Placental angioarchitecture in monochorionic twin pregnancies with twin-twin transfusion syndrome. Perinatal outcomes
Objective. To investigate the role of arteriovenous (AV), arterioarterial (AA) and venovenous (VV) anastomoses in the development and progression of twin-twin transfusion syndrome (TTTS).Bugerenko A.E., Sukhanova D.I., Donchenko Ya.S., Panina O.B., Sichinava L.G.
Material and methods. Seventy-four patients with TTTS underwent laser photocoagulation of placental anastomoses. The number and types of anastomoses were analyzed. The patients were divided into 2 groups based on perinatal outcomes: group 1 (n = 54) with at least one surviving twin and group 2 (n = 20) with no surviving twins. The imbalance in blood flow between the twins was evaluated.
Results. The number of large AA and VV anastomoses in the two groups was not significantly different, but they differed in the number of large AV anastomoses (p = 0.03).
Conclusion. In patients with TTTS, the presence of more than three AV placental anastomoses predicts adverse perinatal outcomes. AA and VV anastomoses do not play a protective role and are not associated with TTTS severity.
Keywords
In recent decades, there has been a significant increase in the incidence of multiple pregnancies. Multiple, especially monochorionic, pregnancies are strongly associated with major obstetrics complications and greater maternal morbidity [1, 2]. One of the most serious complications associated with monochorionic placentation is twin-twin transfusion syndrome (TTTS), which occurs in 10–35% of cases [2]. TTTS usually manifests in the second trimester at 15–25 weeks of gestation. If progressive TTTS left without intrauterine treatment, 80–100% of pregnancies would result in adverse fetal outcomes [3].
Placental angioarchitecture plays a proven role in the development of TTTS because of inter-twin vascular anastomoses that connect the 2 fetal circulation systems. Due to hemodynamic imbalance, blood is transferred disproportionately from one twin (the “donor”) to the other (the “recipient”), which causes the donor twin to have decreased urinary output, oligohydramnios, and hypotension, with the possible development of anemia, selective fetal growth restriction and anhydramnios. In contrast, the recipient develops polyuria, polyhydramnios, hemoconcentration, arterial hypertension and heart failure.
There are three types of placental anastomoses: arteriovenous (AV), arterioarterial (AA) and venovenous (VV), which are located on the placental plate and connect the twins’ umbilical cords [4]. AV anastomoses play a major role in the pathogenesis of TTTS, while the role of AA and VV anastomoses remains unclear and is actively debated in the scientific literature [5, 6]. The blood flow through the AV anastomoses is unidirectional, the artery and vein of the two fetuses are connected through common cotyledon, and blood flows from artery to vein [7].
The direction of blood flow in the AA and VV anastomoses depends on the pressure gradient in the fetal circulatory systems; some researchers suggest that these anastomoses can compensate for unbalanced blood flow through the AV anastomoses. The possible protective role of AA anastomoses in the pathogenesis of TTTS is also attributed to the fact that they are more often present in monochorionic placentas not complicated by TTTS [6]. At the same time, placental VV anastomoses are more often detected in pregnancies complicated by TTTS [8]. According to the literature, the presence of VV anastomoses is an independent risk factor for the development and progression of TTTS in the absence of placental AA anastomoses [9].
Currently, the only pathogenetic treatment for TTTS is fetoscopic laser photocoagulation (FLP). The procedure is performed at more than 15–16 weeks’ gestation in patients with stage II-IV TTTS by Quintero staging system or in cases of acutely increasing polyhydramnios in patients with Quintero stage I TTTS [10, 11]. There are three modifications of FLP: nonselective laser coagulation with the coagulation of all placental anastomoses, selective laser coagulation, and the Solomon technique that involves initially completing coagulation of all anastomoses and then coagulation to connect the anastomoses’ ablation sites from one edge of the placenta to the other.
However, it is sometimes difficult to observe all of the anastomoses on the surface of the placenta by fetoscopy [12]. The literature reported on “partially hidden” AA and VV anastomoses that are partially located under the vascular equator inside the chorionic plate [13].
The study aimed to investigate the role of arteriovenous, arterioarterial, and venovenous placental anastomoses in the development and progression of twin-twin transfusion syndrome in monochorionic twin pregnancies.
Materials and methods
We analyzed the course and outcomes of 74 patients with monochorionic twin pregnancies complicated by Quintero stage II – III TTTS who underwent FLP at 17 to 25 weeks’ gestation. FLP of placental anastomoses and patients’ follow-up were carried out from 2015 to 2017at the Center for Family Planning and Reproduction of the Moscow City Health Department (Head Physician – Ph.D. O.A. Latyshkevich) and the Lapino Clinical Hospital (Head - Academician of the RAS M.A. Kurtser). The age of patients ranged from 18 to 39 years. Among the study participants, 50 (68%) were primiparous, of which 43 (86%) were primigravida; 11 (14%) women were multiparous. Pregnancy occurred spontaneously in 51 patients, 11 women achieved pregnancy with ovulation stimulation, and 12 after IVF and embryo transfer.
Depending on the perinatal outcomes, the patients were divided into two groups:
- group 1 - favorable perinatal outcome, that is, the birth of at least one living child (n = 54);
- group 2 - adverse perinatal outcome - the death of both twins (n = 20).
The indication for fetoscopic laser photocoagulation of placental anastomoses was the presence of stage II - III TTTS by the Quintero classification [10]. Ultrasound examination and Doppler assessment of the placental and fetal circulations were performed using a Siemens Antares expert-class ultrasound machine.
FLP procedures were performed with equipment and instruments from Karl Storz, Dornier YAG laser, using an 11F introducer. The coagulation of the placental anastomoses was performed with a surgical laser.
FLP was performed under epidural anesthesia and continuous ultrasound guidance, an 11F introducer was percutaneously inserted through the anterior abdominal wall into the uterine cavity followed by a fetoscope insertion; the fetal surface of the placenta, parts of the fetus, the amniotic septum was examined. Before coagulation, the interfetal septum was visualized and all vessels crossing it was carefully monitored. The course of the vessels was viewed as far as possible up to the zone of anastomoses; attention was paid not only to the number of anastomoses but also to their type (arterioarterial, venovenous, arteriovenous) and the diameter. The specific type of anastomosis was determined by its morphological signs: the artery is dark blue, the veins are bright red. Qualitatively, the size of vessels (large or small) was determined by matching the size of the vessel to the size of the optical fiber of known diameter (0.2 mm): vessels with diameters smaller or equal to the diameter of the optical fiber were classified as small; vessels with twice as big diameters were considered large. The procedure was completed by amnioreduction to the maximal depth of the vertical pocket of the amniotic fluid according to given gestational age. 10–15 minutes after the extraction of the introducer, an ultrasound was performed to exclude bleeding from the access and maternal and fetal complications [14].
The total number of AV, AA and VV placental anastomoses was calculated using FLP records.
After birth, all placentas were examined for residual anastomoses. To do this, immediately after placental expulsion, all the vessels of one of the umbilical cords were filled with dye (milk) that filled small vessels on the surface of the placenta providing clear visualization of all angioarchitecture of the first umbilical cord. In the presence of residual anastomoses (superficial and deep), the dye, passing through them, also filled the vascular system of the second umbilical cord (Fig. 1).

Statistical analysis of continuous variable “total of anastomoses” not showing normal distribution (assessed by Kolmogorov-Smirnov test) by groups of perinatal outcomes and stages TTTS was performed using nonparametric tests (Mann-Whitney U-test) for independent groups. Also, the Mann-Whitney test was used to assess differences in the numbers of large AV, AA, and VV anastomoses. Quantitative continuous variables, namely the number of large and small AV, AA and VV anastomoses from donor to recipient and from recipient to donor, and perinatal outcomes as a grouping variable were used as a dependent variable when analyzing the possible statistical significance of differences in groups: 1 - favorable, 2 - unfavorable, as well as II and III stages TTTS by Quintero classification.
Calculations were performed using MedCalc 11.5 software (MedCalc Software BVBA, Ostend, Belgium). Differences between the groups were considered statistically significant at p<0.05. When quantitative variables were not normally distributed, the results were expressed as the median (Me), 1 and 3 quartiles (Q1; Q3). All data were tested for normality using the Kolmogorov-Smirnov test.
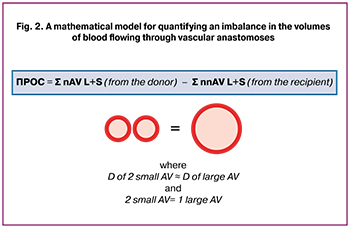 To facilitate the analysis, a mathematical model was developed, which made it possible to approximately estimate the difference in the volumes of the counter-flow of blood between the twins. A dimensionless relative value was introduced that quantitatively reflects the imbalance of blood flow between the twins. This value had no units and was defined as the difference between the total number of large and small AV anastomoses from donor to recipient and from recipient to donor, where 2 small AV anastomoses were taken as 1 large AV anastomosis (Fig. 2) and we designated it as an approximate difference in the volumes of discharged blood (DVDB). For example, in a patient with 2 large and 6 small AV anastomoses from donor to recipient and 2 small AV anastomoses from the recipient to donor without large AV anastomoses, DVDB was calculated as ((2×2) +6) – ((0×2) +2) and equal to 8.
To facilitate the analysis, a mathematical model was developed, which made it possible to approximately estimate the difference in the volumes of the counter-flow of blood between the twins. A dimensionless relative value was introduced that quantitatively reflects the imbalance of blood flow between the twins. This value had no units and was defined as the difference between the total number of large and small AV anastomoses from donor to recipient and from recipient to donor, where 2 small AV anastomoses were taken as 1 large AV anastomosis (Fig. 2) and we designated it as an approximate difference in the volumes of discharged blood (DVDB). For example, in a patient with 2 large and 6 small AV anastomoses from donor to recipient and 2 small AV anastomoses from the recipient to donor without large AV anastomoses, DVDB was calculated as ((2×2) +6) – ((0×2) +2) and equal to 8.
Results and discussion
No significant differences were found between the study groups with different perinatal outcomes regarding age, medical history, non-obstetric diseases. Thirty-six (49%), 32 (43%), and 6 (8%) FLP procedures were performed at gestational ages 17–19, 20–22, and 23–25 weeks, respectively. Therefore, the majority (92%) of patients with TTTS received FLP before 23 weeks’ gestation (Table 1).
Table 2 shows the clinical and demographic characteristics of patients in the study groups. The median (Me) age of the patients in group 1 and 2 was 27 years (25.2; 31) and 29.5 (26; 31) years, respectively. The total number of anastomoses in group 1 and 2 was 9 (7; 12.75) and11.5 (9.25; 14); median DVDB in group 1 and 2 was 6.5, (3; 9) and 9 (4.5; 12), respectively (Table 2).

Our analysis showed that the total number and types of anastomoses on the surface of a monochorionic placenta in pregnancies complicated by TTTS are not associated with perinatal outcomes and do not determine the degree of TTTS severity (Fig. 3). According to the literature, the number of anastomoses also does not affect perinatal outcomes [7].

The differences in the total number of anastomoses between perinatal outcomes were statistically insignificant by the Mann-Whitney test (p = 0.25).
Also, no significant differences in the total number of anastomoses were found between the study participants categorized by the Quintero staging system (p = 0.79).
Therefore, the degree of hemodynamic disturbances in TTTS is determined by the qualitative characteristics of the vessels, and not by their number.
To investigate the possible protective role of AA anastomoses in the progression of TTTS and the prediction of perinatal outcomes, differences in the number of large AA anastomoses in different outcomes and stages of TTTS were analyzed. According to the literature, AA anastomoses are present less often in TTTS placentas compared with placentas not complicated by TTTS, but their number has not been studied at different stages of the syndrome [6]. In our study, the difference between the two groups in the number of large AA anastomoses was statistically insignificant (p = 0.19); however, a higher number of large AA anastomoses were observed in placentas with a higher number of large AV anastomoses in patients with more severe stages of TTTS and adverse perinatal outcomes (Table 3).
A possible relationship between imbalance (DVDB) in AV anastomoses and the presence of AA anastomoses was also investigated. We hypothesized that patients with AA anastomoses will have a smaller imbalance since the presence of these anastomoses can compensate for this imbalance. Differences in the two groups according to the Mann-Whitney test were not statistically significant (p = 0.76). The number of AA anastomoses on the surface of a monochorionic placenta was not related to the number of large AV anastomoses between the donor and the recipient, as well as to the magnitude of the approximate divergence in the volume of blood discharge from the donor to the recipient.
Differences between the number of large VV anastomoses in groups with different perinatal outcomes were even less significant (p = 0.87). This suggests that the presence or absence of AA and VV placental anastomoses do not affect perinatal outcomes in pregnant women with TTTS. Differences in the number of AA and VV anastomoses in both study groups were insignificant, and bidirectional surface vessels were observed more often and in greater numbers in patients with adverse perinatal outcomes. Therefore, we concluded that AA anastomoses do not exert a protective effect in TTTS and do not improve perinatal outcomes (Table 4).
To assess the pathogenetic role of AV anastomoses in the development and progression of TTTS, a comparison was made between the number of large AV anastomoses and the Quintero TTTS stage at the time of the intervention, as well as their total number and perinatal outcomes.
Patients with favorable perinatal outcomes had more large AV anastomoses than patients with adverse outcomes (p <0.05). Differences between the two groups by the number of anastomoses in patients with favorable and adverse outcomes according to the Mann-Whitney test were statistically insignificant (p = 0.03) (Table 4).
Differences between the two groups in terms of the magnitude of the approximate difference in the volume of blood discharge (DVDB) by AV anastomosis were statistically significant (p = 0.07) (Table 4).
Our findings confirm the key role of deep unidirectional placental AV anastomoses from donor to the recipient in the pathogenesis of TTTS. The number of AV anastomoses from donor to recipient is associated with perinatal outcomes after FLP. A higher number of large AV anastomoses (> 3) from donor to recipient predetermines adverse perinatal outcomes.
Therefore, the study findings suggest that in patients with TTTS the presence of more than three AV placental anastomoses predicts adverse perinatal outcomes. AA anastomoses do not play a protective role, moreover, in higher number they aggravate the course of the disease; VV anastomoses are not associated with TTTS severity. To predict perinatal outcomes after intrauterine TTTS correction, all types of placental anastomoses need to be assessed during FLP.
References
- Сичинава Л.Г., Панина О.Б., Гамсахурдиа К.Г. Дискордантный рост плодов у беременных с монохориальной двойней. Акушерство, гинекология и репродукция. 2015; 9(1): 6-12. [Sichinava L.G., Panina OB, Gamsakhurdia K.G. Discordant growth of fruits in pregnant women with monochorial twins. Obstetrics, gynecology and reproduction. 2015; 9 (1): 6-12. (in Russia)].
- Костюков К.В., Гладкова К.А. Диагностика фето-фетального трансфузионного синдрома, синдрома анемии-полицитемии при монохориальной многоплодной беременности. Акушерство и гинекология. 2016; 1: 10-5. [Kostyukov K.V., Gladkova K.A. Diagnosis of feto-fetal transfusion syndrome, anemia-polycythemia syndrome in monochorial multiple pregnancy. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2016; 1: 10-5. (in Russian)].
- Baschat A., Chmait R.H., Deprest J., Gratacós E., Hecher K., Kontopoulos E. et al.; WAPM Consensus Group on Twin-to-Twin Transfusion. Twin-to-twin transfusion syndrome (TTTS). J. Perinat. Med. 2011; 39(2): 107-12.
- Lewi L., Deprest J., Hecher K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. Am. J. Obstet. Gynecol. 2013; 208(1): 19-30.
- Couck I., Lewi L. The placenta in twin-to-twin transfusion syndrome and twin anemia polycythemia sequence. Twin Res. Hum. Genet. 2016; 19(Special 3): 184-90.
- de Villiers S.F., Slaghekke F., Middeldorp J.M., Walther F.J., Oepkes D., Lopriore E. Arterio-arterial vascular anastomoses in monochorionic placentas with and without twin–twin transfusion syndrome. Placenta.2012; 33(8): 652-4.
- Denbow M., Cox P., Taylor M., Denbow M., Hammal D., Fisk N. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am. J. Obstet. Gynecol. 2000; 182(2): 417-26.
- Lewi L., Cannie M., Blickstein I., Jani J., Huber A., Hecher K. et al. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am. J. Obstet. Gynecol. 2007; 197(6): 587-92. e1-8.
- de Villiers S.F., Zhao D.P., Cohen D. Correlation between veno-venous anastomoses, TTTS and perinatal mortality in monochorionic twin pregnancies. Placenta. 2015; 36(5): 603-6.
- Sago H., Ishii K., Sugibayashi R., Ozawa K., Sumie M., Wada S. Fetoscopic laser photocoagulation for twin-twin transfusion syndrome. J. Obstet. Gynaecol. Res. 2018; 32(6): 813-8.
- Simpson L.L.; Society for Maternal-Fetal Medicine. Twin-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2013; 208(1): 3-18.
- Peeters S.H., Akkermans J., Westra M., Lopriore E., Middeldorp J.M., Klumper F.J. et al. Identification of essential steps in laser procedure for twin-twin transfusion syndrome using the Delphi methodology: SILICONE study. Ultrasound Obstet. Gynecol. 2015; 45(4): 439-46.
- Zhao D.P., Dang Q., Haak M.C., Middeldorp J.M., Klumper F.J., Oepkes D., Lopriore E. Superficial” anastomoses in monochorionic placentas are not always superficial. Placenta. 2015; 36(9): 1059-61.
- Бугеренко А.Е., Курцер М.А., Сичинава Л.Г., Суханова Д.И. Синдром фето-фетальной трансфузии. Фетоскопическая лазерная коагуляция анастомозов. Акушерство и гинекология. 2013; 10: 40-5. [Bugerenko A.E., Kurtser M.A., Sichinava L.G., Sukhanova D.I. Feto-fetal transfusion syndrome. Fetoscopic laser coagulation of anastomoses. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2013; 10: 40-5. (in Russian)].
Received 30.10.2018
Accepted 07.12.2018
About the Authors
Bugerenko, Andrei E., PhD, associate professor, Department of Obstetrics and Gynecology, Faculty of Fundamental Medicine, M.V. Lomonosov MSU.119991, Russia, Moscow, GSP-1, Leninskie gory 1, p. 51. Tel.: +7499268553. Center for Family Planning and Reproduction.
117209, Russia, Moscow, Sevastopolsky Avenue, 24 «A». Tel.: +79166884513. E-mail: jeddit@yandex.ru
Sukhanova, Dar’ya I., PhD student, Department of Obstetrics and Gynecology, Faculty of Fundamental Medicine, M.V. Lomonosov MSU.
119991, Russia, Moscow, GSP - 1, Leninskie Gory 1, p. 51. Tel.: +7499268553. Perinatal Medical Center.
117209, Russia, Moscow, Sevastopolsky Avenue, 24 K. 1. Tel.: +79857284805. E-mail: dashenka-sukhanova@yandex.ru
Donchenko, Yakov S., clinical resident, Department of Obstetrics and Gynecology, Faculty of Fundamental Medicine, M.V. Lomonosov MSU.
119991, Russia, Moscow, GSP - 1, Leninskie gory 1, p. 51. Tel.: +7499268553. Center for Family Planning and Reproduction.
117209, Russia, Moscow, Sevastopolsky Avenue, 24 «A». Tel.: +79055458196. Email: jacob8264@gmail.com
Panina, Ol’ga B., MD, professor, Department of Obstetrics and Gynecology, Faculty of Fundamental Medicine, M.V. Lomonosov MSU.
119991, Russia, Moscow, GSP - 1, Leninskie gory 1, p. 51. Tel.: +7499268553. Center for Family Planning and Reproduction.
117209, Russia, Moscow, Sevastopolsky Avenue, 24 «A». Tel.: +79857284805. E-mail: olgapanina@yandex.ru
Sichinava, Lali G., MD, professor, Department of Obstetrics and Gynecology, pediatric faculty N.I. Pirogov RNRMU.
117997, Russia, Moscow, ul. Ostrovityanova 1. Tel.: +74957183473. Center for family planning and reproduction. 117209, Russia, Moscow, Sevastopolsky Avenue, 24 «A». Tel.: +79035491817. E-mail: lalisichinava@gmail.com
For citation: Bugerenko A.E., Sukhanova D.I., Donchenko Ya.S., Panina O.B., Sichinava L.G. Placental angioarchitecture in monochorionic twin pregnancies with twin-twin transfusion syndrome. Perinatal outcomes. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (5): 63-9. (in Russian)
https://dx.doi.org/10.18565/aig.2019.5.63-69




