Cerebrovascular disorders associated with severe preeclampsia and eclampsia
Aim: The aim of the research was to study the pathomorphological and immunohistochemical features of brain damage in cases of the most severe forms of preeclampsia and eclampsia that ended with death.Sidorova I.S., Nikitina N.A., Ageev M.B., Kokin A.A.
Materials and methods: Tissue samples taken from 10 women during autopsy, who died from from preeclampsia and eclampsia, were examined, as well as tissue samples for comparison from 3 women, who died from other reasons. The markers of mature neurons (γ-NSE) and endotheliocytes (CD-34) were used for immunohistochemistry of postmortem brain tissue.
Results: The study of autopsy material from patients, who died from severe preeclamsy and eclampsy detected some features of the pathogenesis of cerebrovascular complications associated with preeclampsia: 1) pronounced vasogenic and cytotoxic cerebral edema with multiple foci of ischemia, subarachnoid and intracerebral hemorrhages; 2) the signs of severe ischemic encephalopathy, death of cortical neurons and glial cells; 3) Brain capillary endothelium destructive damage with impaired blood-brain barrier (BBB) integrity and the release of neurospecific proteins from the brain tissue into the peripheral blood flow.
Conclusion: The major pathophysiological mechanisms of cerebrovascular complications of preeclampsia and eclampsia are disruption of cerebral blood flow autoregulation, destructive damage of brain capillary endothelium, impaired BBB integrity, and possibly neuroinflammation.
Keywords
Preeclampsia is a specific complication in the second half of pregnancy, which initially develops as a multisystem lesion and is characterized by generalized vascular dysfunction.
The brain is one of the target organs that are affected by preeclampsia and eclampsia. Until recently, it was believed, that eclampsia and severe preeclampsia in medical history do not have long-term outcomes, and after delivery, all clinical symptoms disappear. However, much of the latest published data suggests otherwise. A group of cerebrovascular complications of preeclampsia was described, including posterior reversible encephalopathy syndrome (PRES), reversible cerebral vasoconstriction syndrome (RCVS), hemorrhagic and ischemic stroke, vascular dementia caused by the damage of small arteries in the brain [1–4].
The complexity of studying cerebrovascular disorders in preeclampsia is due to many factors, for example: preeclampsia is a unique complication, which is specific only to human pregnancy; it is often impossible to use neurovisualization method, and applicability of in vitro models in the clinical practice is limited, as well as other factors. Acute cerebrovascular disorders, including PRES, RCVS, ischemic and hemorrhagic stroke, cerebral venous sinus thrombosis (CVST) are the most severe complications of preeclampsia, which can lead to disability or even death of a mother. The risk for the development these complications in pregnant women with preeclampsia is 1 case per 500 births, while the overall risk of such complications during pregnancy is approximately 30 cases per 100,000 births [5].
According to the published data, the percentage of ischemic strokes in the structure of maternal mortality is 12% [6]. The incidence rate for strokes during pregnancy is 2–3 times higher than in the general population (about 30 cases per 100,000 births) [7], the risk is especially high in the third trimester and during the first 6–12 weeks after delivery [8]. The risk of stroke in pregnant women with hypertensive disorders increases by 4–5 times compared to healthy pregnant women [9].
The etiology and pathophysiology of cerebral complications of preeclampsia and eclampsia are not clear enough, but it is believed, that these conditions are the result of impaired cerebrovascular regulation, leading mainly to vasogenic cerebral edema and, sometimes to cytotoxic cerebral edema, mainly in the parietal and occipital lobes of the brain. A possible mechanism of these conditions may be cerebral vasoconstriction, impaired autoregulation with forced dilatation of cerebral arteries and endothelial dysfunction [10].
Neurological symptoms of severe preeclampsia and eclampsia (headache, visual impairment, changes in consciousness, convulsive syndrome, less often - focal neurological disorders) essentially correspond to the clinical symptoms of PRES [11, 12]. It is believed, that obstetric PRES is caused by severe hypertension with impaired permeability of the blood-brain barrier (BBB) and development of vasogenic (usually symmetric) cerebral edema in the parietal and occipital lobes, which in most cases regresses after appropriate therapy. However, in some cases, during CTG and MRI, in addition to vasogenic edema, infarctions in the subcortical white matter areas and in the adjacent zones of the cerebral cortex are found [13]. This may contribute to the progression of PRES and damage to the brain parenchyma with residual neurological deficits.
The initial concept of the cause of PRES – the concept of cerebral vasoconstriction was proposed at the end of the last century. It was based on the results of angiography, which showed a small diameter of cerebral arteries in eclampsia. It was assumed, that cerebral arteries vasoconstriction occurs in response to arterial hypertension with the subsequent activation of compensatory mechanisms of autoregulation of cerebral blood flow. These changes lead to hypoxia, endothelial dysfunction and further to the development of vasogenic and cytotoxic cerebral edema [14].
However, currently, the scientific community adheres to another concept – loss (disruption) of cerebral autoregulation and cerebral hyperperfusion.
The current theory explaining the loss of cerebral autoregulation of blood flow is based on vascular paralysis and dilation of cerebral vessels with decreased cerebrovascular resistance to increased systemic blood pressure (BP). Dilation of cerebral arteries results in increased cerebral blood flow, increased pressure in the blood vessel wall, and in turn, causes vasogenic cerebral edema [10, 15]. However, this process can occur only when the upper limit of cerebral blood flow autoregulation is reached (with BP ≥ 170–180 mmHg), and it is not always observed in women with eclampsia and preeclampsia [16]. The reason for disruption of cerebral blood flow autoregulation in pregnant women with severe preeclampsia and eclampsia and with blood pressure within the normal range or with moderate hypertension is poorly studied.
One of the consequences of impaired cerebral blood flow autoregulation is increased permeability of the BBB, which can result in cerebral edema [17].
During pregnancy, there are adaptive changes in blood-brain barrier. In 2012 Cipolla M.J. et al. found that, when serum of pregnant animals is applied directly to isolated hippocampal slices, it causes increased excitability of neurons and the seizure activity, as well as morphological changes, which are specific for microglia upon activation [18]. However, the authors did not detect the increased BBB permeability in their study. They concluded that the BBB plays a role in brain protection from various neuroinflammatory substances circulating in the bloodstream of healthy pregnant women.
Impaired permeability of the BBB due to complications, such as severe preeclampsia, eclampsia, HELLP syndrome, accompanied by neurological symptoms, is critical for development of cerebral edema and seizures in pregnant women [19]. It seems that the increased BBB permeability in pregnant women with these complications is due to increased number of damage factors in the circulating blood that affect endothelium, including the endothelial component of the BBB. The results obtained by Amburgey O.A. et al. are interesting. They showed, that the experimental effect of the blood plasma of patients with preeclampsia on cerebral blood vessels in rats has little effect on the reactivity of cerebral arteries, but significantly increases the BBB permeability (by 18 times compared to plasma of healthy pregnant women) [20].
Molecular mechanisms that lead to seizure and eclampsia are not fully studied yet. Many authors emphasize, that cerebral microglial activation likely contributes to seizures. Microglial cells are brain-resident macrophages, which are widely distributed in the brain parenchyma. These cells are the first that respond to pathological stimuli. Microglia activation is characterized by a number of specific features, including morphological transformation and the production of pro-inflammatory cytokines (for example, TNFα) [21], which effect neurons via GABA receptors, changing neuronal excitability.
Despite the remaining uncertainty in the problem of cerebrovascular impairment in severe preeclampsia and eclampsia, the aim of our study was to investigate the features of brain damage in cases of the above complications of pregnancy using autopsy material.
Materials and methods
A retrospective analysis of medical documentation (including anatomic pathology reports) as well as an immunohistochemical study of brain tissues from 10 women, who died of severe preeclampsia (n=5) and eclampsia (n=5), comprising the main group, was performed. The patients with brain damage not associated with preeclampsia (traumatic, tumor, infectious, toxic, neurodegenerative, medically induced, etc.) were not included in the study.
Unfortunately, we had no opportunity to compare the changes between those, which were described in anatomic pathology reports and intravital braing imaging, as autopsy material was provided to us by the Research Institute of Human Morphology of the Russian Academy of Sciences (the research was performed in the Laboratory of Female Reproductive System Pathology headed by M.D., Professor A.P. Milovanov).
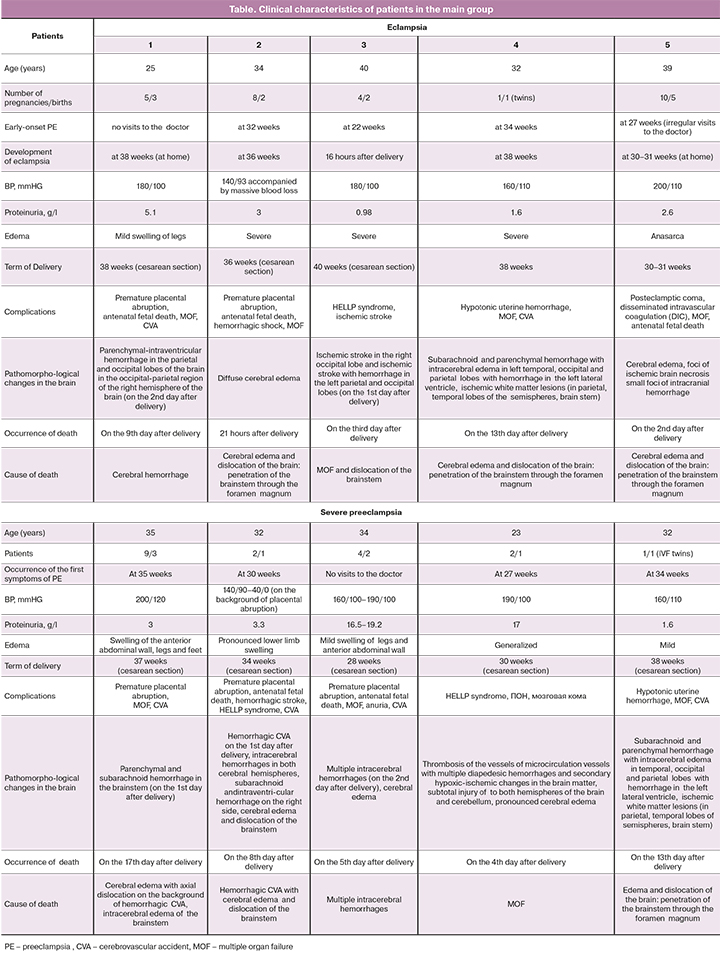
The below Table shows the main clinical characteristics of the patients. The comparison group was comprised of 3 women who died and were not diagnosed for preeclampsia. The causes of death were: uterine rupture and development of hemorrhagic shock at the 38th week of pregnancy (38 years old, the 6th pregnancy, the 4th birth); anaphylactic shock and cardiac arrest after introduction of ketamine (33 years old, the 3rd pregnancy, the 2nd birth); acute lymphoblastic leukemia with severe hemorrhagic syndrome at the 32nd week of pregnancy (39 years old, the 2nd pregnancy, the 2nd birth).
The autopsy material was fixed in 10% neutral buffed formalin and embedded in paraffin according to standard procedure. Microtome was used to cut the sections of 3–5 μm thickness, which were stained with hematoxylin and eosin. Among the viewed slides, we selected those that were promising for immunohistochemical studies. Deparaffined sections were placed on polylysine-coated glass slides. The antibodies (“Novocastra”, Germany) for deparaffined sections were used: 1) γ-NSE (γ-neuron specific enolase, clone 5E2) – a marker for neurons and neuroendocrine cells; 2) CD-34 (clone QBEND/10) – vascular endothelial cells marker. After deparaffining, the sections were incubated with the above primary antibodies for 15 minutes at T 25°С. When performing the assay detection Kit (NCL-RTV) was used according to standard technology application.
Results
Examination of the brain tissue in the comparison group
Routine pathomorphological examination found cerebral edema in cortical, subcortical and cerebellar areas, pronounced perivascular edema, multiple ischemic lesions (Fig. 1a). This confirms that hemorrhagic shock plays an important role in thanatogenesis.
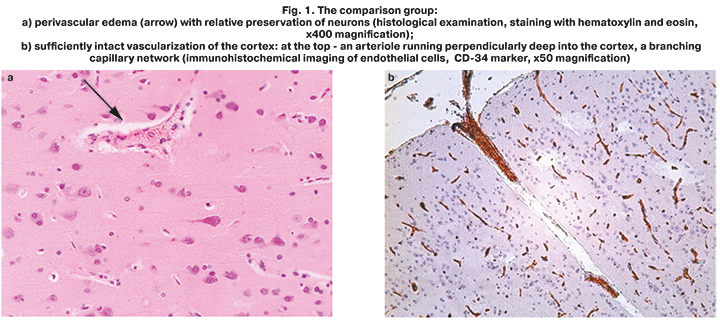
Immunohistochemical analysis, where CD-34 was used as endothelial cells marker in the tissues of the cerebral cortex, showed that high density of blood flow distribution was visualized in capillary network (Fig. 1b). Cerebral arteriole coming off pia mater and branches into dense capillary network.
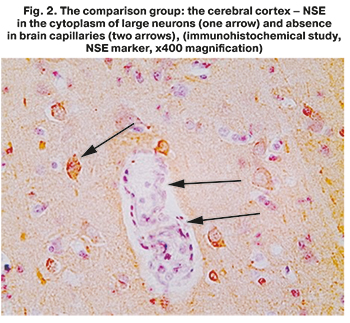 To assess the state of neurons in detail, an immunohistochemical study of brain tissue was carried out using neurospecific enolase (NSE), a marker of mature differentiated neurons. In the comparison group, NSE was clearly visualized in the cytoplasm of large neurons, outlining the contours of the preserved nuclei. NSE was also detected around neurons, in a dense network of neuron processes (dendrites and axons) (Fig. 2). However, assessment of NSE distribution showed pathological changes in neurons: the marginal membrane was indistinct, absence of Nissl body (or tigroid substance) in the cytoplasm, i.e. an accumulation of cisterns of the granular endoplasmic reticulum (Fig. 2). This was indicative of impaired synthesis of various neuroproteins in these cells. Astrocytes, oligodendrocytes and other non-neuronal (neuroepithelial) cells were NSE-negative.
To assess the state of neurons in detail, an immunohistochemical study of brain tissue was carried out using neurospecific enolase (NSE), a marker of mature differentiated neurons. In the comparison group, NSE was clearly visualized in the cytoplasm of large neurons, outlining the contours of the preserved nuclei. NSE was also detected around neurons, in a dense network of neuron processes (dendrites and axons) (Fig. 2). However, assessment of NSE distribution showed pathological changes in neurons: the marginal membrane was indistinct, absence of Nissl body (or tigroid substance) in the cytoplasm, i.e. an accumulation of cisterns of the granular endoplasmic reticulum (Fig. 2). This was indicative of impaired synthesis of various neuroproteins in these cells. Astrocytes, oligodendrocytes and other non-neuronal (neuroepithelial) cells were NSE-negative.
Thus, in the comparison group, the described changes in the brain clearly indicated severe progressive hypoxic-ischemic encephalopathy on the background of shock. Despite the destructive changes in some neurons, their total number was preserved, and the network of brain capillaries was widespread. This confirmed the intact vascularization of the cerebral cortex up to the development of shocks. The above presented pathological disorders in the cerebral cortex reflected the mechanisms of thanatogenesis in the comparison group and were not direct causes of death of these patients.
The study of brain tissue in preeclampsia and eclampsia
The results of pathomorphological examination of the cerebral cortex of women, who died from preeclampsia and eclampsia, largely overlapped with the results obtained in the comparison group and indicated severe hypoxic-ischemic encephalopathy, but were more pronounced.
 Microscopic analysis found pronounced cerebral edema in 100% of microspecimens, ischemic foci of various sizes – in 60% of microspecimens, and subarachnoid and intracerebral hemorrhages in 60% of microspecimens.
Microscopic analysis found pronounced cerebral edema in 100% of microspecimens, ischemic foci of various sizes – in 60% of microspecimens, and subarachnoid and intracerebral hemorrhages in 60% of microspecimens.
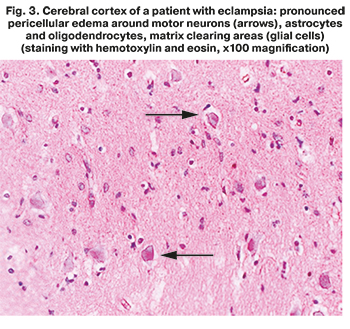
Routine microscopic examination using hematoxylin and eosin stain found large zones of perivascular and pericellular edema with vacuolar degeneration of neurons. In contrast to the comparison group, decreased number of neurons was found in all zones of the neocortex (especially in layer V), that indicated the death of a large volume of these cells due to ischemia (Fig. 3). Microcirculatory thrombosis with multiple ischemic changes in the surrounding tissues was found in 60% of microspecimens.
Analysis of vascularization features with of the cerebral cortex of women, who died from preeclampsia/eclampsia was performed using CD-34 marker. It showed that vascularization features had significant differences compared to the comparison group. Sharply decreased density of cerebral capillaries distribution was found in all layers of the cortex (especially in layers 1, 2, 3) (Fig. 4a). Only single small capillaries were visualized in some studied zones.
Apparently, this was due to the specific features of the cellular composition in the cerebral cortex, which were found by us. Thus, even more severe ischemic injury of motor neurons and other cells was detected with NSE marker in almost all layers of the cortex. Layer 1 (superficial molecular layer) of the cerebral cortex was thickened (Fig. 4b – 1). There was a reduced number of small pyramidal cells in layer 2 (external granular layer) (Fig. 4b – 2), only the contours of anucleated pyramidal neurons with accumulation of NSE in the perikarion were found. Single pyramidal and granular cells with weak NSE immunoexpression in the form of small granular accumulation were isualized in layers 3 and 4, and in the layer of pyramidal neurons (рис. 4b – 3) and in the internal granular layer (Fig. 4b – 4). Also, only single large neurons (Betz cells) with branched axons were found in layer 5 (internal pyramidal/ganglionic layer) (Fig. 4b – 5). Considering that collaterals of Betz cell axons send inhibitory impulses to the cortex, such a sharp "depletion" of the cerebral cortex by motor neurons may be one of the mechanisms of the onset of convulsive seizures.
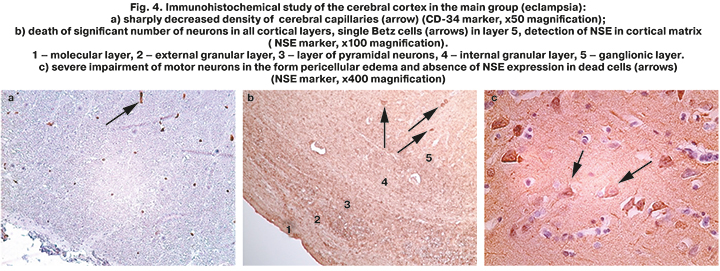
Different stages of motor neurons death were traced at high magnification (Fig. 4c), such as loss of clarity of cell contours, different intensity of NSE expression (from weak to complete absence), vacuolization of the perikaryon up to the death of individual neurocytes. NSE-positive material from damaged neurons was freely detected in the cortical matrix in the form of granules.
There were also marked ischemic damage in astrocytes and oligodendrocytes with cytoplasmic clearing, the absence of clear contours of their nuclei.
An important fact was the finding of a large number of NSE-positive inclusions in the dendritic masses and endotheliocytes of cerebral capillaries in this group (Fig. 2). Moreover, an excessive concentration of NSE in the desquamated endothelium was noted on the background of pronounced perivascular edema around most capillaries and thickening of their basement membrane (Fig. 5a, b). At high magnification, in addition to desquamation of endothelial cells from the basement membrane, we detected other signs of severe cerebral capillary endotheliosis – swelling and vacuolization of endothelial cells, swelling of edematous endothelial cells into the lumen of capillaries up to joining with cells of the opposite vascular wall (Fig. 5b). There were no such changes in the comparison group.
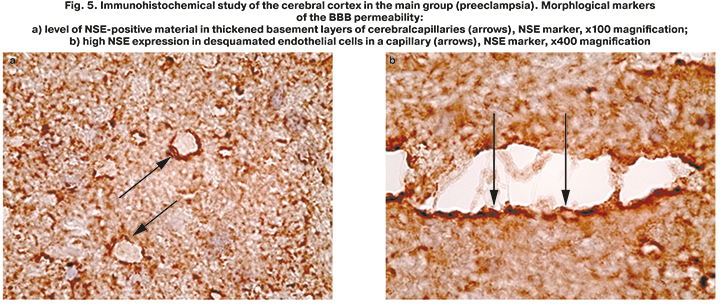
Analysis of NSE distribution features in the brain tissues made it possible to ascertain the fact of the BBB permeability in patients, who died from severe preeclampsia and eclampsia. Elevated level of NSE in the damaged endothelium of cerebral capillaries compared to the surrounding pyramidal neurons was found (Fig. 5b). This confirmed that basement membrane in the capillaries, that are important component of the BBB, was injured. Normal localization of NSE was only in neurons. Determination of this marker outside these cells is possible in case of neuronal necrosis and the release of NSE from the intracellular compartment of cortical neurons into the surrounding matrix. Further, NSE passes through the damaged basement membrane of cerebral capillaries and accumulates in endothelial cells.
Thus, in severe preeclampsia and eclampsia, due to the BBB permeability, neurospecific proteins from dead neurons penetrate into the cortical matrix and accumulate in a totally damaged endothelium. In such situation, further release of NSE into cerebral blood flow is possible with subsequent activation of maternal immune system in response to her own brain proteins in the absence of immunologica tolerance to them.
Discussion
The recent clinical studies showed that the true incidence of cerebral disorders in preeclampsia is much higher than it is assumed. MRI was performed in patients with preeclampsia in the first 48 hours after delivery. MRI findings showed the presence of PRES in every fourth of them (in 26.6% of women) even when there were no pronounced symptoms of preeclampsia, in the group with eclampsia the signs of PRES were in 86.7% of women [22]. Timely treatment could lead to a rapid regression of morphological changes inherent in PRES and reduce the risk of long-term neurological consequences.
The published data showed that even 5–15 years after preeclampsia, MRI detects increased white matter hyperintensity, which is a marker of cerebral small vessels disease and directly correlates with an increased risk of stroke, cognitive impairment and dementia at a later age [3, 23]. Moreover, it became clear that PRES can be found not only in the posterior regions of the brain, but also in the parietal or frontal regions [24].
Brain damage commonly described by pathologists in eclampsia includes massive cerebral edema and white matter changes, which are specific for posterior reversible leukoencephalopathy syndrome, as well as extensive necrotic foci [25].
It is believed, that morphological signs of impaired function of brain tissue are not strictly specific for preeclampsia. Similar changes are described in other pathological conditions with the signs of generalized systemic vascular endothelium injury (thrombotic microangiopathy, atypical hemolytic-uremic syndrome, antiphospholipid syndrome, etc.) [26].
Our study showed that in general, the morphological picture of the brain in the group of women, who died from preeclampsia/eclampsia corresponds to very severe hypoxic-ischemic encephalopathy (pronounced cerebral edema, ischemic foci, subarachnoid and intracerebral hemorrhages).
Microscopic examination detected large zones of perivascular and pericellular edema in both white and gray matter in the brain, microcirculatory thrombosis with multiple ischemic changes. Significantly higher volume of ischemic neuronal necrosis, astrocytes and oligodendrocytes in all layers of the cerebral cortex was found than in patients in the comparison group. This partly reflects the pathophysiological mechanisms of impaired autoregulation of cerebral blood flow, and to a certain degree – the process of thanatogenesis. The death of neurons and other cells of the cerebral cortex in severe preeclampsia and eclampsia, a sharp decrease in the density of cerebral capillaries distribution can be a long-term process with changes in cognitive functions in the patient's subsequent life, up to vascular dementia described in the literature.
Different authors in recent studies indirectly confirm the changes in the cellular composition of the cerebral cortex, which we detected in our study. Raman M.R. et al. showed a decrease in the volume of gray matter in the posterior parts of the brain in women with preeclampsia in medical history, and called it cortical atrophy [27].
Application of special methods of morphological research using specific markers showed severe brain damage in the main group of women.
Immunohistochemical study with endothelial cells marker CD-34 in the comparison group detected relatively intact vascularization of the cerebral cortex with a widespread capillary network. On the contrary, a sharp decrease in the density of the capillary network distribution was found in cortex layers 1, 2, 3, and in some fields, only single small capillaries were visualized in the main group. On the one part, these changes were due to massive cerebral edema, on the other part, apparently due to a long-term antiangiogenic state, which is specific for preeclampsia on the background of increased levels of such antiangiogenic factors as sFlt-1.
The results of our study showed, that cerebral edema in preeclampsia and eclampsia is of combined character, i.e. vasogenic and cytotoxic. The first is caused by BBB impairment and subsequent leakage of extracellular fluid of the brain into the intercellular space. Cytotoxic edema is associated with increased fluid flow into brain cells through aquaporin channels, resulting in edema and cell swelling. In this case the integrity of BBB is not impaired.
An important result of our study was detection of extreme degree of destructive endotheliosis of cerebral vessels in preeclampsia and eclampsia: most cerebral capillaries were surrounded by perivascular edema, their basement membranes were edematous, thickened, and there was desquamation of endothelial cells from the basement membrane. At high magnification, the bulging of edematous vacuolated endothelial cells into capillary lumen up to complete occlusion of vessel lumen was visualized.
The modern understanding of endothelial dysfunction is a systemic pathological condition of the endothelium, characterized by the loss of physiological response to vasodilators and vasoconstrictors, both of endothelial and non-endothelial genesis Endothelial dysfunction in the modern sense is a systemic pathological condition of the endothelium, characterized by the loss of its physiological response to vasodilators and vasoconstrictors , both endothelial and non-endothelial genesis [28].
Generalized damage of the vascular endothelium of all organs, including the brain, is a central link in the pathogenesis of preeclampsia. In this case, endothelial cells of cerebral vessels are one of the key components of BBB. The phenotype of BBB endothelial cells differs from the phenotype of peripheral endothelial cells, as they express a large number of tight junction proteins, transmembrane transporters and metabolic enzymes. Forming tight intercellular junction, they limit BBB permeability, therefore, it understandable that their damage leads to loss of BBB integrity observed in preeclampsia/ eclampsia.
The detected specific features of distribution of neuronal marker NSE with the most intensive immunoexpression in the damaged capillary endothelium, but not in the surrounding neurons, suggest the damage of basement membrane of cerebral microvessels BBB and penetration of NSE into peripheral bloodstream. Such changes were not visualized in the comparison group.
The results of immunohistochemical studies made it possible to trace the chain of events in pathogenesis: severe hypoxic neuronal injury up to necrosis – release of neurospecific proteins (NSE) from neurons into the surrounding tissues – penetration of NSE through the damaged BBB and accumulation in the cerebral capillary endothelial cells – determination of NSE in the peripheral blood flow. However, neurospecific proteins are normally located only in the brain tissue and are separated from BBB immune system from the period of intrauterine ontogenesis, and therefore, there is no physiological immune tolerance to them. Penetration of neuronal proteins into the bloodstream triggers immune responses to eliminate foreign antigens, increasing the pro-inflammatory imbalance in preeclampsia.
Thus, the obtained data showed the BBB permeability in patients with severe preeclampsia and eclampsia, which is an adverse prognostic factor for pregnancy outcomes in women with these complications.
Recent studies by other authors are consistent with our obtained data on the increased BBB permeability for brain proteins in preeclampsia, and these changes persist up to one year after delivery [29].
However, the true cause of the increased BBB permeability in preeclampsia has not been adequately studied. It is believed, that ischemic placenta is a source of factors damaging the endothelial cells of the cerebral capillaries in the BBB. This hypothesis was confirmed by Warrington et al. [30], who reported the increased permeability of cerebral vessels in pregnant rats with induced placental ischemia. The authors concluded that factors released from the ischemic placenta can increase the sensitivity of cerebral vessels to changes in blood pressure and lead to changes in the BBB permeability. The authors made a conclusion, that the factors released from the ischemic placenta can increase the sensitivity of cerebral vessels to the changes in blood pressure and lead to the changes in the BBB permeability.
Many authors described, that a possible mechanism of the increased BBB permeability is high levels of pro-inflammatory cytokines in preeclampsia [30–32], effects of some angiogenic and other factors [33–36].
The last fact allows to suppose that the preventive effect of magnesium sulfate on eclampsia, in part, may be associated with stabilization of the BBB. The experimental model showed, that magnesium sulfate actually contributes to reduction in the BBB permeability, decreased neuroinflammatory response, and modulates the activity of excitatory GABA receptors [37].
The increased BBB permeability can lead not only to release of neuropeptides from the brain tissue into the peripheral bloodstream, but also to the penetration of dangerous blood components (proteins, cytokines, antibodies, complement components, etc.) into the brain parenchyma with the development of neuroinflammatory reaction, convulsive syndrome and other severe neurological complications.
The issue of possible development of eclampsia in cases of moderate hypertension or even in The question of the possibility of developing eclampsia in moderate hypertension or even in normotensive patients remains open [38]. It should be noted, that we have never observed seizures on the background of normal blood pressure, however, some authors reported on them. It seems that impaired autoregulation of the cerebral blood flow at lower blood pressure occurs on the background of impaired remodeling of cerebral vessels with primary damage of endothelium due to inadequate response of cerebral vessels to a high or sharp rise in blood pressure, increased BBB permeability, and possible penetration of plasma components into the brain tissue. Ischemic encephalopathy with severe damage of cortical neurons and glial cells undoubtedly contributes to the regulation of brain homeostasis.
High rate of intracerebral hemorrhages in severe preeclampsia/eclampsia (according to some data up to 92% [1]) is largely due to severe vasculopathy (with destructive endotheliosis) of cerebral vessels in combination with thrombocytopenia and sudden changes in blood pressure.
Thus, impaired autoregulation of the cerebral blood flow, severe damage of the endothelium of cerebral vessels and impaired integrity of the BBB, and possible neuroinflammation are closely interrelated pathophysiological mechanisms of cerebrovascular complications of preeclampsia and eclampsia.
Conclusion
Cerebral complications of preeclampsia continue to be a significant cause of maternal morbidity and mortality worldwide. To achieve Sustainable Development Goal 5 of the WHO, aimed at reducing maternal mortality, it is undoubtedly necessary to use interdisciplinary approach to increasing knowledge of vascular changes in the brain in preeclampsia/eclampsia, as well as fundamental scientific and clinical research in this area is required.
It is important to study and develop the tools for predicting cerebrovascular complications in preeclampsia, which are currently not available.
The accumulated data, that confirm the increased risk of cebrovascular diseases in subsequent life of women preeclampsia/eclampsia in history, dictate the need to develop strategies for their prevention.
References
- Miller E.C. Preeclampsia and cerebrovascular disease. Hypertension. 2019; 74(1): 5-13. https://dx.doi.org/10.1161/HYPERTENSIONAHA.118.11513.
- McDermott M., Miller E.C., Rundek T., Hurn P.D., Bushnell C.D. Preeclampsia: Association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018; 49(3): 524-30. https://dx.doi.org/10.1161/STROKEAHA.117.018416.
- Siepmann T., Boardman H., Bilderbeck A., Griffanti L., Kenworthy Y., Zwager C. et al. Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 2017; 88(13): 1256-64. https://dx.doi.org/10.1212/WNL.0000000000003765.
- Basit S., Wohlfahrt J., Boyd H.A. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018; 363: k4109. https://dx.doi.org/10.1136/bmj.k4109.
- Miller E.C., Gatollari H.J., Too G., Boehme A.K., Leffert L., Marshall R.S. et al. Risk factors for pregnancy-associated stroke in women with preeclampsia. Stroke. 2017; 48(7): 1752-9. https://dx.doi.org/10.1161/STROKEAHA.117.017374.
- James A.H., Bushnell C.D., Jamison M.G., Myers E.R. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet. Gynecol. 2005;106(3):509-16. https://dx.doi.org/10.1097/01.AOG.0000172428.78411.b0.
- Grear K.E., Bushnell C.D. Stroke and pregnancy: clinical presentation, evaluation, treatment, and epidemiology. Clin. Obstet. Gynecol. 2013; 56(2): 350-9. https://dx.doi.org/10.1097/GRF.0b013e31828f25fa.
- Hovsepian D.A., Sriram N., Kamel H., Fink M.E., Navi B.B. Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke. 2014; 45(7): 1947-50. https://dx.doi.org/10.1161/STROKEAHA.114.005129.
- Roth J., Deck G. Neurovascular disorders in pregnancy: A review. Obstet. Med. 2019; 12(4): 164‐7. https://dx.doi.org/10.1177/1753495X19825699.
- Bergman L., Torres-Vergara P., Penny J., Wikström J., Nelander M., Leon J. et al. Investigating maternal brain alterations in preeclampsia: the need for a multidisciplinary efort. Curr. Hypertens. Rep. 2019;21(9):72. https://dx.doi.org/10.1007/s11906-019-0977-0.
- Dong X.Y., Bai C.B., Nao J.F. Clinical and radiological features of posterior reversible encephalopathy syndrome in patients with pre-eclampsia and eclampsia. Clin. Radiol. 2017; 72(10): 887-95. https://dx.doi.org/10.1016/j.crad.2017.06.009.
- Sundin C.S., Johnson M.L. Posterior reversible encephalopathy syndrome. MCN Am. J. Matern. Child Nurs. 2018; 43(2): 77-82. https://dx.doi.org/10.1097/NMC.0000000000000409.
- Kutlesič M.S., Kutlesič R.M., Koratevič G.P. Posterior reversible encephalopathy syndrome in eclamptic patients: neuroradiological manifestation, pathogenesis and management. Med. Pregl. 2015; 68(1-2): 53-8. https://dx.doi.org/10.2298/mpns1502053k.
- Trommer B.L., Homer D., Mikhael M.A. Cerebral vasospasm and eclampsia. Stroke. 1988; 19(3):326-9. https://dx.doi.org/10.1161/01.str.19.3.326.
- Jones-Muhammad M., Warrington J.P. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci. 2019; 9(9). pii: E224. https://dx.doi.org/10.3390/brainsci9090224.
- Zeeman G.G., Cipolla M.J., Cunningham F.G. Cerebrovascular (patho)physiology in preeclampsia/eclampsia. In: Roberts J., Cunningham F., Lindheimer M., eds. Chesley’s hypertensive disorders in pregnancy. 3rd ed. Academic Press; 2009: 227-47. https://dx.doi.org/10.1016/B978-0-12-374213-1.00013-6.
- Hammer E.S., Cipolla M.J. Cerebrovascular dysfunction in preeclamptic pregnancies. Curr. Hypertens. Rep. 2015; 17(8): 64. https://dx.doi.org/10.1007/s11906-015-0575-8.
- Cipolla M.J., Pusic A.D., Grinberg Y.Y., Chapman A.C., Poynter M.E., Kraig R.P. Pregnant serum induces neuroinflammation and seizure activity via TNFα. Exp. Neurol. 2012; 234(2): 398-404. https://dx.doi.org/10.1016/j.expneurol.2012.01.005.
- Cipolla M.J., Sweet J.G., Chan S.L. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J. Appl. Physiol. 2011; 110: 329-39. https://dx.doi.org/10.1152/japplphysiol.01159.2010.
- Amburgey O.A., Chapman A.C., May V., Bernstein I.M., Cipolla M.J. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010; 56(5): 1003-8. https://dx.doi.org/10.1161/HYPERTENSIONAHA.110.158931.
- Hulse R.E., Swenson W.G., Kunkler P.E., White D.M., Kraig R.P. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J. Neurosci. 2008; 28(47): 12199-211. https://dx.doi.org/10.1523/JNEUROSCI.3856-08.2008.
- Basavarajappa D.H., Saha P.K., Bagga R., Khandelwal N., Modi M. Neuroradiological perspectives of severe preeclampsia and eclampsia spectrum – Correlation from posterior reversible encephalopathy syndrome. Pregnancy Hypertens. 2020; 20: 119-23. https://dx.doi.org/10.1016/j.preghy.2020.04.003.
- Ijomone O.K., Shallie P., Naicker T. Changes in the structure and function of the brain years after pre-eclampsia. Ageing Res. Rev. 2018; 47: 49‐54. https://dx.doi.org/10.1016/j.arr.2018.06.006.
- Brewer J., Owens M.Y., Wallace K., Reeves A.A., Morris R., Khan M. et al. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am. J. Obstet. Gynecol. 2013; 208(6): 468. e1-468. e4686. https://dx.doi.org/10.1016/j.ajog.2013.02.015.
- Schwartz R.B., Feske S.K., Polak J.F., DeGirolami U., Iaia A., Beckner K.M. et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000; 217(2): 371‐6. https://dx.doi.org/10.1148/radiology.217.2.r00nv44371.
- Hecht J.L., Ordi J., Carrilho C., Ismail M.R., Zsengeller Z.K., Karumanchi S.A., Rosen S. The pathology of eclampsia: An autopsy series. Hypertens. Pregnancy. 2017; 36(3): 259-68. https://dx.doi.org/10.1080/10641955.2017.1329430.
- Raman M.R., Tosakulwong N., Zuk S.M., Senjem M.L., White W.M., Fields J.A. et al. Influence of preeclampsia and late-life hypertension on MRI measures of cortical atrophy. J. Hypertens. 2017; 35(12): 2479-85. https://dx.doi.org/10.1097/HJH.0000000000001492.
- Brunner H., Cockcroft J.R., Deanfield J., Donald A., Ferrannini E., Halcox J. et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005; 23(2): 233-46. https://dx.doi.org/10.1097/00004872-200502000-00001.
- Bergman L., Zetterberg H., Kaihola H., Hagberg H., Blennow K., Åkerud H. Blood-based cerebral biomarkers in preeclampsia: Plasma concentrations of NfL, tau, S100B and NSE during pregnancy in women who later develop preeclampsia – A nested case control study. PLoS One. 2018; 13(5): e0196025. https://dx.doi.org/10.1371/journal.pone.0196025
- Warrington J.P., Fan F., Murphy S.R., Roman R.J., Drummond H.A., Granger J.P. et al. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol. Rep. 2014; 2(8): e12134. https://dx.doi.org/10.14814/phy2.12134.
- LaMarca B.D., Ryan M.J., Gilbert J.S., Murphy S.R., Granger J.P. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr. Hypertens. Rep. 2007; 9(6): 480-5. https://dx.doi.org/10.1007/s11906-007-0088-1.
- Warrington J.P., Drummond H.A., Granger J.P., Ryan M.J. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: role of TNF-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015; 309(11):R1425-31. https://dx.doi.org/10.1152/ajpregu.00372.2015.
- Cindrova-Davies T., Sanders D.A., Burton G.J., Charnock-Jones D.S. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc. Res. 2011; 89(3): 671-9. https://dx.doi.org/10.1093/cvr/cvq346.
- Griessenauer C.J., Chua M.H., Hanafy K.A., Baffour Y.T., Chen R., LeBlanc R.H. et al. Soluble Fms-Like Tyrosine Kinase 1 (sFlt-1) and risk of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2017; 108: 84-9. https://dx.doi.org/10.1016/j.wneu.2017.08.128.
- Cross S.N., Ratner E., Rutherford T.J., Schwartz P.E., Norwitz E.R. Bevacizumab-mediated interference with VEGF signaling is sufficient to induce a preeclampsia-like syndrome in nonpregnant women. Rev. Obstet. Gynecol. 2012; 5(1): 2-8.
- Jiang S., Xia R., Jiang Y., Wang L., Gao F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS One. 2014; 9(2): e86407. https://dx.doi.org/10.1371/journal.pone.0086407.
- Johnson A.C., Tremble S.M., Chan S.L., Moseley J., LaMarca B., Nagle K.J. et al. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLoS One. 2014; 9(11): e113670. https://dx.doi.org/10.1371/journal.pone.0113670.
- Martin J.N. Jr., Thigpen B.D., Moore R.C., Rose C.H., Cushman J., May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet. Gynecol. 2005; 105(2): 246-54. https://dx.doi.org/10.1097/01.AOG.0000151116.84113.
Received 12.03.2021
Accepted 22.04.2021
About the Authors
Iraida S. Sidorova, Dr. Med. Sci., Professor, Academician of the RAS, Merited Scholar of the Russian Federation, Department of Obstetrics and Gynecology № 1,N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), sidorovais@yandex.ru,
https://orcid.org/0000-0003-2209-8662, 119991, Russia, Moscow, Trubetskaya str., 8-2.
Natalya A. Nikitina, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology № 1, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), natnikitina@list.ru, https://orcid.org/ 0000-0001-8659-9963, 119991, Russia, Moscow, Trubetskaya str., 8-2.
Mikhail B. Ageev, Ph.D., Teaching Assistant at the Department of Obstetrics and Gynecology №1, N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), mikhaageev@yandex.ru, https://orcid.org/ 0000-0002-6603-804X, 119991, Russia, Moscow, Trubetskaya str., 8-2.
Albert A. Kokin, Head of Anesthesiology and Intensive Care Unit, Maternity Hospital affiliated to the V.V. Veresaev Clinical Hospital, Moscow City Health Department,
Alberkokin@yandex.ru, 127247, Russia, Moscow, 800th anniversary of Moscow str., 22.
Authors’ contributions: Sidorova I.S., Nikitina N.A. – the concept and design of the study; writing the article; Nikitina N.A.,
Ageev M.B., Kokin A.A. – material collection and examination, statistical data processing; Sidorova I.S. – editing the text
of the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was conducted without any sponsorship.
Gratitude: The authors express gratitude to Prof. Milovanov A.P, MD, Professor of Medicine, for his assistance in conduction of this study.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sidorova I.S., Nikitina N.A., Ageev M.B., Kokin A.A. Cerebrovascular disorders associated with severe preeclampsia and eclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 81-92 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.81-92



