В последние годы зафиксировано значительное снижение показателей заболеваемости раком шейки матки (РШМ) во многих развитых странах мира вследствие планомерной работы программ цервикального скрининга [1]. Этому способствует организованное, а не оппортунистическое проведение скрининга на РШМ с вовлечением в него не менее 80% женского населения [2, 3] и совершенствование скрининговых программ путем внедрения новых и современных технологий. В Российской Федерации цервикальный скрининг носит оппортунистический характер и охватывает не более 30% женского населения [4].
Распространенным скрининговым методом является традиционная цитология из-за более низкой стоимости [5], однако большое количество исследований показало низкую чувствительность цитологических методов в выявлении патологии шейки матки (ШМ) [6, 7], при этом прогностическая ценность традиционной и жидкостной цитологии сопоставима [8]. В последние годы многие страны стали активно включать тестирование на вирус папилломы человека высокого канцерогенного риска (ВПЧ-ВКР) в программы цервикального скрининга, что связано с доказанной этиологической ролью папилломавируса в цервикальном канцерогенезе [2, 9]. По мнению экспертов, тестирование на ДНК ВПЧ позволит снизить заболеваемость РШМ в течение 4– лет и смертность от РШМ в течение 8 лет по сравнению с цитологическим скринингом [2]. Исследователи из разных стран сделали заключение, что ВПЧ-тестирование без дополнительной цитологии обладает достаточной чувствительностью для первичного скрининга РШМ [1, 10, 11]. Внедрение ВПЧ-тестирования в скрининговые программы обусловило высокую значимость кольпоскопического исследования, а также увеличило число диагностируемых поражений из группы CIN III+ и РШМ, требующих немедленного лечения [12]. Для привлечения к участию в цервикальном скрининге большего числа женщин в последние годы были разработаны устройства для самостоятельного забора вагинального отделяемого для ВПЧ-теста, а также исследованы их диагностическая ценность, экономическая эффективность, удобство применения и возможность включения в государственные скрининговые программы [13–16].
Цель исследования – определить эффективность традиционной цитологии и ВПЧ-ВКР-тестирования в выявлении цервикальной интраэпителиальной неоплазии высокой степени.
Материалы и методы
В проспективное исследование включены 200 женщин 18–45 лет, обратившихся для прохождения периодического медицинского осмотра. У 84/200 (42%) пациенток была выявлена ДНК ВПЧ-ВКР, что обусловило их участие в исследовании через 6 месяцев. На 2 этапе исследования всем женщинам (n=84) выполняли: традиционное цитологическое исследование с окраской по Романовскому–Гимзе; ВПЧ-тестирование путем самостоятельного забора влагалищного содержимого устройством Qvintip и врачебного взятия секрета цервикального канала урогенитальным зондом типа «А»; расширенную кольпоскопию. Методом полимеразно-цепной реакции (ПЦР) определяли 12 генотипов ВПЧ высокого канцерогенного риска (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59). Интерпретацию цитологических результатов проводили согласно классификации Бетесда (The Bethesda system, TBS, 2001 г.): NILM, ASCUS, LSIL, HSIL. Кольпоскопические признаки интерпретировали согласно классификации кольпоскопических терминов, принятой в Рио-де-Жанейро от 2011 г. При получении аномальной кольпоскопической картины, с согласия женщин, проводили радиоволновую биопсию ШМ с гистологической верификацией диагноза (n=64). Для статистической обработки данных использовали пакет программ StatSoft Statistica 10 Advanced (Statsoft Ins., США; лицензия № AXAR507G794202FA-B). Анализ количественных признаков осуществляли при помощи вычисления среднего арифметического, дисперсии и 95% доверительного интервала (ДИ). При проверке статистических гипотез о различиях долей в двух или нескольких независимых выборках использовали критерий хи-квадрат (χ2) Пирсона. Значения считали статистически достоверными при величине χ2>3,84 и при р≤0,05. Оценку показателей эффективности диагностических методов в выявлении HSIL выполняли на основании гистологических заключений при помощи расчета следующих показателей:
1. чувствительность (Se) – мера вероятности того, что болезнь будет идентифицирована с помощью теста: Se=а/(а+с)×100% ;
2. специфичность (Sp) – мера вероятности правильной идентификации людей, не имеющих болезни, с помощью теста: Sp=d/(b+d)×100%,
где a – больные, выявленные с помощью теста (истинно положительные);
b – здоровые с положительным результатом теста (ложноположительные);
c – больные, не выявленные с помощью теста (ложноотрицательные);
d – здоровые с отрицательным результатом теста (подлинно отрицательные).
Результаты и обсуждение
В когорте исследуемых нами женщин обеими методиками забора образцов ДНК ВПЧ-ВКР обнаружена у 36/84 (42,8%). Ранжирование типов ВПЧ-ВКР представлено на рисунке.
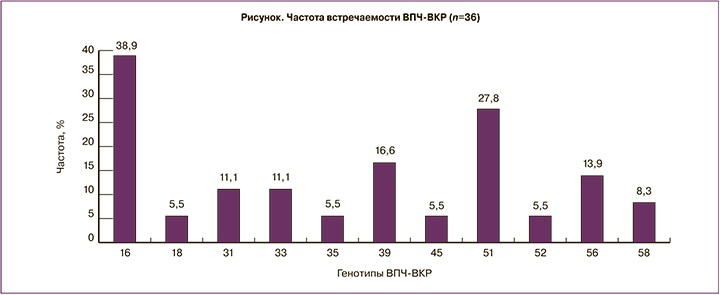
Через 6 месяцев от начала исследования с большей частотой встречались 16, 51 и 39 типы ВПЧ-ВКР, 59 тип на данном этапе исследования не идентифицировался. Только врачебным забором (при отрицательном результате самозабора) выявлен 1 ВПЧ-положительный образец, в то время как методом самозабора (при отрицательном результате врачебного забора) было получено 14 ВПЧ-позитивных тестов (2,8% vs 38,9%; χ2=8,2; p=0,004; ОШ=14,0; ДИ 1,7–110,7).
Патологические признаки по данным традиционной цитологии выявлены в 21/84 (25%) случаев: LSIL – в 19/84 (22,6%), HSIL – в 2/84 (2,4%).
При проведении расширенной кольпоскопии аномальная кольпоскопическая картина выявлена у 66/84 (78,6%). Аномальная картина 1 степени диагностирована у 44/84 (52,4%); 2 степени – у 17/84 (20,2%). Неудовлетворительная картина в сочетании с другими аномальными признаками визуализировалась у 4/84 (4,8%) пациенток. У 1/84 (1,2%) обнаружено подозрение на инвазию.
Интраэпителиальные поражения верифицированы при помощи гистологического исследования у 47/64 (73,4%): LSIL – у 37/64 (57,8%) и HSIL – у 10/64 (15,6%) пациенток. Средний возраст исследуемых был сопоставим и составил 30,21±7,28 года у женщин с LSIL и 31,35±7,28 года – c HSIL (p>0,05). Следует отметить, что пациентки с HSIL имели аномальные цитологические признаки, соответствующие LSIL в 6/10 (60%), и нормальные цитологические заключения в 7/10 (70%) случаев.
В исследовании Koliopoulos G. и соавт. [7] установлено, что один отрицательный ВПЧ-тест более надежен в отсутствии HSIL в сравнении с отрицательным цитологическим тестом. Toliman P. и соавт. [17] указывают на то, что чувствительность тестирования на ДНК ВПЧ (88–100%) существенно выше цитологического исследования (68–86%), при этом специфичность ВПЧ-теста (68–97%) незначительно уступает цитологии (78–99%). Высказано мнение, что высокая частота получения ложноотрицательных цитологических результатов может быть обусловлена неверным взятием образца или неадекватной интерпретацией полученных результатов [5]. В нашем исследовании чувствительность традиционной цитологии в выявлении HSIL оказалась низкой и составила 10%, при этом специфичность метода достигала 98,1%, что означает наличие заболевания при получении положительного результата данного диагностического теста.
В обзоре De Thurah L. и соавт. [18] продемонстрировано, что выявление ДНК ВПЧ обладает достаточной точностью в диагностике HSIL независимо от метода тестирования. Высокая чувствительность (81–89%) самостоятельного взятия влагалищного образца для последующего тестирования на ДНК ВПЧ выявлена в ходе многочисленных исследований [19–21]. Мы установили, что взятие образца врачом для ВПЧ-ВКР теста обладает средними показателями чувствительности (50%) и специфичности (74,1%), при этом наибольшее количество истинно положительных результатов показал метод самостоятельного забора образца для ВПЧ-ВКР-теста, что обусловило его чувствительность в 70%. Специфичность данной методики составила 57,4%.
 В некоторых странах выявление ДНК ВПЧ используется в качестве ко-тестирования с цитологическим методом для повышения точности выявления предраковых заболеваний («Совместное или двойное тестирование»), а также как самостоятельный скрининговый тест, за которым, при положительном ответе, следует развернутое обследование [22]. Примерно в 50% случаев цитологическое исследование не выявляет CIN II+ у молодых женщин 25–29 лет, однако ко-тест цитологии с идентификацией ВПЧ увеличивает вероятность обнаружения CIN II+ на 28% [11]. Blatt A.J. и соавт. [23] в своей работе указали на высокую чувствительность ко-теста цитологии и выявления ДНК ВПЧ в обнаружении CIN III+ у женщин 30–65 лет. Нами оценена эффективность ко-тестирования традиционной цитологии с ВПЧ-ВКР-типированием в диагностике HSIL (при положительном результате обоих методов случай SIL считался выявленным), представленная в табл. 1.
В некоторых странах выявление ДНК ВПЧ используется в качестве ко-тестирования с цитологическим методом для повышения точности выявления предраковых заболеваний («Совместное или двойное тестирование»), а также как самостоятельный скрининговый тест, за которым, при положительном ответе, следует развернутое обследование [22]. Примерно в 50% случаев цитологическое исследование не выявляет CIN II+ у молодых женщин 25–29 лет, однако ко-тест цитологии с идентификацией ВПЧ увеличивает вероятность обнаружения CIN II+ на 28% [11]. Blatt A.J. и соавт. [23] в своей работе указали на высокую чувствительность ко-теста цитологии и выявления ДНК ВПЧ в обнаружении CIN III+ у женщин 30–65 лет. Нами оценена эффективность ко-тестирования традиционной цитологии с ВПЧ-ВКР-типированием в диагностике HSIL (при положительном результате обоих методов случай SIL считался выявленным), представленная в табл. 1.
В ходе нашего исследования выявлено, что применение ВПЧ-ВКР-тестирования в дополнение к цитологическому методу позволяет диагностировать большее число пациенток с HSIL. Комбинация традиционной цитологии с ВПЧ-тестированием путем самостоятельного взятия влагалищного образца показала наибольший показатель чувствительности – 70%.
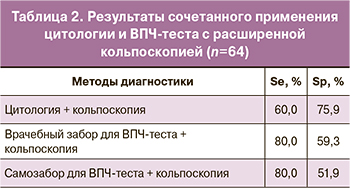 В метаанализе Mustafa R.A. и соавт. [24] показана чувствительность кольпоскопии, достигающая 95%, и специфичность 42%. Нами выявлена средняя чувствительность расширенной кольпоскопии в диагностике HSIL – 60% и высокая специфичность – 75,9%. В табл. 2 вынесены результаты комбинированного применения цитологии и ВПЧ-теста с кольпоскопией.
В метаанализе Mustafa R.A. и соавт. [24] показана чувствительность кольпоскопии, достигающая 95%, и специфичность 42%. Нами выявлена средняя чувствительность расширенной кольпоскопии в диагностике HSIL – 60% и высокая специфичность – 75,9%. В табл. 2 вынесены результаты комбинированного применения цитологии и ВПЧ-теста с кольпоскопией.
Высокий показатель чувствительности (80%) выявлен у сочетания расширенной кольпоскопии с ВПЧ-тестированием независимо от методики забора материала, в то время как высокоспецифичным оказалось ко-тестирование цитологии с кольпоскопией (75,9%).
Заключение
Традиционная цитология обладает низкой чувствительностью (10%) и высокой специфичностью (98,1%) в выявлении интраэпителиальной неоплазии высокой степени. ВПЧ-тестирование путем врачебного взятия образца обладает средними показателями чувствительности (50%) и специфичности (74,1%). При применении метода самостоятельного забора образца для ВПЧ-теста показатель чувствительности достигает 70%. Самотестирование с определением ДНК ВПЧ как при сочетанном использовании с традиционной цитологией, так и в качестве одиночного метода позволяет диагностировать большее количество пациенток с HSIL.



