Association of COL1A1, ESR1, and VDR gene polymorphisms with the risk of pelvic floor dysfunction in postpartum women
Mikhelson A.A., Lukianova K.D., Lazukina M.V., Varlamova A.L., Tretyakova T.B., Varaksin A.N., Konstantinova E.D., Maslakova T.A.
Objective: To investigate the influence of collagen type 1 gene polymorphisms, estrogen receptors, and vitamin D receptors on early development of pelvic floor dysfunction in postpartum women.
Materials and methods: This prospective cohort study enrolled 120 women who delivered naturally. The patients were divided into two groups. Group 1 (study group) consisted of 67 women who developed pelvic floor dysfunction, such as pelvic organ prolapse and urinary incontinence, six months postpartum. Group 2 (control group) included 53 women who did not develop pelvic floor dysfunction within six months postpartum. Molecular genetic typing and determination of COL1A1 gene polymorphisms -1997 C>A and 1546 G>T, ESR1 gene -397 T>C and
-351 G>A, and VDR gene 283 A>G and 2 A>G were conducted for all women.
Results: Among patients who developed pelvic floor dysfunction after delivery, carriage of the minor allele G of the ESR1:A-351G polymorphic marker was more common than that in healthy women, where the predominant allele was ESR1:-351 A. The presence of the variant allele -397 C of the ESR1 gene in the homozygous or heterozygous state of the genotype is associated with an increased risk of developing this pathology.
Conclusion: The polymorphism of the ESR1 gene at loci -351 A>G and -397 T>C is associated with the development of pelvic organ prolapse and urinary incontinence 6 months after natural childbirth. No association was found between COL1A1 and VDR gene polymorphisms and the early development of pelvic floor dysfunction after childbirth.
Authors' contributions: Mikhelson A.A., Lukianova K.D., Lazukina M.V. – conception and design of the study; Lukianova K.D. – material collection and processing; Varaksin A.N., Maslakova T.A., Konstantinova E.D. – statistical analysis; Lukianova K.D., Lazukina M.V., Varlamova A.L. – drafting of the manuscript; Mikhelson A.A., Tretyakova T.B., Varaksin A.N. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Mikhelson A.A., Lukianova K.D., Lazukina M.V., Varlamova A.L., Tretyakova T.B., Varaksin A.N., Konstantinova E.D., Maslakova T.A. Association of COL1A1, ESR1, and VDR gene polymorphisms
with the risk of pelvic floor dysfunction in postpartum women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (5): 82-91 (in Russian)
https://dx.doi.org/10.18565/aig.2024.18
Keywords
The issue of pelvic floor disorders is significant because of its prevalence and substantial negative impact on the quality of life of women across all age groups. Pelvic floor dysfunction (PFD) includes urinary incontinence (UI), fecal incontinence, pelvic organ prolapse (POP), and sexual disorders. A combination of these disorders occurs frequently; however, in 25% of cases, a single clinically significant disorder has been identified [1]. Pregnancy and delivery play pivotal roles in PFD development and are the primary risk factors for this pathology [2, 3]. Pregnancy induces anatomical and physiological changes in the pelvic floor with potential progression during the postpartum period [4]. Many authors report obstetric factors contributing to pelvic dysfunction, including delivery of a large fetus, improper head positioning, breech presentation, rapid labor, perineal trauma, and vaginal surgical delivery, particularly involving obstetric forceps [5, 6].
Additionally, there are non-reproductive-related risk factors for PFD development, including age, increased body mass index (BMI), physical inactivity, smoking, and chronic diseases leading to elevated intra-abdominal pressure [7, 8].
According to Artymuk N.V. et al. (2018), after childbirth, 46.6% of women have POPs, 49.7% have urinary problems, and 43.3% have colorectal and anal problems [9]. Diagnosing pelvic floor disorders in the initial stages is challenging because of their asymptomatic nature. Consequently, delayed diagnosis leads to delayed treatment, often resorting to surgical correction years after disease onset [10]. Hence, the identification of diagnostic markers of PFD development after childbirth is crucial.
Currently, researchers focus on identifying molecular genetic predictors of pelvic floor dysfunction formation. Connective tissue pathology significantly influences PFD development, and is the primary structural component of the pelvic floor. Three extensive meta-analyses have underscored the substantial effect of family history on POP development or recurrence, with odds ratios ranging from 1.84 to 2.64 in affected first-degree relatives [11–13]. Familial forms of POP constitute 28% of cases, with autosomal dominant inheritance and high penetrance in such families [14, 15]. Approximately 32 genetic determinants have been described, with polymorphisms causing functional pelvic organ disorders associated with genital prolapse. In 2021, a genome-wide study identified four significant genes that contribute to genital prolapse: ALL1, GDF7, TBX5, and TBX5 [16]. Moreover, a large meta-analysis from 2015 to 2020 in the USA presented candidate genes associated with genital prolapse development, including rs2228480 in the ESR1 gene, rs12589592 in the FBLN5 gene, rs484389 in the PGR gene, and rs1800012 in the COL1A1 gene [17].
Scientific opinions vary regarding the influence of collagen type I and III gene polymorphisms, COL1A1 and COL3A1 as the main structural components of connective tissue, on POP development [18–21]. There is also evidence of the impact of ESR1 gene polymorphism, with a 75% frequency in women with POP and minor forms of undifferentiated connective tissue disease (UCTD) compared to 53% in patients with POP devoid of CTD signs [22]. Furthermore, studies have explored the effects of vitamin D and its deficiency on PFD development. A study by domestic scientists Mekhtieva E.R. et al. (2017) revealed associations between insertion-deletion polymorphisms and polymorphisms, such as varying numbers of tandem repeats of the collagen gene Col3A1, Col1A1 gene polymorphism, and vitamin D receptor gene, and the prediction of pelvic floor incompetence [23].
Most published studies have focused on data from POP and severe UI studies in older patients. Therefore, we aimed to investigate the influence of collagen type 1 gene polymorphisms, estrogen, and vitamin D receptors on the early development of pelvic floor dysfunction in postpartum women.
Materials and methods
This open prospective cohort study included 120 women who underwent natural childbirth between 2020–2021. The patients were divided into two groups. The study group (group 1, n=67) included 67 women who developed PFD in the form of POP and UI 6 months after delivery. The control group (group 2, n=53) included patients who did not develop PFD 6 months after delivery. The study was reviewed and approved by the Research Ethics Committee of the Ural Research Institute for Maternal and Child Care, Ministry of Health of Russia.
All women underwent a molecular genetic study in a genetics laboratory to determine polymorphisms of the genes encoding proteins involved in the formation of connective tissue, including the structure of type I collagen (COL1A1: -197 C>A [rs1107946], COL1A1:1546 G> T [rs1800012]), estrogen receptor gene (ESR1:-397 T>C [rs2234693], ESR1:-351 A>G [rs9340799]), and vitamin D receptor gene (VDR:2 A>G [rs 10735810], VDR:283 G>A [rs1544410]). DNA was isolated from venous blood (0.5 ml of venous blood collected in a test tube with EDTA as an anticoagulant using Proba GS-Genetics reagents (LLC NPO DNA Technology). To estimate the amount of isolated genomic DNA, a set of KVM reagents were used to control the collection of material for the polymerase chain reaction (PCR) method ("DNA-Technology", Russia). At least 1.0 ng of genomic DNA per reaction was used in the study. Genotyping of samples for allelic variants of the studied genes was carried out using allele-specific real-time PCR by recording the melting curves of amplification products. PCR analysis was carried out in automatic mode using the detection amplifier DT-96 ("DNA-Technology", Russia).
To identify the symptoms of POP, all women underwent a gynecological examination at rest and with straining (lying on a gynecological chair). To identify UI symptoms, in addition to the subjective complaints of patients, functional tests were performed to determine the presence or absence of urination disorders, including the cough test and Valsalva maneuver (with a full bladder).
Statistical analysis
Statistical analysis was performed using Excel, SPP Statistics 22.0, Statistica for Windows 10 (TIBCO Software Inc., Palo Alto, CA, USA). For genotype prevalence indicators, counts and percentages were reported, and the χ2 test was used to test statistical hypotheses.
Results and discussion
The ages of the patients in the study groups ranged from 18 to 45 years. There were no significant differences in anthropometric characteristics between the patients in the study groups. Women in both groups had a normal body weight, height, and body mass index (BMI) (Table 1).
All patients in the study groups underwent vaginal delivery without vaginal surgery (vacuum extraction of the fetus and obstetric forceps). Most of the patients who developed PFD were multiparous. An analysis of obstetric risk factors for the development of pelvic dysfunction in the study groups is presented in Table 2. The mean birth weight of the newborns in the group of patients with PFD was significantly higher (3555.08±428.10 g than in group 2 (3393.21±305.1 g (p<0.05).
Results of the molecular genetic typing of polymorphisms of genes regulating the synthesis of type I collagen fibers of connective tissue (COL1A1:-1997 C>A, COL1A1:1546 G>T), alpha estrogen receptor genes (ESR1:-397 T>C; ESR1: -351 A>G), and vitamin D (VDR:283 G>A, VDR:2 A>G), indicated that the nature of the distribution of alleles and genotypes for polymorphic variants of the genes studied in both subgroups was consistent with Hardy–Weinberg equilibrium (Table 3).
The possible influence of genotype on the development of early PFD after childbirth was assessed using general, multiplicative, dominant, and recessive models.
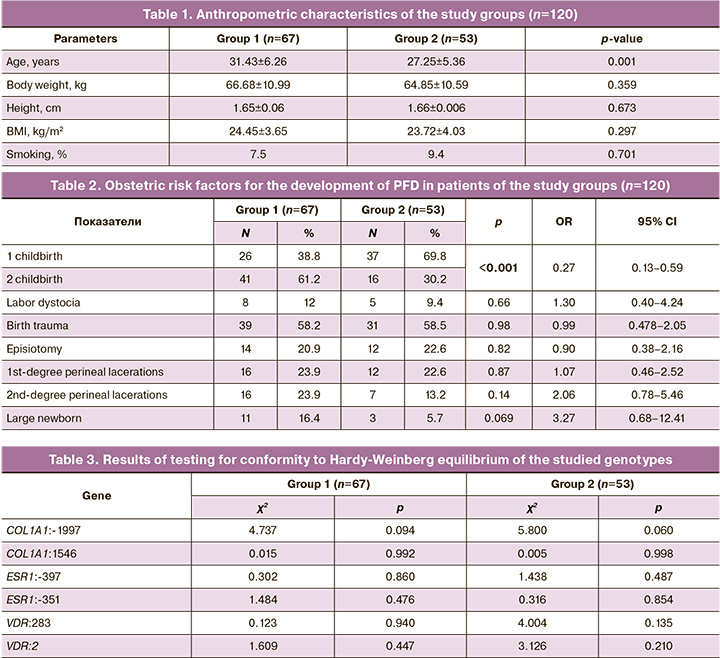
Analysis of the distribution of genotypes by polymorphic markers of the studied genes according to the general model revealed that the estrogen receptor gene variant ESR1:A-351G GG was registered significantly more often in the study group than in the control group (OR=7.36 (95% CI 1.60–33.81); p=0.004). In contrast, genotypes -351 A>G AA (OR=0.40 (95% CI 0.19–0.83); p<0.05) and -397 T>C TT (OR=0.41 (95% CI 0.18–0.90); p<0.05) of the ESR1 gene were significantly more common among healthy women than among patients in the study group (Table 4).
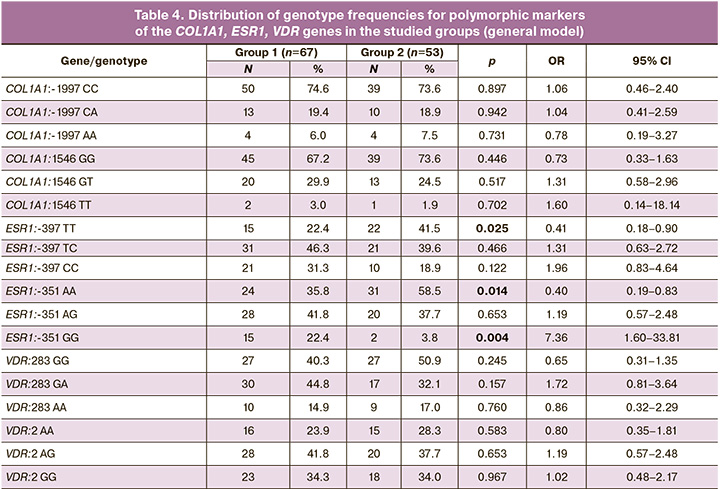
The use of a multiplicative genetic model made it possible to establish that among patients who developed PFD after childbirth, carriage of the minor allele G of the ESR1:A-351G polymorphic marker is more common than among healthy women (OR=2.61 (95% CI 1.48–4.61 ), p<0.001), who have a predominant carriage of the ESR1:-351 A allele (OR=0.38 (95% CI 0.22–0.68), p<0.001). Carriage of the minor allele C of the polymorphic marker ESR1:T-397C was significantly more often found in patients in the study group (OR=1.90 (95% CI 1.13–3.19), p=0.015); women in the control group showed a predominant carriage of the T allele of the polymorphic marker ESR1:T-397C (OR=0.53 (95% CI 0.31–0.89), p=0.015) (Fig. 1).
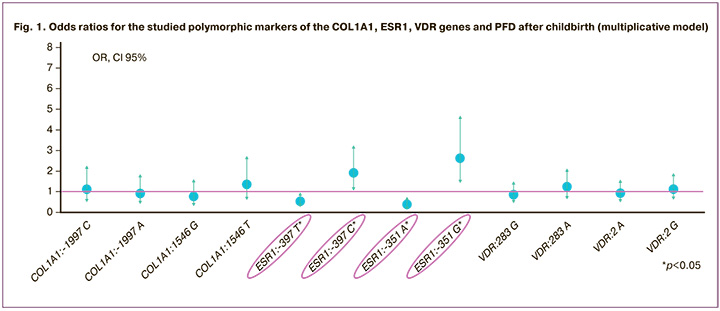
Analysis of the recessive model showed that carriage of the ESR1:-351 GG genotype was more often observed in women with PFD (OR=7.36; (95% CI 1.60–33.81), p=0.004). In healthy women, there were genotypes containing the ESR1:-351 A allele in the homo- or heterozygous state (OR=0.14 (95% CI 0.03–0.63), p=0.004) (Fig. 2).
Since an association between the ESR1:A-351G polymorphism and the development of PFD has been identified, the -351 A>G GG genotype of ESR1 can be regarded as a “risk” genotype for the development of this pathology after childbirth. In contrast, the presence of the variant allele -351 A of the ESR1 gene in a homo- or heterozygous state reduces the chance of developing PFD in women after childbirth.
The results of the analysis of the dominant model of genotype frequency distribution revealed the significance of the ESR1:T-397C polymorphic marker in the risk of developing PFD; the presence in the genotype of the variant allele -397C of the ESR1 gene in a homo- or heterozygous state increases the risk of developing this pathology (OR=2.46 (95% CI 1.11–5.44), p<0.05). The -397 TT genotype of the estrogen receptor gene, on the contrary, provides a protective effect against the development of PFD in women after childbirth (OR=0.41 (95% CI 0.18–0.90), p<0.05). A similar trend was observed in the distribution of genotypes and alleles for ESR1:-351 A>G polymorphism. The presence of the variant allele -351 G of the ESR1 gene in the genotype in a homo- or heterozygous state increased the risk of developing PFD in women after childbirth (OR=2.53 (95% CI 1.20–5.29), p<0.05). In contrast, the presence of the AA genotype of ESR1:A-351G reduced the risk of developing this pathology (OR=0.40 (95% CI 0.19–0.83), p<0.05) (Fig. 3).
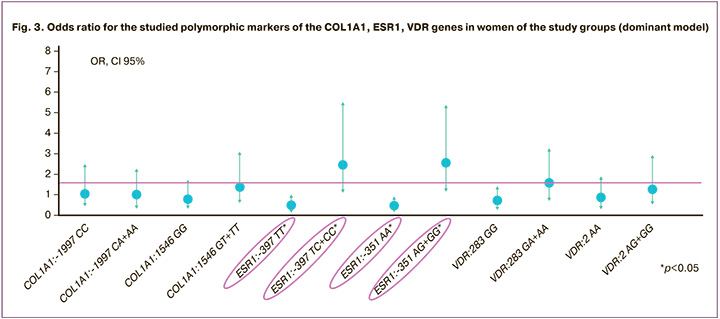
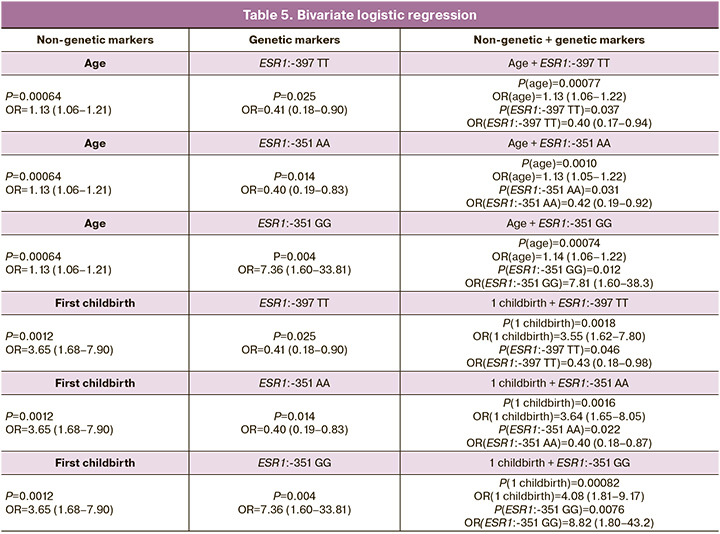
Logistic regression was used to calculate the influence of nongenetic and genetic markers on the development of PFD after childbirth (Table 5). The first column shows the p-values and ORs for the association of non-genetic markers (age and parity) with the development of PFD (univariate logistic regression). According to our calculations, three polymorphic markers were significantly associated with PFD development: ESR1:-397 TT; ESR1:-351 AA; and ESR1:-351 GG. The second column presents the p-values and ORs for the association of genetic markers with the development of this pathology (univariate logistic regression). The third column represents p-values and OR in the logistic regression, containing two indicators: non-genetic and genetic markers (bivariate logistic regression).
This analysis showed that the inclusion of non-genetic markers in logistic regression did not lead to the disappearance of the influence of genetic markers on the development of PFD. Accordingly, genetic polymorphisms that had statistically significant associations with PFD in univariate approximation retained their statistical significance in the presence of statistically significant non-genetic risk factors.
It is believed that PFD in women is a result of UCTD and that pathological changes in the connective tissue contribute more to the development of this pathology than childbirth and obstetric trauma to the pelvic floor. When developing a computer model, the phenotypic signs of UCTD (joint hypermobility, tendency to dislocation, and sprain of the ligaments of the joints) were identified as statistically significant risk factors for predicting PFD in women after childbirth [24]. As a rule, UCTD is of genetic origin, and disruption of the tissue structure leads to pathology in the formation of organs and systems [25].
Type I collagen is a structural component of the vaginal wall, uterosacral ligament, and parietal fascia of the pelvis. In turn, type III collagen in the pelvis predominates in the walls of the large blood vessels and in the loose connective tissue surrounding the vagina and other internal organs. These fibril-forming types of collagen impart elasticity and mechanical strength to the connective tissue and influence the growth and differentiation of cells in the extracellular matrix. Type I collagen is a heterodimer consisting of two α1 chains and one α2 chain encoded by the COL1A1 and COL1A2 genes, respectively. COL1A1 encodes the pro-alpha1 chains of type I collagen, the triple helix of which consists of two alpha1 chains and one alpha2 chain. This gene is located on chromosome 17 (17q21.33) and comprises 51 exons. It is believed that genetic variants in the COL1A1 gene affect the ratio of α1 and α2 collagen chains, which, if altered, can lead to damage to the connective tissue structure and weakening of the pelvic floor.
Collagen fibers play an important role in the formation of the ligamentous apparatus of the pelvic floor. However, domestic research by Vishnevskii L.V. et al. (2020) did not show an association between the COL1A1, COL3A1, and COL18A1 polymorphisms, which are responsible for the synthesis of collagen, and the development of pelvic dysfunction [26].
Our analysis of the distribution of COL1A1 genotypes also did not reveal differences between patients who developed PFD and the control group. We obtained results indicating that the polymorphisms of the COL1A1 gene -1997 C>A and 1546 G>T do not have a significant effect on the development of PFD in women six months after natural birth.
In connective tissue, unlike vaginal epithelial cells, the estrogen receptor content remains constantly high. Therefore, collagen, which is the main structural component, appears to be the estrogen-sensitive component of the pelvic floor [27]. Estrogens increase collagen formation in connective tissues. Estrogen activity also affects other organs and tissues, including the skeletal muscle. Estrogens are small lipophilic substances that can directly enter the nucleus or cytosol of target cells, where they bind to estrogen receptors (ERα and ERβ). The estrogen receptor is a ligand-dependent transcription factor that shares a basic common structure among steroid hormone receptors, as well as the thyroid hormone, retinoic acid, and vitamin D receptors. This gene is located on the long arm of chromosome 6 (6q25.1-q25.2) and contains 22 exons. Estrogen and its receptors may play a role in collagen metabolism and maintain the integrity of the extracellular matrix [28]. The role of estrogen receptors in the development of POP was noted in the results of a study by Lang J.H. et al., where a decrease in their concentration was found in the suspensory ligaments of the pelvic floor in premenopausal women [29]. This provides the basis for studying the effect of polymorphism of the estrogen receptor gene ESR1 on the development of PFD.
Many modern studies have confirmed the influence of estrogen receptor gene polymorphisms on the development of POP. Essentially, the studies are aimed at investigating the molecular genetic nature of the pathology in patients with severe prolapse and its recurrence after surgical treatment. A study by Nakad B. et al (2017) confirmed that ESR1 gene polymorphisms were significantly more common in patients with stage 3–4 POP according to the POP-Q classification than in women in the control group [30]. In addition, based on the results of a study of 196 patients (China, 2020), ESR1 gene polymorphisms rs17847075 and rs2234693 were found to be significantly associated with the risk of developing genital prolapse [31].
The results of our study showed that a predictor of the early development of PFD after childbirth is the carriage of the ESR1 gene polymorphism -397 T>C and -351 A>G, which can be considered as candidate genes for pelvic floor insufficiency.
Support for the association of estrogen receptor gene polymorphisms with POP was found in a large systematic review by Allen-Brady K. et al. (2022), where predictor genes associated with the development of genital prolapse were identified: rs2228480 in the ESR1 gene, rs1800012 in the COL1A1 gene, rs12589592 in the FBLN5 gene of the fibulin protein involved in the synthesis and integration of elastic fibers, and rs484389 in the PGR gene of the progesterone receptor [17].
The hormonally active metabolite of vitamin D, calcitriol 1, 25(OH)2D, has a pleiotropic effect, which is mediated through vitamin D receptors (VDR). The role of vitamin D in the body is not limited to its participation in phosphorus-calcium metabolism. Its receptors are also located in the muscles, intestines, kidneys, heart, mammary gland, pancreas, and the immune system. Estrogens, prolactin, and somatotropic hormones stimulate the synthesis of calcitriol. During growth, pregnancy, and lactation, the need for calcium increases, which leads to an increase in 1α-hydroxylase activity. Reportedly, 62.5% of pregnant women experience vitamin D insufficiency and deficiency [32].
Modern scientists have begun to search for an answer to the question of whether vitamin D and its deficiency influence the development of PFD. A group of researchers in 2022 found that vitamin D deficiency may be an important factor associated with genital prolapse [33]. An analysis of 150 women showed that vitamin D deficiency contributes to a significant decrease in the strength of the pelvic floor muscles, but no significant correlation was found between these conditions [34].
The year 2022 marks 100 years since the discovery of vitamin D, and according to epidemiological studies, its deficiency remains a widespread problem, both in Russia and abroad. In recent years, multicenter cohort studies have reported the role of VDR gene polymorphisms in the development of pathology in various human organs and systems has been studied. The vitamin D receptor is a nuclear receptor of the ligand-activating transcription factor family that induces both genomic and non-genomic regulation of various biological functions, the precise mechanism of which is currently under investigation. VDR is located on the short arm of chromosome 12 (12q13.1) and consists of nine exons and eight introns. It has a high variability of polymorphic sequences that occur in both coding and non-coding regions of the gene, leading to changes in its function due to the activation of gene expression, and less often, protein structure.
Molecular genetic analysis of VDR gene showed that rs7975232 and rs1544410 polymorphisms were significantly associated with PFD in vitamin D deficiency and insufficiency groups [35]. In our study, we did not find an association between the VDR 283 G>A and 2 A>G gene polymorphisms and the early development of PFD in postpartum patients.
Conclusion
The search for molecular genetic markers revealed an association between ESR1 gene polymorphisms at the -351 A>G and -397 T>C loci and the development of POP and UI in women at six months postpartum. There was no association between COL1A1 and VDR gene polymorphisms and early development of postpartum PFD. The discovery of carriage of the rs2234693 and rs9340799 polymorphisms of the candidate gene ESR1, associated with the risk of developing postpartum PFD, allows obstetrician-gynecologists to identify a group at increased risk of this pathology in their practice. This facilitates a personalized approach to postpartum rehabilitation, including additional recommendations for conservative methods, such as the use of vaginal simulators, electrical muscle stimulators, or dynamic quadripolar radiofrequency, in addition to standard postpartum care. Women in this group require mandatory follow-up with an obstetrician-gynecologist at the end of the postpartum period and at 6 and 12 months postpartum. Further research is needed to diagnose and prevent the early development of PFD, with the goal of improving the quality of life of women of reproductive age.
References
- Adjoussou S.A., Bohoussou E., Bastide S., Letouzey V., Fatton B., de Tayac R. Prévalence des troubles fonctionnels et associations anatomo-fonctionnelles chez les femmes présentant un prolapsus génital [Functional symptoms and associations of women with genital prolapse]. Prog. Urol. 2014; 24(8): 511-7. (in French)]. https://dx.doi.org/10.1016/j.purol.2013.11.015.
- Радзинский В.Е., ред. Перинеология. Болезни женской промежности в акушерско-гинекологических, сексологических, урологических, проктологических аспектах. Радзинский В.Е., Дурандин Ю.М., Гагаев Ч.Г., Токтар Л.Р., Марилова Н.А., Тотчиев Г.Ф., Шалаев О.Н. М.: МИА; 2006; 336с. [Radzinsky V.E., ed. Perineology. Diseases of the female perineum in obstetrician-gynecological, sexological, urological, proctological aspects. Radzinsky V.E., Durandin Yu.M., Gagaev Ch.G., Toktar L.R., Marilova N.A., Totchiev G.F., Shalaev O.N. Moscow: MIA; 2006; 336p. (in Russian)].
- Сухих Г.Т., Данилов А.Ю., Боташева Д.А. Роль иммуногистохимических и генетических факторов в уточнении этиологии и патогенеза пролапса гениталий у женщин. Российский вестник акушера-гинеколога. 2012; 12(2): 47-50. [Sukhikh G.T., Danilov A.Yu., Botasheva D.A. Role of immunohistochemical and genetic factors in specifying the etiology and pathogenesis of genital prolapse in women. Russian Bulletin of Obstetrician-Gynecologist. 2012; 12(2): 47-50. (in Russian)].
- Reimers C., Staer-Jensen J., Siafarikas F., Saltyte-Benth J., Bø K., Ellström Engh M. Change in pelvic organ support during pregnancy and the first year postpartum: a longitudinal study. BJOG. 2016; 123(5): 821-9. https://dx.doi.org/10.1111/1471-0528.13432.
- Chi X., Yu S., Zhu K., Chen Y., Chu .Y, Chen X. Influence of different obstetric factors on early postpartum pelvic floor function in primiparas after vaginal delivery. Int. J. Womens Health. 2023; 22(15): 81-90. https://dx.doi.org/10.2147/IJWH.S390626.
- Urbankova I., Grohregin K., Hanacek J., Krcmar M., Feyereisl J., Deprest J. et al. The effect of the first vaginal birth on pelvic floor anatomy and dysfunction Int. Urogynecol. J. 2019; 30(10): 1689-96. https://dx.doi.org/10.1007/s00192-019-04044-2.
- Gillor M., Saens P., Dietz H.P. Demographic risk factors for pelvic organ prolapse: do smoking, asthma, heavy lifting or family history matter? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021; 261: 25-8. https://dx.doi.org/10.1016/j.ejogrb.2021.04.006.
- Kawahara T., Ito H., Yao M., Uemura H. Impact of smoking habit on overactive bladder symptoms and incontinence in women. Int. J. Urol. 2020; 27(12):1078-86. https://dx.doi.org/10.1111/iju.14357.
- Артымук Н.В., Хапачева С.Ю. Распространенность симптомов дисфункции тазового дна у женщин репродуктивного возраста. Акушерство и гинекология. 2018; 9: 99-105. [Artymuk N.V., Khapacheva S.Yu. The prevalence of pelvic floor dysfunction symptoms in reproductive-aged women. Оbstetrics and Gynecology. 2018; (9): 99-105. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.9.99-105.
- Данилина О.А., Волков В.Г. Распространенность пролапса тазовых органов среди женщин репродуктивного возраста. Вестник новых медицинских технологий. 2022; 29(1): 29-33. [Danilina O.A., Volkov V.G. Prevalence of pelvic organ prolapse among women of reproductive age. Journal of New Medical Technologies. 2022; 29(1): 29-33. (in Russian)]. https://dx.doi.org/10.24412/1609-2163-2022-1-29-33.
- Friedman T., Eslick G.D., Dietz H.P. Risk factors for prolapse recurrence: systematic review and meta-analysis. Int. Urogynecol. J. 2018; 29(1): 13-21. https://dx.doi.org/10.1007/s00192-017-3475-4.
- Lince S.L., van Kempen L.C., Vierhout M.E., Kluivers K.B. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int. Urogynecol. J. 2012; 23(10): 1327-36. https://dx.doi.org/10.1007/s00192-012-1704-4.
- Samimi P., Jones S.H., Giri A. Family history and pelvic organ prolapse: a systematic review and meta-analysis. Int. Urogynecol. J. 2021; 32(4): 759-74. https://doi.org/10.1007/s00192-020-04559-z.
- Lince S.L., van Kempen L.C., Vierhout M.E., Kluivers K.B. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int. Urogynecol. J. 2012; 23(10): 1327-36. https://dx.doi.org/10.1007/s00192-012-1704-4.
- Mothes A.R., Radosa M.P., Altendorf-Hofmann A., Runnebaum I.B. Risk index for pelvic organ prolapse based on established individual risk factors. Arch. Gynecol. Obstet. 2016; 293(3): 617-24. https://dx.doi.org/10.1007/s00404-015-3863-2.
- Cox C.K., Pandit A., Zawistowski M., Dutta D., Narla G., Swenson C.W. Genome-wide association study of pelvic organ prolapse using the Michigan Genomics Initiative. Female Pelvic Med. Reconstr. Surg. 2021; 27(8): 502-6. https://dx.doi.org/10.1097/SPV.0000000000001075.
- Allen-Brady K., Chua J.W.F., Cuffolo R., Koch M., Sorrentino F., Cartwright R. Systematic review and meta-analysis of genetic association studies of pelvic organ prolapse. Int. Urogynecol. J. 2022; 33(1): 67-82. https://dx.doi.org/10.1007/s00192-021-04782-2.
- Cartwright R., Kirby A.C., Tikkinen K.A., Mangera A., Thiagamoorthy G., Rajan P. et al. Systematic review and metaanalysis of genetic association studies of urinary symptoms and prolapse in women. Am. J. Obstet. Gynecol. 2015; 212(2): 199.е1-e24. https://dx.doi.org/10.1016/j.ajog.2014.08.005.
- Li L., Sun Z., Chen J., Zhang Y., Shi H., Zhu L. Genetic polymorphisms in collagen-related genes are associated with pelvic organ prolapse. Menopause. 2020; 27(2): 223-9. https://dx.doi.org/10.1097/GME.0000000000001448.
- Ashikari A., Suda T., Miyazato M. Collagen type 1A1, type 3A1, and LOXL1/4 polymorphisms as risk factors of pelvic organ prolapse. BMC Res. Notes. 2021; 14(1): 15. https://dx.doi.org/10.1186/s13104-020-05430-6.
- Устюжина А.С., Солодилова М.А., Полоников А.В., Пахомов С.П., Шокирова У.Г., Матросова А.В. Влияние генов COL1A1 и COL3A1 на пролапс тазовых органов у женщин. Здравоохранение Таджикистана. 2020; 2: 54-61. [Ustyuzhina A.S., Solodilova M.A., Polonikov A.V., Pakhomov S.P., Shokirova U.G., Matrosova A.V. The effect of polymorphisms of the COL1A and COL3A genes on pelvic organ prolapse. Health Care of Tajikistan. 2020; (2): 54-61. (in Russian)].
- Ли Е.С., Каппушева Л.М., Караева К.Ю. Влияние полиморфизмов генов коллагена III типа и рецептора эстрогена альфа на исход хирургической коррекции генитального пролапса. Доктор. Ру. Гинекология. 2015; 11(112): 32-8. [Li E.S., Kappusheva L.M., Karaeva K.Yu. Polymorphisms in collagen type III gene and estrogen receptor alpha gene: impact on outcomes of surgical correction of genital prolapse. Doctor.Ru. Gynecology. 2015; 11(112): 32-8. (in Russian)].
- Мехтиева Э.Р., Ящук А.Г., Зaйнуллина Р.М., Мусин И.И. Роль полиморфизма генов коллагена 1-го и 3-го типов, гена рецепторов витамина Д в возникновении несостоятельности тазового дна у женщин. Практическая медицина. 2017; 7: 102-5. [Mekhtieva E.R., Yashchuk A.G., Zainullina R.M.,
- Musin I.I. Role of polymorphisms of collagen gene 1 and 3 types, gene of vitamin D receptor in the insolvency of pelvic floor in women. Practical Medicine. 2017; (7): 102-5. (in Russian)].
- Суханов А.А., Кукарская И.И. Ранняя профилактика и лечение дисфункции тазового дна. Масштаб заболевания в современном мире. Уральский медицинский журнал. 2018; 6: 107-17. [Sukhanov A.A., Kukarskaya I.I. Early prevention and treatment of pelvic floor dysfunction. The scale of the disease in the modern world. Ural Medical Journal. 2018; (6): 107-17. (in Russian)]. https://dx.doi.org/10.25694/URMJ.2018.04.104.
- Мартынов А.И., Нечаева Г.И., Акатова Е.В., Вершинина М.В., Викторова В.А., Громова О.А. и др. Национальные рекомендации Российского научного медицинского общества терапевтов по диагностике, лечению и реабилитации пациентов с дисплазиями соединительной ткани. Медицинский вестник Северного Кавказа. 2016; 11(1): 2-76. [Martynov A.I. Nechaeva G.I., Akatova E.V., Vershinina M.V., Viktorova V.A., Gromova O.A. et al. National guidelines of the Russian Scientific Medical Society of Therapists for the diagnosis, treatment and rehabilitation of patients with connective tissue dysplasia. Medical News of North Caucasus. 2016; 11(1): 2-76. (in Russian)]. https://dx.doi.org/10.14300/mnnc.2016.11001.
- Вишневский Л.В., Акуленко Л.В., Касьян Г.Р., Тупикина Н.В., Пушкарь Д.Ю. Роль генов коллагеновых белков в развитии дисфункции тазового дна у женщин. Медицинская генетика. 2020; 19(8): 70-1. [Vishnevskii L.V., Akulenko L.V., Kas’yan G.R., Tupikina N.V., Pushkar’ D.Yu. The role of collagen protein genes in the development of pelvic floor dysfunction in women. Medical Genetics. 2020; 19(8): 70-1. (in Russian)]. https://dx.doi.org/10.25557/2073-7998.2020.08.70-71.
- Доброхотова Ю.Э., Нагиева Т.С., Ильина И.Ю., Слободянюк Б.А., Зрагус Е.С., Карева Е.Н., Кочина Н.А., Шахмартова И.А., Краснощок Е.В., Шипилова С.Ю. Влияние эстриола на обмен коллагена в слизистой оболочке влагалища после пластической операции у пациенток послеродового периода. Экспериментальная и клиническая фармакология. 2019; 82(8): 17-21. [Dobrokhotova Yu.E., Nagieva T.S., Il’ina I.Yu., Slobodyanyuk B.A., Zragus E.S., Kareva E.N., Kochina N.A., Shakhmartova I.A., Krasnoshchok E.V., Shipilova S.Yu. The effect of estriol on collagen metabolism in the vaginal mucosa after plastic surgery in postpartum patients. Experimental and Clinical Pharmacology. 2019; 82(8): 17-21. (in Russian)]. https://dx.doi.org/10.30906/0869-2092-2019-82-8-17-21.
- Ikeda K., Horie-Inoue K., Inoue S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 2019; 191: 105375. https://dx.doi.org/10.1016/j.jsbmb.2019.105375.
- Lang J.H., Zhu L., Sun Z.J., Chen J. Estrogen levels and estrogen receptors in patients with stress urinary incontinence and pelvic organ prolapse. Int. J. Gynaecol. Obstet. 2003; 80(1): 35-9. https://dx.doi.org/10.1016/s0020-7292(02)00232-1.
- Nakad B., Fares F., Azzam N., Feiner B., Zilberlicht A., Abramov Y. Estrogen receptor and laminin genetic polymorphism among women with pelvic organ prolapse. Taiwan. J. Obstet. Gynecol. 2017; 56(6): 750-4. https://dx.doi.org/10.1016/j.tjog.2017.10.008.
- Abulaizi A., Abula A., Ababaikeli G., Wan X., Do R., Zhakeer A. Identification of pelvic organ prolapse risk susceptibility gene SNP locus in Xinjiang women. Int. Urogynecol. J. 2020; 31(1): 123-30. https://dx.doi.org/10.1007/s00192-019-04039-z.
- Полуэктова А.Ю., Мартынова Е.Ю., Фатхутдинов И.Р., Демидова Т.Ю., Потешкин Ю.Е. Генетические особенности чувствительности к витамину D и распространенность дефицита витамина D среди пациентов поликлиники. РМЖ. Мать и дитя. 2018; 1(1): 11-7. [Poluektova A.Yu., Martynova E.Yu., Fatkhutdinov I.R., Demidova T.Yu., Poteshkin Yu.E. Genetic features of sensitivity to vitamin D and the prevalence of vitamin D deficiency among clinic patients. RMJ. Mother and Child. 2018; 1(1): 11-7. (in Russian)]. https://dx.doi.org/10.32364/2618-8430-2018-1-1-11-17.
- Legan M., Barbič M., Osredkar J., Blaganje M. Association of vitamin D deficiency and pelvic organ prolapse in postmenopausal women: a cross-sectional study. Womens Midlife Health. 2022; 8(1): 9. https://dx.doi.org/10.1186/s40695-022-00078-7.
- Aydogmus H., Demirdal U.S. Vitamin D deficiency and lower urinary tract symptoms in women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 228: 48-52. https://dx.doi.org/10.1016/j.ejogrb.2018.06.009.
- Ahn J.H., Noh Y.H., Um K.J., Kim H.S., Cho S. Vitamin D status and vitamin D receptor gene polymorphisms are associated with pelvic floor disorders in women. J. Menopausal Med. 2018; 24(2): 119-26. https://dx.doi.org/10.6118/jmm.2018.24.2.119.
Received 26.01.2024
Accepted 24.04.2024
About the Authors
Anna A. Mikhelson, Dr. Med. Sci., Associate Professor, Head of the Scientific Department for the Preservation of Reproductive Function, Head of the Gynecological Department, Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, 620028, Russia, Yekaterinburg, Repina str., 1, ann_tolmik@mail.ru, https://orcid.org/0000-0003-1709-6187Ksenia D. Lukianova, Obstetrician-Gynecologist, Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, 620028, Russia, Yekaterinburg, Repina str., 1, k.d.lukianova@mail.ru, https://orcid.org/0000-0001-7142-8547
Maria V. Lazukina, PhD, Researcher, Obstetrician-Gynecologist, Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia,
620028, Russia, Yekaterinburg, Repina str., 1, masha_balueva@mail.ru, https://orcid.org/0000-0002-0525-0856
Anastasia L. Varlamova, PhD Student, Obstetrician-Gynecologist, Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, 620028, Russia, Yekaterinburg, Repina str., 1, https://orcid.org/0009-0008-7703-4248
Tatyana B. Tretyakova, PhD, Senior Researcher at the Genetic Research Group, Biochemical Research Methods Division, Laboratory Geneticist, Urals Scientific Research Institute for Maternal and Child Care, Ministry of Health of Russia, 620028, Russia, Yekaterinburg, Repina str., 1, https://orcid.org/0000-0002-5715-7514
Anatoly N. Varaksin, Dr. Sci. (Physics and Mathematics), Professor, Chief Researcher at the Laboratory of Mathematical Modeling in Ecology and Medicine, Institute of Industrial Ecology, Ural Branch of the Russian Academy of Sciences, 620108, Russia, Yekaterinburg, Sofia Kovalevskaya str., 20, varaksin@ecko.uran.ru,
https://orcid.org/0000-0003-2689-3006
Ekaterina D. Konstantinova, PhD, Senior Researcher, Head of the Laboratory of Mathematical Modeling in Ecology and Medicine, Institute of Industrial Ecology, Ural Branch of the Russian Academy of Sciences, 620108, Russia, Yekaterinburg, Sophia Kovalevskaya str., 20, https://orcid.org/0000-0002-2260-744X
Tatyana A. Maslakova, PhD, Researcher at the Laboratory of Mathematical Modeling in Ecology and Medicine, Institute of Industrial Ecology, Ural Branch of the Russian Academy of Sciences, 620108, Russia, Yekaterinburg, Sofia Kovalevskaya str., 20, https://orcid.org/0000-0001-6642-9027



