Association between the 657T>C polymorphism of the chromosome segregation gene SYCP3 and idiopathic recurrent pregnancy loss in the Kazakh population
Aim. To investigate the association between the 657T>C polymorphism of the synaptonemal complex SYCP3 gene (rs769825641) and the development of idiopathic recurrent pregnancy loss (iRPL) in ethnically homogeneous Kazakh population.Svyatova G.S., Berezina G.M., Murtazalieva A.V.
Material and methods. Three thousand two patients with iRPL and 300 women with normal reproductive function underwent independent replicative TagMan genotyping.
Results. The study findings support the association between the studied polymorphism and the development of iRPL in the Kazakh population. The frequency of carrying the unfavorable C allele in the study group (3,97%) was significantly higher than in women with normal reproduction function (2,0%) (χ2 = 4, 04; p < 0.05).
Conclusions. Synaptonemal complex proteins play an important role in regulating meiosis in iRPL, suggesting the possibility of considering the studied polymorphism as a possible genetic risk factor for iRPL of unknown cause.
Keywords
Recurrent pregnancy loss (RPL) is the most common female reproductive problem that is defined as the spontaneous loss of two or more pregnancies [1-3]. RPL affects 0.5–3% of married couples and can be caused by various factors including chromosomal or anatomical abnormalities, infections, and immunological disorders. It also may be of unknown origin [1–4] termed as idiopathic RPL (iRPL).
Most researchers consider RPL polygenic disorder associated with both genetic and environmental factors, but the monogenic etiology of RPL is not excluded. As a candidate gene, the SYCP3 gene was studied, which encodes the synaptonemal complex proteins (OMIM 270960) mediating correct segregation and recombination of chromosomes during meiosis I or II. An impairment of this process is associated with azoospermia, high aneuploidy rates, and a decrease in the oocyte pool [5–7].
It was shown that the carriage of a 657T > C mutation in SYCP3 gene frequently leads to chromosomal abnormalities of the embryo and causes RPL [5, 6].
Due to the complexity of meiosis, many diverse genes involved in this process, and the low prevalence of the 657T>C polymorphism of the SYCP3 gene, some researchers have expressed doubts that a mutation in one gene may be associated with RPL in many patients [8].
Mizutani E. et al. [9] noted that future studies are needed to understand the molecular mechanisms involved in RPL and provide additional candidate genes to be screened in recurrent miscarriage patients and embryos with genetic factors.
Since the results of population studies of adverse polymorphisms vary depending on racial and geographical factors, we studied an ethnically homogeneous population of Kazakhs. To exclude the possible additive effects of many factors known to be associated with RPL, we selected a group of women with recurrent pregnancy loss of unknown cause.
This study aimed to investigate the population prevalence and possible genetic contribution of the 657T>C polymorphism of the synaptonemal complex SYCP3 gene (rs769825641) to the development of iRPL in an ethnically homogeneous Kazakh population.
Materials and methods
This was a prospective study conducted at the Outpatient Department of the Research Center for Obstetrics, Gynecology, and Perinatology (RC for OG&P) and the Medical Center for Molecular Medicine. The study was conducted with the informed consent of participating women to the use of their blood samples and medical history data and was approved by the Research Ethics Committee of RC for OG&P.
The study group comprised 302 women with iRPL of Kazakh nationality aged 18–45 years who had a history of spontaneous loss of two or more pregnancies prior to 12 completed weeks of gestational age. The control group included 300 Kazakh women with normal reproductive function, having at least one child, without a history of spontaneous of RPL.
Inclusion criteria for the study group of iRPL patients were as follows: Kazakh nationality by grandparents on the maternal and paternal side; age 18–45 years; a history of two or more early spontaneous miscarriages; the pregnancy confirmed by ultrasound and/or a pregnancy hormone test.
Exclusion criteria included luteal phase insufficiency detected by endometrial biopsy, anatomical uterine abnormalities detected by ultrasound, hysterosalpingography, hysteroscopy or sono-hysteroscopy, carriage of balanced chromosomal abnormalities by karyotyping of both spouses, the presence of antiphospholipid syndrome (APS), confirmed by cardiolipin antibody test (IgM/IgM ), lupus anticoagulant; sexually transmitted infections confirmed by two different analyzes of biological materials (enzyme-linked immunosorbent assay, polymerase chain reaction), thyroid dysfunction diagnosed by TSH and thyroid antibodies test.
The study material of the population control group was DNA from 700 conditionally healthy individuals of Kazakh nationality that was stored in the Miras Biobank of the RC for OG&P. The Miras Biobank was established in the EU 7th Framework project «Genetic studies of pre-eclampsia in Central Asian and European populations» under grant agreement No. 282540.
The Miras DNA biobank is designed to store DNA samples. It contains genomic and clinical information about more than 10,000 individuals of Kazakh nationality. All subjects were informed of the objectives of the project and signed informed consent to participate. Criteria for selection in the population control group were Kazakh ethnicity, including grandparents; age 18 years and older; the ability of the subject to make an independent decision to participate in the project. Exclusion criteria were a medical history of hypertension, stroke, type 1 or 2 diabetes requiring medical treatment confirmed by medical documents.
DNA was isolated by separating M-PVA magnetic particles on a Prepitto automatic analyzer (PerkinElmer) to isolate ChemagicPrepito nucleic acids (Wallac, Finland) using the PrepitoDNACytoPure reagent kit.
Molecular genetic analysis was conducted using TaqMan with site-specific amplification and real-time genotyping (Real-Time PCR) using the TestGen test system (TestGen, Russia) for molecular genetic studies.
Statistical analysis was performed using the PLINK software [10]. Testing the association between the carriage of polymorphic gene variants and the risk of developing RPL was done by the Chi-square (χ2) test with the calculation of the odds ratio (OR) with 95% confidence interval (95% CI). Statistical significance was assumed at p < 0.05.
Statistical analysis using the PLINK program includes the calculation of associative interactions between the studied groups based on various models: allelic (ALLELIC), genotypic (GENO), additive (TREND), dominant (DOM) and recessive (REC).
The deviation from the Hardy-Weinberg equilibrium was verified using the PLINK software based on calculations of the observed (Ho) and expected (He) heterozygosity [10].
Results
The SYCP3 gene (rs769825641, 657T> C) is located on the long arm of chromosome 12 (12q23.2); it encodes the synaptonemal complex protein 3, the physical distance in base-pair position is101729109 [11].
We investigated characteristic features of the distribution of frequencies of genotypes and alleles of the SYCP3 657T>C polymorphism in the Kazakh population in the population control. The population frequencies of the SYCP3 gene polymorphism alleles were calculated for 700 DNA samples. According to the National Center for Biotechnology Information [11], in many world populations, the unfavorable C allele was not found in population samples from 15,000 to 130,000; the data are presented in Table 1.
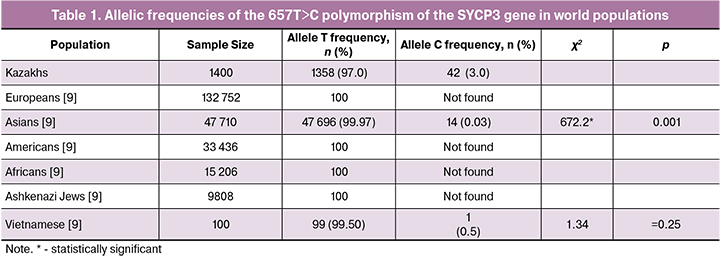
The frequency of TT genotype in the population control was 94.0%, the heterozygous ST genotype was 6.0%, the T allele was 97.0%, the unfavorable C allele in the SYCP3 gene (657T> C) in the Kazakh population was 3.0%, which turned out to be 3.0% significantly higher (p <0.001) compared with the same indicator for Asian populations (0.03%) [11] (Table 1).
The frequencies of the 657T>C polymorphism of the SYCP3 gene calculated using the PLINK program showed the observed (0.113) and expected (0.144) heterozygosity and discrepancy from Hardy-Weinberg Equilibrium (р<0.001). This discrepancy may be attributable to insufficient sample size for this polymorphism and historical patterns of the formation of the gene pool of the Kazakh population.
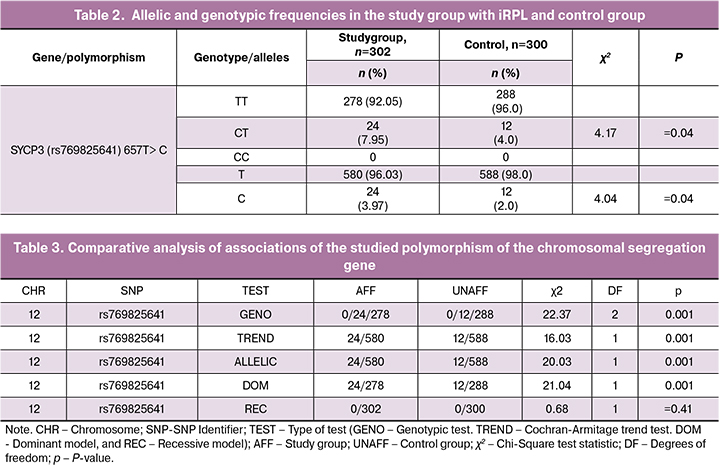
A comparative analysis of allelic and genotypic frequencies of SYCP3 (rs769825641) 657T> C polymorphism among patients with iRPL and control subjects is presented in Table. 2. As seen from Table 2, the prevalence of the unfavorable C allele in the study group was 4.0%, which was twice as high as that among women with normal reproduction (2.0%) (χ2 = 4.04; p = 0.04). It should be noted that a homozygous unfavorable genotype С657С was not found in the studied sample, which is probably due to the low prevalence of this allele in the Kazakh population. In the iRPL group, the favorable homozygous T657T genotype was significantly less common (92.0%), while the frequency of the unfavorable heterozygous C657T genotype was significantly higher (7.95%). These frequencies were significantly different from that among women with normal reproduction (96.0 and 4.0 %, respectively) (χ2 = 4.17; p = 0.04), which was confirmed by OR of 2.1; 95% CI (1.02-4.22).
Our results are consistent with data reported by Bolor H. et al. [5], who found that 3% out of 26 Japanese women with RPL carried heterozygous C657T genotype of the SYCP3 gene, the carrier frequency of which in the main group was 3.0%, which was present among a group of 150 fertile women. This observation allowed the authors to suggest that the C657 mutation in the gene of the synaptonemal complex SYCP3 can be considered as a predisposing genetic factor for RPL.
Similar findings were published in a study investigating T657C polymorphism of the SYCP3 gene in 100 Iranian women with iRPL and 100 normal fertile women having at least one healthy child. The frequency of the unfavorable heterozygous genotype (9.0%) was significantly higher in women with RPL than in the control group (4%) (p <0.005) [7].
However, Mizutani E. et al. [9], who studied the SYCP3 657T>C mutation in 101 patients with RPL and 82 fertile controls, reported that SYCP3 657T>C mutation was identified in one patient with RPL and one fertile woman. An analysis of abortion karyotypes showed that patients with a mutation carrier and a normal genotype did not have significant differences in the frequency of chromosomal abnormalities in abortion material, which suggests no association of this mutation with RPL caused by aneuploidy.
Associations of the SYCP3 gene polymorphism evaluated by various tests are presented in Table. 3.
Statistical analysis using the PLINK program involves calculating associations based on various models. The allelic model (ALLELIC) is based on an assessment of the strength of the association of allelic frequencies, is the simplest test that does not take into account the common genotype of two chromosomes, therefore we used more accurate models of genotypic tests. The genotypic test (GENO) of associations of SNP polymorphisms with iRPL risk is based on using the frequencies of three possible genotypes in the main and control groups. The unit of calculation is not an allele, but three possible genotypes with df = 2. According to the conditions of the additive (TREND trend) model, the presence of two copies of the minor allele in the homozygous unfavorable genotype is more strongly associated with the development of iRPL than the presence of one allele in the heterozygous genotype. The additive mathematical model is based on the rule that the more copies of the minor allele are in the study group, the greater the adverse effect on the risk of developing iRPL have heterozygotes with phenotypes lying between two homozygotes. This test has 1 df and is known as the Cochran-Armitage Trend Test.
The Dominant Model (DOM) suggests that disease only appears if there is at least one copy of an unfavorable allele. All subjects are classified into two groups, depending on whether or not there is a minor allele; the dominant test has 1 df. The recessive model (REC) suggests that the effect on the phenotype is manifested only if the subject has two copies of the minor allele; the number of degrees of freedom is 1. The most significant, if we are not sure of the genetic model of the association between the genotype and phenotype, is the additive model, which is less based on the principle of inheritance, but is statistically less effective due to the additional degree of freedom.
Table 3 summarizes the results of a comparative analysis of the associations of the studied polymorphism with iRPL based on the use of several models with statistical significance assumed at p ≤0.05 for multiple testing.
As seen from Table 3, statistically significant iRPL associations were found for the studied polymorphism of the chromosome segregation gene, which implies a specific relationship between the genotype and phenotype, including statistically significant differences in the genotypic, additive (trend), dominant and allelic models (p <0.001).
No significant associations were identified using the recessive model, which suggests a random trend associated with a low frequency of an unfavorable minor allele in this polymorphism, which amounted to 4.0%.
Thus, our results indicate the possible significance of the 657T>C polymorphism of the SYCP3 chromosomal segregation gene in the development of iRPL. Carriage of the unfavorable C allele is associated with a 2-fold higher risk of iRPL development by a factor of 2, as evidenced by the odds ratio [OR = 2.03; 95% CI (1.0-4.09)].
Discussion
The genetic aspects of iRPL are still not fully understood; replicative studies of the 657T>C polymorphism of the SYCP3 chromosome segregation gene in various ethnic populations are few and inconclusive. Possible explanations include differences in the iRPL recruiting criteria, the small sample sizes, ethnicity, and the extremely low population prevalence of heterozygous genotypes [7, 12, 13].
The prevalence of carrying the unfavorable C allele in the SYCP3 gene (rs769825641) in the Kazakh population was significantly higher (p <0.0001) than in other Asian populations [11].
A comparative analysis of allelic and genotypic frequencies of SYCP3 (rs769825641) 657T> C polymorphism among 302 women with iRPL and 300 normal fertile women showed significant differences with higher rates of the unfavorable C allele in the group with iRPL (3.97%) compared with the control subjects ( 2.0%). The frequencies of the heterozygous CT genotype in patients with iRPL and fertile women were 8.0% and 4.0%, respectively (p = 0.042).
The results obtained in an ethnically homogeneous Kazakh population indicate the possible genetic contribution of the polymorphism (657T> C) of the SYCP3 chromosome segregation gene to the etiology of iRPL, which was confirmed by a statistically significant odds ratio of 2.03.
Unfortunately, due to the extremely low prevalence of carriage of the SYCP3 657C mutation in most human populations, there is no clinical utility of screening families with RPL for the carriage of this mutation.
Conclusion
Based on the study findings, it can be logically concluded that synaptonemal complex proteins play an important role in regulating meiosis in iRPL, suggesting the possibility of considering the studied polymorphism as a possible genetic risk factor for iRPL of unknown cause. Due to the complexity of meiosis and numerous different genes involved in this process, a mutation in only one gene seems unlikely to cause recurrent miscarriage in many patients. Further studies are needed to explore other polymorphisms of the meiotic recombination genes.
References
- Pfeifer S., Goldberg J., Lobo R., Thomas M., Widra E., Licht M., Collins J., Cedars M., Vernon M., Davis O., Gracia C., Catherino W., Thornton K., Rebar R., La Barbera A. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility and Sterility. 2013; 99(1): 63.doi: 10.1016/j.fertnstert.2012.09.023.
- Сидельникова В.М. Невынашивание беременности – современный взгляд на проблему. Акушерство и гинекология. 2007; 5: 4–27. [Sidelnikova V.M. Miscarriage: thepresentviewoftheproblem.AkusherstvoiGinekologiya/Obstetricsandgynecology. 2007; 5: 4–27.(in Russian)]
- Беспалова О.Н. Генетика невынашивания беременности. Журнал акушерства и женских болезней. 2007; LVI(1): 81. [Bespalova O.N. Genetics of pregnancy miscarriage. Zhurnal akusherstva i zhenskih boleznej. 2007; LVI(1): 81. (in Russian)].
- Branch D.W., Gibson M., SilverR.M. Clinical practice. Recurrent miscarriage. N. Engl. J. Med. 2010; 363(18): 1740–7. doi: 10.1056/NEJMcp1005330.
- Bolor H., Mori T., Nishiyama S., Ito Y., Hosoba E., Inagaki H., et al. Mutations of the SYCP3 gene in women with recurrent pregnancy loss. Am. J. Hum. Genet. 2009; 84(1): 14–20. doi: 10.1016/j.ajhg.2008.12.002.
- Miyamoto T., Hasuike S., Yogev L., Maduro M.R., Ishikawa M., Westphal H., et al. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet. 2003; 362(9397): 1714–9.doi: 10.1016/S0140-6736(03)14845-3
- Sazegari A., Kalantar S.M., Pashaiefar H., Mohtaram S., Honarvar N., Feizollahi Z., Ghasemi N.The T657C polymorphism on the SYCP3 gene is associated with recurrent pregnancy loss. J Assist Reprod Genet. 2014; 31(10): 1377–81. doi: 10.1007/s10815-014-0272-6
- Hanna C.W., Blair J.D., Stephenson M.D,. Robinson W.P. Absence of SYCP3 mutations in women with recurrent miscarriage with at least one trisomic miscarriage. Reprod. Biomed. Online. 2012; 24(2): 251–3. doi: 10.1016/j.rbmo.2011.10.013.
- Mizutani E., Suzumori N., Ozaki Y., Oseto K., Yamada-Namikawa C., Nakanishi M., et al. SYCP3 mutation may not be associated with recurrent miscarriage caused by aneuploidy. Human Reprod (Oxford, England). 2011; 26(5): 1259–66. doi: 10.1093/humrep/der035.
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81(3): 559–75. doi: 10.1086/519795.
- National Center for Biotechnology Information.https://www.ncbi.nlm.nih.gov/snp/rs769825641#frequency_tab
- Martínez J., Bonache S., Carvajal A., Bassas L., Larriba S.Mutations of SYCP3 are rare in infertile Spanish men with meiotic arrest. FertilSteril. 2007; 88(4): 988–9.doi: 10.1016/j.fertnstert..11.163 ·
- Gurkan H., Aydin F., Kadıoglu A., Palanduz S. Investigation of mutations in the synaptonemal complex protein 3 (SYCP3) gene among azoospermic infertile male patients in the Turkish population. Andrologia. 2013; 45(2): 92–100. doi: 10.1111/j.1439-0272.2012.01317.x
Received 06.09.2019
Accepted 04.10.2019
About the Authors
Gulnara S. Svyatova, MD, professor, head of the Republican Medical and Genetic Consultation of the Scientific Center of Obstetrics, Gynecology and Perinatology Joint Stock Company, ORCID ID 0000-0001-5092-3143, A25D6G4, 125 Dostyk Ave., Almaty, Kazakhstan; tel.: +7 (727) 300-45-61, e-mail: gsvyatova1@mail.ruGalina M. Berezina, Doctor of Biological Sciences, Associate Professor, Specialist of the Laboratory, Republican Medical Genetic Consultation of the Scientific Center of Obstetrics, Gynecology and Perinatology, JSC, ORCID ID 0000-0002-5442-4461, A25D6G4, 125 Dostyk Ave., Almaty, Kazakhstan;
tel.: +7 (727) 300-45-62, e-mail: gberezina54@mail.ru
Alexandra V. Murtazalieva, geneticist of the Republican Medical Genetic Consultation of the Scientific Center of Obstetrics, Gynecology and Perinatology Joint-Stock Company, ORCID ID 0000-0001-9156-5944, A25D6G4, Dostyk Ave. 125, Almaty, Kazakhstan; tel.: +7 (727) 300-45-62, e-mail: alexmurtazalieva@gmail.com
For citation: Svyatova G.S., Berezina G.M., Murtazalieva A.V. Association between the 657t>c polymorphism of the chromosome segregation gene SYCP3 and idiopathic recurrent pregnancy loss in the Kazakh population.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2019; 12: 105-10. (In Russian).
https://dx.doi.org/10.18565/aig.2019.12.105-110



