assisted hatching with partial dissection of the zona pellucida is the complete removal of the zona pellucida, which may be a factor explaining the distinction between these techniques in different groups of patients. Perhaps, the quality of intercellular contacts between individual blastomeres changes in embryos of poor quality. This may be the cause of the reduced pronase hatching efficacy.
The use of HA-enriched culture medium (EmbryoGlue) increased the pregnancy rate in a subgroup of patients aged 38 years and older, as well as in the presence of 3 or more ineffective embryo transfer cycles in the anamnesis, which corresponded to the published data [26].
Conclusion
None of the additional embryological techniques had been shown to be effective for routine use. Moreover, the use of such techniques in the absence of indications can lead to a decrease in the efficacy of ART. Further research should be aimed at identifying the groups of patients for whom certain modern embryological techniques, as well as their combinations, should be unambiguously recommended.
References
- Сыркашева А.Г., Казакова В.В., Долгушина Н.В., Романов А.Ю., Андреева М.Г., Яроцкая Е.Л. Реализация программ вспомогательных репродуктивных технологий у пациенток с агрегатами гладкого эндоплазматического ретикулума в цитоплазме ооцитов. Акушерство и гинекология. 2016; 7: 54-9. [Syrkasheva A.G., Kazakova V.V., Dolgushina N.V., Romanov A.Yu., Andreeva M.G., Yarotskaya E.L. Implementation of assisted reproductive technology programs in patients with smooth endoplasmic reticulum aggregates in the cytoplasm of oocytes. Obstetrics and Gynecology. 2016; (7): 54-9.(in Russian)]. https://dx.doi.org/10.18565/aig.2016.7.54-59.
- Ковальская Е.В., Сыркашева А.Г., Романов А.Ю., Макарова Н.П., Долгушина Н.В. Современные представления о компактизации эмбрионов человека в условиях in vitro. Технологии живых систем. 2017; 1: 25-35. [Kovalskaya E.V., Syrkasheva A.G.,Romanov A.Yu., Makarova N.P., Dolgushina N.V. Modern concepts of compaction of human embryos in vitro. Journal Technologies of Living Systems. 2017; (1): 25-35.(in Russian)].
- Романов А.Ю., Ковальская Е.В., Макарова Н.П., Сыркашева А.Г., Долгушина Н.В. Использование цейтраферной съемки для оценки качества эмбрионов человека в программах экстракорпорального оплодотворения. Цитология. 2017; 59(7): 462-6. [Romanov A.Yu., Kovalskaya E.V., Makarova N.P., Syrkasheva A.G., Dolgushina N.V. Use of time-lapse imaging to assess the quality of human embryos in the IVF cycles. Cytology. 2017; 59(7):462-6. (in Russian)].
- Романов А.Ю., Силачев Д.Н., Макарова Н.П., Долгушина Н.В. Влияние механической микровибрации на качество эмбрионов человека при культивировании in vitro и исходы программ вспомогательных репродуктивных технологий. Клеточные технологии в биологии и медицине. 2018; 2: 86-90. [Romanov A.Yu., Silachev D.N., Makarova N.P., Dolgushina N.V.The influence of mechanical microvibration on the quality of human embryos during in vitro cultivation and the outcomes of assisted reproductive technologies programs. Cell technologies in biology and medicine. 2018; (2):86-90.(in Russian)].
- Romanov A.Yu., Silachev D.N., Makarova N.P., Dolgushina N.V. Effect of Mechanical Microvibration on the Quality of Human Embryos during In Vitro Culturing and Outcomes of Assisted Reproduction Technologies. Bull Exp Biol Med. 2018; 165(4):544-7.
- Романов А.Ю., Фролова А.М., Макарова Н.П., Долгушина Н.В. Первый российский опыт применения управляемой механической микровибрации при культивировании эмбрионов человека в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2019; 12: 120-5. [Romanov A.Yu., Frolova A.M., Makarova N.P., Dolgushina N.V. The first Russian experience of using controlled mechanical microvibration in the cultivation of human embryos in programs of assisted reproductive technologies. Obstetrics and Gynecology. 2019; (12):120-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.120-125.
- Datta A.K., Campbell S., Deval B., Nargund G. Add-ons in IVF programme – hype or hope? Facts Views Vis. ObGyn. 2015; 7(4):241-50.
- Harper J., Jackson E., Sermon K., Aitken R.J., Harbottle S., Mocanu E. et al. Adjuncts in the IVF laboratory: where is the evidence for “add-on” interventions? Hum. Reprod. 2017; 32(3): 485-91. https://dx.doi.org/10.1093/humrep/dex004.
- Zemyarska M.S. Is it ethical to provide IVF add-ons when there is no evidence of a benefit if the patient requests it? J. Med. Ethics. 2019; 45(5): 346-50. https://dx.doi.org/10.1136/medethics-2018-104983.
- Gleicher N., Kushnir V.A., Barad D.H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open. 2019; 2019(3): hoz017. https://dx.doi.org/ 10.1093/hropen/hoz017.
- Наими З.М.С., Калинина Е.А., Донников А.Е., Алиева K.У., Дударова А.Х., Тухватуллина Я.А. Эффективность программ вспомогательных репродуктивных технологий при переносе эмбрионов в стимулированном цикле по сравнению с переносом криоконсервированных/размороженных эмбрионов. Акушерство и гинекология. 2016; 6: 11-7.[Naimi Z.M.S., Kalinina E.A., Donnikov A.E., Alieva K.Y., Dydarova A.Kh., Tukhvatullina Ya.A. Efficiency of assisted reproductive technology programs in stimulated-cycle embryo transfer versus cryopreserved-thawed embryo transfer. Obstetrics and Gynecology.2016; (6): 11-7. (in Russian)]. https://dx.doi.org/ 10.18565/aig.2016.6.11-17.
- Кравчук Я.Н., Калугина А.С., Зубова Ю.Г. Применение методов криоконсервации эмбрионов в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2012; (8-2): 80-4. [Kravchuk Ya.N., Kalygina A.S., Zybova Yu.G. Application of methods of cryopreservation of embryos in programs of assisted reproductive technologies.Obstetrics and Gynecology. 2012; (8–2): 80-4. (in Russian)].
- Rienzi L.F., Iussig B., Dovere L., Fabozzi G., Cimadomo D., Ubaldi F.M. Perspectives in gamete and embryo cryopreservation. Semin. Reprod. Med. 2018; 36(5): 253-64. https://dx.doi.org/10.1055/s-0038-1677463.
- Levi-Setti P.E., Patrizio P., Scaravelli G. Evolution of human oocyte cryopreservation: slow freezing versus vitrification. Curr. Opin. Endocrinol. Diabetes Obes. 2016; 23(6): 445-50. https://dx.doi.org/ 10.1097/MED.0000000000000289.
- Konc J., Kanyó K., Kriston R., Somoskői B., Cseh S. Cryopreservation of embryos and oocytes in human assisted reproduction. Biomed. Res. Int. 2014; 2014: 307268. https://dx.doi.org/10.1155/2014/307268.
- Kaye L., Will E.A., Bartolucci A., Nulsen J., Benadiva C., Engmann L. Pregnancy rates for single embryo transfer (SET) of day 5 and day 6 blastocysts after cryopreservation by vitrification and slow freeze. J. Assist. Reprod. Genet. 2017; 34(7): 913-9. https://dx.doi.org/10.1007/s10815-017-0940-4.
- Edgar D.H., Gook D.A. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum. Reprod. Update. 2012; 18(5): 536-54. https://dx.doi.org/10.1093/humupd/dms016.
- Pandian Z., Templeton A., Serour G., Bhattacharya S. Number of embryos for transfer after IVF and ICSI: a Cochrane review. Hum. Reprod. 2005; 20(10): 2681-7. https://dx.doi.org/10.1093/humrep/dei153.
- Wang H., Ou Z., Chen Z., Yang L., Sun L. Influence of different post-thaw culture time on the clinical outcomes of different quality embryos. Adv. Clin. Exp. Med. 2019; 28(4): 523-7. https://dx.doi.org/10.17219/acem/91010.
- Ибрагимова Э.О., Долгушина Н.В., Сыркашева А.Г., Романов А.Ю., Языкова О.И., Макарова Н.П. Роль вспомогательного хетчинга в программах лечения бесплодия методами вспомогательных репродуктивных технологий: обзор литературы. Гинекология. 2016; 18(2): 44-7. [Ibragimova E.O., Dolgushina N.V., Syrkasheva A.G., Romaniv A.Yu., Yazikova O.I., Makarova N.P. The role of assisted hatching in assisted reproductive technology treatment programs for infertility: a literature review. Gynecology. 2016; 18(2): 44-7. (in Russian)].
- Шафеи Р.А., Сыркашева А.Г., Романов А.Ю., Макарова Н.П., Долгушина Н.В., Семенова М.Л. Хетчинг бластоцисты у человека. Онтогенез. 2017; 48(1): 8-20. [Schaffei R.A., Syrkasheva A.G., Romanov A.Yu., Makarova N.P., Dolgushina N.V., Semenova M.L. Blastocyst hatching in humans. Ontogenez. 2017; 48(1):8-20. (in Russian)].
- Syrkasheva A.G., Dolgushina N.V., Romanov A.Yu., Burmenskaya O.V., Makarova N.P., Ibragimova E.O. et al. Cell and genetic predictors of human blastocyst hatching success in assisted reproduction. Zygote. 2017; 25(5): 631-6. https://dx.doi.org/10.1017/S0967199417000508.
- Shafei R.A., Syrkasheva A.G., Romanov A.Y., Makarova N.P., Dolgushina N. V., Semenova M.L. Blastocyst hatching in humans. Russ. J. Dev. Biol. 2017; 48(1): 5-15. https://dx.doi.org/10.1134/S1062360417010106.
- Долгушина Н.В., Ибрагимова Э.О., Романов А.Ю., Макарова Н.П., Довгань А.А., Сыркашева А.Г., Калинина Е.А. Роль проназного хетчинга в повышении эффективности программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2018; 3: 70-4. [Dolgushina N.V., Ibragimova E.O., Romanov A.Yu., Makarova N.P., Dovgan A.A., Syrkasheva A.G. The role of pronase hatching in improving the efficiency of assisted reproductive technology programs. Obstetrics and Gynecology. 2018; (3):70-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.3.70-74.
- Долгушина Н.В., Ибрагимова Э.О., Романов А.Ю., Бурменская О.В., Макарова Н.П., Шафеи Р.А., Сыркашева А.Г. Предикторы эффективности спонтанного хетчинга бластоцист человека в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2018; 2: 88-95. [Dolgushina N.V., Ibragimova E.O., Romanov A.Yu., Bourmenskaya O.V., Makarova N.P., Schaffei R.A. Predictors of the effectiveness of spontaneous hatching of human blastocysts in assisted reproductive technology programs. Obstetrics and Gynecology. 2018; (2):88-95. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.2.88-95.
- Романов А.Ю., Макарова Н.П., Долгушина Н.В., Калинина Е.А. Культуральная среда, обогащенная гиалуроновой кислотой, при переносе эмбрионов в программах вспомогательных репродуктивных технологий: механизм действия и показания к применению. Акушерство и гинекология. 2018; 12: 12-6. [Romanov A.Yu., Makarova N.P., Dolgushina N.V., Kalinina E.A. Culture medium enriched with hyaluronic acid for embryo transfer in programs of assisted reproductive technologies: mechanism of action and indications for use. Obstetrics and Gynecology. 2018; (12):12-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.12.12-16.
- Li Y.J., Chen J.H., Sun P., Li J.J., Liang X.Y. Intrafollicular soluble RAGE benefits embryo development and predicts clinical pregnancy in infertile patients of advanced maternal age undergoing in vitro fertilization. J. Huazhong Univ. Sci.Technolog. Med. Sci. 2017; 37(2): 243-7. https://dx.doi.org/10.1007/s11596-017-1722-z.
- Munné S. Status of preimplantation genetic testing and embryo selection. Reprod. Biomed. Online. 2018; 37(4): 393-6. https://dx.doi.org/10.1016/j.rbmo.2018.08.001.
- García-Ferreyra J., Hilario R., Dueñas J. High percentages of embryos with 21, 18 or 13 trisomy are related to advanced paternal age in donor egg cycles. JBRA Assist. Reprod. 2018; 22(1): 26-34. https://dx.doi.org/10.5935/1518-0557.20180004.
- Kristensen S.G., Humaidan P., Coetzee K. Mitochondria and reproduction: possibilities for testing and treatment. Panminerva Med. 2019; 61(1): 82-96.
Received 26.03.2020
Accepted 01.04.2020
About the Authors
Yana A. Petrosyan, postgraduate student, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. E-mail: Yana_petrosyan86@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Anastasiya G. Syrkasheva, M.D., PhD., Senior researcher of ART Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. Tel.: +7(926)363-17-20. E-mail: anast.syrkasheva@gmail.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Andrey Yu. Romanov, postgraduate student, specialist of R&D Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. Tel.: +7(903)158-94-00. E-mail: romanov1553@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya P. Makarova, PhD, Leading Researcher of IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology
Ministry of Healthcare of Russian Federation. E-mail: np_makarova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Elena A. Kalinina, M.D., PhD, Head of IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology
Ministry of Healthcare of Russian Federation. E-mail: e_kalinina@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Petrosyan Ya.A., Syrkasheva A.G., Romanov A.Yu., Makarova N.P., Kalinina E.A. Differentiated approach to the embryological stage in frozen-thawed embryo transfer.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 11: 107-113 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.107-113



