Clinical and laboratory characteristics of patients with embryonic arrest in the early embryonic period of in vitro fertilization programs.
Pogosyan M.T., Nazarenko T.A., Gaysin E.A.
Objective: To investigate the clinical and hormonal parameters and outcomes of in vitro fertilization (IVF) programs in patients with impaired embryo cleavage compared to those in patients with embryos suitable for transfer.
Materials and methods: Patients meeting the inclusion and non-inclusion criteria were divided into two groups. Group 1 (study group) included 287 patients with arrested embryogenesis, that is fertilized oocytes were present, and the embryos stopped fragmenting in the early or late stages of culture. Group 2 (control group) included 483 patients with the presence of a blastocyst on the 5th or 6th day of culture. Ovarian stimulation was conducted according to standard protocols using gonadotropin-releasing hormone agonists or antagonists. Fertilization of the aspirated oocytes was performed using IVF or ICSI.
Results: Clinical factors associated with impaired embryo development in women of reproductive age included late reproductive age of 38 (34.5; 41) and 35 (32;39) years in groups 1 and 2, respectively; reduced ovarian reserve in 71.8% and 24.4% patients in groups 1 and 2, respectively; history of missed miscarriage in 59.6% and 16% of women in groups 1 and 2 among patients with a history of pregnancy; infertility of unknown origin in 40.1% and 12%, respectively; extragenital endometriosis in 16% and 8.9% of women in groups 1 and 2, respectively.
Conclusion: The development of embryos in the early stages of culture and the frequency of blastocyst formation depend on the number of mature oocytes and zygotes obtained, which in turn are determined by the age of the patient and the state of the ovarian reserve. A history of missed miscarriage, infertility of unknown origin, extragenital endometriosis, and impaired embryo development in previous IVF attempts may be risk factors for embryonic arrest in young women, even if at least 5 mature oocytes and at least 3 zygotes are obtained.
Authors’ contributions: Nazarenko T.A. – editing of the manuscript; Pogosyan M.T. – creation of a clinical database of patients, drafting of the manuscript; Gaysin E.A. – collection and processing of material (fertilization of oocytes, cultivation of embryos, cryopreservation of embryos).
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Pogosyan M.T., Nazarenko T.A., Gaysin E.A. Clinical and laboratory characteristics of patients with embryonic arrest in the early embryonic period of in vitro fertilization programs.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (2): 89-96 (in Russian)
https://dx.doi.org/10.18565/aig.2023.271
Keywords
Oocytes obtained in in vitro fertilization (IVF) programs exhibit varying fertilization potential, and embryos develop differently during the cleavage period. Consequently, only 5% of oocytes produce embryos, and transfer of these embryos results in live births [1]. The relationship between oocytes and embryo quality has been confirmed in several studies, demonstrating that early embryo development failures are predominantly due to maternal factors [2–5]. Numerous defects in embryo maturation arise even before fertilization within the corresponding oocyte and largely depend on intracellular mechanisms of epigenetic modifications of the genome [6–8]. The process of acquiring oocyte competence remains poorly understood and develops during oogenesis [9]. Of interest is the study of the mechanisms leading to embryonic arrest, where an embryo suitable for transfer stops developing. Depending on the stage at which embryonic arrest occurs, we may observe the inability of the zygote to initiate the cleavage process, cessation of cleavage on the 2nd or 3rd day (at the two- to eight-cell embryo stage), failure to form a morula, and subsequently a blastocyst. The presented publications analyzed possible reasons for the arrest of embryo development at different cleavage stages [10–12].
Several studies have summarized the molecular factors that contribute to embryonic arrest at various stages. Embryonic arrest is a multifactorial phenomenon that is influenced by both internal and external factors. The investigation of cause-and-effect relationships determining embryonic arrest revealed maternal and/or paternal factors, embryonic metabolism activity, disruptions in crucial molecular pathways halting the developmental process, genetic abnormalities, and laboratory conditions as contributing factors [13–15].
Fetal factors associated with embryonic arrest include gene variations, mitochondrial DNA copy number, methylation patterns, chromosomal abnormalities, metabolic profiles, and morphological features. Parental factors include gene variations, protein expression levels, and etiology of infertility [16–18]. Despite an extensive analysis of the literature by the authors, no specific causes of embryonic arrest have been established. All of these factors potentially participate in the process, but how they do so remain unclear, given the diversity and heterogeneity of the causes of embryonic arrest during early embryogenesis, as well as their relationships [19].
However, in clinical practice, it is crucial to establish criteria that allow the prediction of the stage of embryonic development during patient examinations with varying degrees of reliability.
This study aimed to investigate the clinical and hormonal parameters and outcomes of in vitro fertilization (IVF) programs in patients with impaired embryo cleavage compared to those in patients with embryos suitable for transfer.
Materials and methods
This observational case-control clinical study included 770 patients who underwent IVF infertility treatment.
To achieve the goal of the study, all patients who met the inclusion and non-inclusion criteria were divided into two groups: Group 1 (study group) consisted of 287 patients whose embryos arrested in developmentat different stages of cleavage during IVF, and Group 2 (control group) included 483 patients with a blastocyst suitable for transfer on the 5th or 6th day of culture.
A total of 770 IVF cycles were performed at the F. Paulsen Research and Educational Center for ART in the Clinical Department of V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation in 2022 in patients of the study groups.
Additionally, representative groups of patients were identified (groups 1a and 2a), which were comparable in age, state of ovarian reserve, and number of oocytes and zygotes obtained. Each group comprised 37 patients. In Group 1a, all embryos arrested in development, and patients in Group 2a received embryos suitable for transfer.
The criteria for inclusion in the study groups were absence of pregnancy for one year or more, normal karyotype of both spouses, and indications for achieving pregnancy using IVF.
The criteria for non-inclusion in the study groups were the use of donor gametes in the studied cycle of assisted reproductive technologies (ART), cryopreserved embryo transfer programs, all conditions that are contraindications to ART, and achieving pregnancy in accordance with the order of the Ministry of Health of Russia dated July 31, 2020, No. 803n.
All married couples underwent full clinical and laboratory examinations in accordance with Order No. 803n of the Russian Ministry of Health, dated July 31, 2020.
Stimulation of ovarian function was performed with gonadotropins (recombinant follicle-stimulating hormone (FSH) and/or human menopausal gonadotropin) according to standard protocols with gonadotropin-releasing hormone agonists or antagonists. Urinary or recombinant forms of human chorionic gonadotropin were used as ovulation triggers. Ultrasound monitoring was performed three to five times during ovarian stimulation. Transvaginal puncture of the follicles was performed 36–37 h after ovulation-trigger administration. Fertilization of aspirated oocytes was performed using IVF or intracytoplasmic sperm injection (ICSI). All embryos were cultured in individual drops of one-step culture medium (G-TL, Vitrolife) in multigas incubators. The quality of the resulting embryos was assessed on the 2nd, 3rd, 4th, 5th and 6th days of culture based on morphological criteria in accordance with Gardner’s grading system (ESHRE 2011 modified classification by D. Gardner) [20].
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation
Statistical analysis
Statistical analysis and graphing were performed using GraphPad Prism v6 (GraphPad Software Inc., USA). The statistical programming language R was used to construct the logistic regression. For qualitative data, counts and percentages were used (N (%)), and Fisher's exact test was used. The distribution of continuous variables was tested for normality using the Shapiro-Wilk test. Numerical variables that were not normally distributed were summarized as medians and interquartile range (Me (Q1; Q3)), and the Mann–Whitney test was used. In order to assess the quality of binary classification, ROC analysis was performed. When performing the ROC analysis, the cutoff threshold, area under the ROC curve (AUC), sensitivity, and specificity were calculated. Regression analysis was performed to determine the relationship between the dependent variables and one or more independent variables. The statistical programming language R was used to build the logistic regression. Multicollinearity was assessed using the Spearman’s correlation test. Differences were considered statistically significant at p<0.05.
Results
Group 1 (study group) included 287 women with a mean age of 38 (34; 41) years, from whom the IVF program obtained three or more (3; 6) mature oocytes and three or more (3; 4) zygotes, but all embryos were arrested in development.
Group 2 (control group) consisted of 483 patients with a mean age of 35 (32; 39) years; eight (5; 13) mature oocytes, six (4; 10) zygotes, and three (2; 6) blastocysts were obtained in the IVF program.
When comparing the clinical characteristics of patients in the study groups, there were no differences in the frequency of primary/secondary infertility, duration of infertility, or number of previous ART attempts (Table 1).
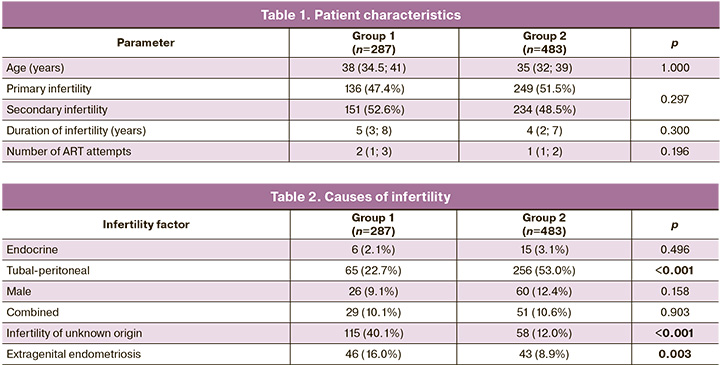
At the same time, analysis of the reproductive function of patients with a history of pregnancy showed that in group 2, 88/234 (37.6%) women had childbirth, 107/234 (45.7%) pregnancies were terminated at the request of the woman, and only 39/234 (16.0%) had spontaneous miscarriages. Among Group 1 patients, only 19/151 (12.5%, p<0.001) had a live birth, 41/151 (27.2%, p<0.001) had an abortion at the patient's request, and 90/151 (59.6%, p<0.001) had at least one missed miscarriage.
A thorough analysis of the causes of infertility in the study groups showed that the tubo-peritoneal factor of infertility occurred in 256/483 (53.0%) patients in group 2 and in 65/287 (22.7%) patients in group 1; infertility of unknown origin was diagnosed in 115/287 (40.1%) patients in group 1 and 58/483 (12. Infertility associated with endometriosis was diagnosed in 46/287 (16.0%) patients in Group 1 and 43/483 (8.9%) women in Group 2. The incidence of the combined forms of infertility and infertility associated with male factors did not differ between the groups (Table 2).
Therefore, the clinical factors associated with impaired embryo development included late reproductive age 38 years (34.5;41) in group 1 and 35 years (32;39) in group 2; missed miscarriage in 59.6% of women in group 1 and 16% in group 2 among patients with a history of pregnancy, infertility of unknown origin in 40.1% and 12%, respectively; and extragenital endometriosis in 16% of women in group 1 and 8.9% in group 2.
Objective confirmation of a possible impairment of embryo development can be the analysis of previous IVF attempts when an embryo suitable for transfer was not obtained. In the present study, almost half of the patients in the study group had a history of repeated IVF attempts, and none of them produced an embryo suitable for transfer.
Significant differences were observed in the state of ovarian reserve of the study participants (Table 3). Patients with embryo development disorders had diminished ovarian reserve rates compared with in those the control group.
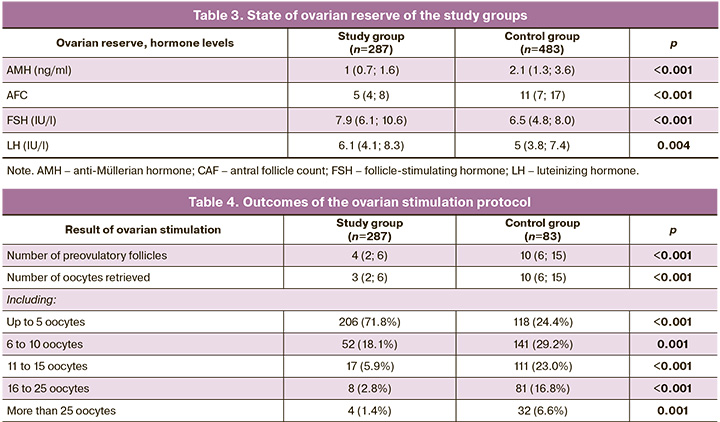
The data obtained confirmed the opinion of many experts that late age is a decisive factor in determining not only the quantity but also the quality of the oocytes obtained [21]. However, it is known that ovarian reserve, in particular the anti-Müllerian hormone (AMH) level, is a predictor of the number of oocytes retrieved but is not associated with their quality and pregnancy rate [22].
The number of oocytes obtained in the study and control groups was analyzed (Table 4).
All results are presented for each IVF attempt. As shown in the table, the probability of embryonic arrest in all embryos was directly related to the number of preovulatory follicles and the number of oocytes retrieved.
The fertilization rate (2PN) in the study groups was 68.4 and 76.3%, respectively.
The presented data demonstrate a relationship between the number of oocytes retrieved and embryonic arrest. Thus, in the study group the mean number of oocytes was 3, while in the control group it was 10; 71.8% of attempts in the study group were characterized by a “poor response” of the ovaries, while in the control group there were 24.4% of such patients. The results obtained were consistent with the clinical and hormonal characteristics of the patients; in the study group, there were more older women with diminished ovarian reserve.
Using a control group of patients as an example, blastocyst yield was calculated as a function of the number of mature oocytes obtained. Blastocyst yield was calculated by dividing the number of blastocysts by the number of oocytes obtained.
Considering that the average percentage of blastulation per oocyte was 31.1%, it was possible to calculate the probability of embryonic arrest when obtaining a small number of oocytes, assuming that this process was random.
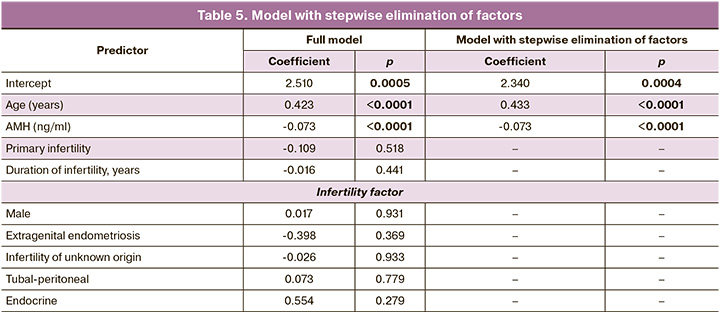
To clarify the influence of individual predictors, logistic regression was performed, and the results of constructing the dependence of the parameters are presented in Table 5. When checking for multicollinearity (by correlation analysis), only a weak negative correlation was found between age and AMH levels (r=-0.172, p=0.004). Embryonic arrest was used as an independent variable. Age, AMH level, type of infertility, duration of infertility, and infertility factors were used as the dependent variables. To eliminate predictors in a stepwise manner, we used the stepAIC function of the MASS package. The regression equation fits the model, as follows:
Y = exp(z)/(1+exp(z));
z = Intercept+0.433*(Age, years)-0.073*(AMH, ng/ml);
Intercept=2,340.
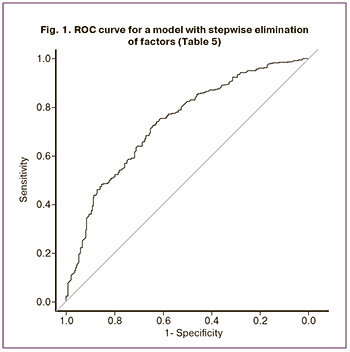
Thus, only patient age and AMH level were significant factors. To determine the reliability of these signs, a receiver operating characteristic (ROC) curve was constructed, the ROC curve is shown in Figure 1. The area under the curve (AUC) was 0.739, the sensitivity was 83.9%, and the specificity was 48.4% (OR = 5.57; 95% CI 3.88–8.01).
The number of oocytes and zygotes obtained are important indicators of the effectiveness of the embryological phase of IVF programs (Fig. 2).
Without denying the dependence of blastocyst formation on the number of oocytes and zygotes, this fact alone cannot be completely justified.
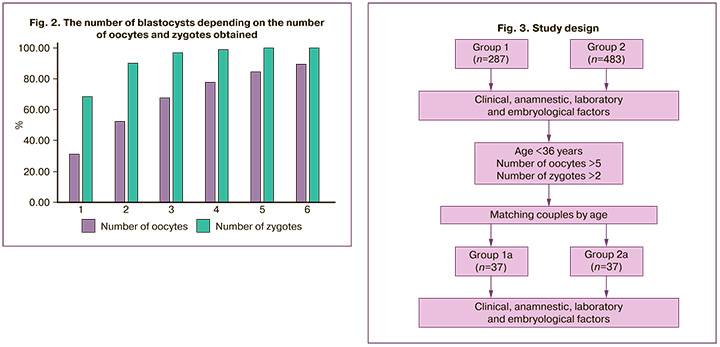
In the present study, in 28.2% of patients with embryonic arrest, a sufficient number of mature oocytes (six or more) were obtained, comparable to the control group, which dictates the need to search for other factors that primarily determine the quality of gametes, and not only their quantity.
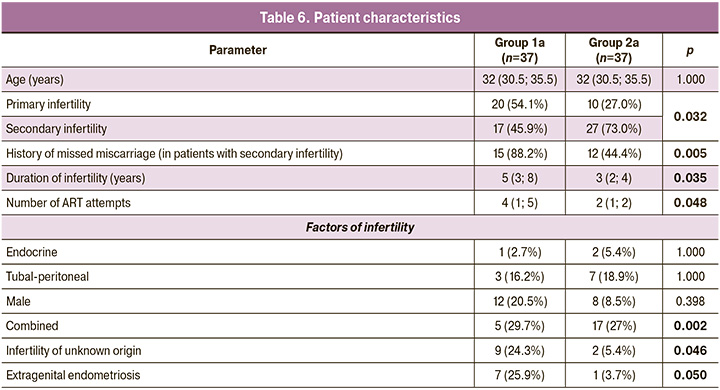
We compared the clinical and laboratory characteristics of selected age-representative patients in the study and control groups (Fig. 3).
In the subsequent analysis, we excluded the factors of age and low number of oocytes received, comparing patients under 36 years of age with more than five oocytes and at least three zygotes received, to identify factors associated with embryonic arrest in the group of patients in whom this outcome was least expected (Table 6) and women with similar characteristics but with at least one blastocyst suitable for transfer. Group 1a (study group) consisted of 37 women aged 32 (30.5; 35.5) years, of whom eight (6; 12) mature oocytes and four (3; 6) zygotes were obtained in the IVF program, but all embryos were arrested in development. Group 2a (control) consisted of 37 patients aged 32 (30.5; 35.5) years, from whom 10 (8; 18) mature oocytes, 8 (5; 10) zygotes, and 4 (3; 5) blastocysts were obtained.
Despite the comparability of patients, the following differences were identified:
- Patients with embryonic arrest had a longer duration of infertility (5 (3; 8) years versus 3 (2; 4) years, respectively; p=0.035).
- Primary infertility occurred in 54.1% and 27.0% of patients in groups Ia and IIa, respectively (p=0.032).
- A severe form of pathozoospermia, in combination with other infertility factors, was present in 17/37 (46.0%) and 6/37 (16.2%) spouses, respectively (p=0.011).
- Infertility of unknown origin was diagnosed in 24.3% of the cases in group Ia versus 5.4% in group IIa (p=0.046).
- Extragenital endometriosis was detected in 25.9% and 3.7% of the patients (p=0.050).
- In group Ia, 88.2% of the patients with secondary infertility had a history of missed miscarriages versus 44.4% in group IIa (p=0.005).
- Patients in group Ia had at least three previous unsuccessful IVF attempts (three (0; 4), and in group IIa, only one (0; 1)) (p=0.048).
Discussion
Embryos arrested in development obtained during IVF can be classified as a physiological phenomenon, a type of natural selection, where non-viable embryos do not develop. It is believed that the normal blastulation rate is 40–45% of the number of zygotes obtained [23]. However, in some cases, it is not possible to obtain an embryo suitable for transfer, and the development of all resulting embryos stops. Unfortunately, this phenomenon is aggravated by the older age of patients and the small number of oocytes obtained. This study confirms the well-known fact that the clinical factors causing impaired embryo development are the woman’s age and the associated decrease in ovarian reserve, as diagnosed by the level of AMH. This study aimed to determine whether blastocyst formation depends on the number of oocytes and zygotes obtained. The identified patterns can be useful in the practical work of a doctor, allowing assessment of the possible number of oocytes obtained during ovarian stimulation to predict the likelihood of a blastocyst suitable for transfer to the uterine cavity. Moreover, if the prognosis for embryo fragmentation is unfavorable, it is possible to change the patient’s management tactics and carry out an early embryo transfer on the 2nd or 3rd day of development, or perhaps at the zygote stage. Few studies have addressed this topic [24–26]. Situations where at least five mature oocytes and at least three zygotes are obtained, and the embryos stop developing, are difficult to explain and make clinical decisions. In this study, we compared the clinical and anamnestic data of women representative of age, state of ovarian reserve, number of mature oocytes, and zygotes obtained in women with impaired parameters of early embryogenesis and patients who had blastocysts suitable for transfer. The observed differences include a greater number of missed miscarriages in group 1a than in group 2a, the presence of infertility of unknown origin, and a high proportion of detected extragenital endometriosis. These factors may be the possible clinical causes of impaired embryo development, as confirmed by the results of previous IVF attempts in patients, where none of the attempts yielded embryos suitable for transfer. The presented results emphasize the necessity for a thorough analysis of the anamnestic and clinical characteristics of patients and the results of previous IVF programs to determine tactics for achieving pregnancy.
Conclusion
The development of embryos in the early stages of culture and the frequency of blastocyst formation depend on the number of mature oocytes and zygotes obtained, which in turn are determined by the age of the patient and the state of the ovarian reserve. A history of missed miscarriages, infertility of unknown origin, extragenital endometriosis, and impaired embryonic development in previous IVF attempts are clinical and anamnestic characteristics that may be risk factors for embryonic arrest in young women, even if at least five mature oocytes are obtained and at least three zygotes are present.
References
- Lemmen J.G., Rodríguez N.M., Andreasen L.D., Loft A., Ziebe S. The total pregnancy potential per oocyte aspiration after assisted reproduction – in how many cycles are biologically competent oocytes available? J. Assist. Reprod. Genet. 2016; 33(7): 849-54. https://dx.doi.org/10.1007/s10815-016-0707-3.
- Xu Y., Shi Y., Fu J., Yu M., Feng R., Sang Q. et al. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am. J. Hum. Genet. 2016; 99: 744-52. https://dx.doi.org/10.1016/j.ajhg.2016.06.024.
- Chen B.,Wang W., Peng X., Jiang H., Zhang S., Li D. et al. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur. J. Hum. Genet. 2019; 27(2): 300-7. https://dx.doi.org/10.1038/s41431-018-0283-3.
- Zheng W., Hu H., Dai J., Zhang S., Gu Y., Dai C. et al. Expanding the genetic and phenotypic spectrum of the subcortical maternal complex genes in recurrent preimplantation embryonic arrest. Clin. Genet. 2021; 99(2): 286-91. https://dx.doi.org/10.1111/cge.13858.
- Zhao L., Xue S., Yao Z., Shi J., Chen B., Wu L. et al. Biallelic mutations in CDC20 cause female infertility characterized by abnormalities in oocyte maturation and early embryonic development. Protein Cell. 2020; 11(12): 921-7.
- Yang Y., Shi L., Fu X., Ma G., Yang Z., Li Y. et al. Metabolic and epigenetic dysfunction underlie the arrest of in vitro fertilized human embryos in a senescent-like state. PLoS Biol. 2022; 20(6): e3001682. https://dx.doi.org/10.1371/journal.pbio.3001682.
- Hsieh R.H., Au H.K., Yeh T.S., Chang S.J., Cheng Y.F., Tzeng C.R. Decreased expression of mitochondrial genes in human unfertilized oocytes and arrested embryos. Fertil. Steril. 2004; 81(Suppl. 1): 912-8. https://dx.doi.org/10.1016/j.fertnstert.2003.11.013.
- Мартиросян Я.О., Назаренко Т.А., Кадаева А.И., Краснова В.Г., Бирюкова А.М., Погосян М.Т. Новые подходы к изучению регуляции преимплантационного развития эмбрионов. Акушерство и гинекология. 2023; 6: 29-37. [Martirosyan Ya.O., Nazarenko T.A., Kadaeva A.I., Krasnova V.G., Biryukova A.M., Pogosyan M.T. New approaches to studying the regulation of preimplantation embryonic development. Obstetrics and Gynecology. 2023; (6): 29-37. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.10.
- Albertini D.F., Sanfins A., Combelles C.M. Origins and manifestations of oocyte maturation competencies. Reprod. Biomed. Online. 6(4): 410-5. https://dx.doi.org/10.1016/s1472-6483(10)62159-1.
- Montjean D., Geoffroy-Siraudin C., Gervoise-Boyer M.J., Boyer P. Competence of embryos showing transient developmental arrest during in vitro culture. J. Assist. Reprod. Genet. 2021; 38(4): 857-63. https://dx.doi.org/10.1007/s10815-021-02090-8.
- Wei Y., Wang J., Qu R., Zhang W., Tan Y., Sha Y. et al. Genetic mechanisms of fertilization failure and early embryonic arrest: a comprehensive review. Hum. Reprod. Update. 2023; 30(1): 48-80. https://dx.doi.org/10.1093/humupd/dmad026.
- McCollin A., Swann R.L., Summers M.C., Handyside A.H., Ottolini C.S. Abnormal cleavage and developmental arrest of human preimplantation embryos in vitro. Eur. J. Med. Genet. 2020; 63(2): 103651. https://dx.doi.org/10.1016/j.ejmg.2019.04.008.
- Sipahi M., Mümüşoğlu S., Coşkun Akçay N., Sever A., Yeğenoğlu H., Bozdağ G., Karakoç Sökmensüer L. The impact of using culture media containing granulocyte-macrophage colony-stimulating factor on live birth rates in patients with a histpry of embryonic developmental arrest in previous in vitro fertilization cycles. J. Turk. Ger. Gynecol. Assoc. 2021; 22(3): 181-6. https://dx.doi.org/10.4274/jtgga.galenos.2021.2020.0168.
- Civico S., Agell N., Bachs O., Vanrell J.A., Balasch J. Increased expression of the cyclin-dependent kinase inhibitor p27 in cleavage-stage human embryos exhibiting developmental arrest. Mol. Hum. Reprod. 2002; 8(10): 919-22. https://dx.doi.org/10.1093/molehr/8.10.919.
- Ming L., Yuan C., Ping L., Jie Q. Higher abnormal fertilization, higher cleavage rate and higher arrested embryos rate were found in conventional IVF than in intracytoplasmic sperm injection. Clin. Exp. Obstet. Gynecol. 2015; 42(3): 372-5.
- Bayram A., Elkhatib I., Arnanz A., Linan A., Ruiz F., Lawrenz B., Fatemi H.M. What drives embryo development? Chromosomal normality or mitochondria? Case Rep. Genet. 2017; 2017: 4397434. https://dx.doi.org/10.1155/2017/4397434.
- Martínez-Moro Á., Lamas-Toranzo I., González-Brusi L., Pérez-Gómez A., Padilla-Ruiz E., García-Blanco J. mtDNA content in cumulus cells does not predict development to blastocyst or implantation. Hum. Reprod. Open. 2022; 2022(3): hoac029. https://dx.doi.org/10.1093/hropen/hoac029.
- Zamora R.B., Sánchez R.V., Pérez J.G., Díaz R.R., Quintana D.B., Bethencourt J.C. Human zygote morphological indicators of higher rate of arrest at the first cleavage stage. Zygote. 2011; 19(4): 339-44. https://dx.doi.org/10.1017/s0967199410000407.
- Sfakianoudis K., Maziotis E., Karantzali E., Kokkini G., Grigoriadis S., Pantou A. et al. Molecular drivers of developmental arrest in the human preimplantation embryo: a systematic review and critical analysis leading to mapping future research. Int. J. Mol. Sci. 2021; 22(15): 8353. https://dx.doi.org/10.3390/ijms22158353.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011; 26(6): 1270-83.
- Janny L., Menezo Y.J. Maternal age effect on early human embryonic development and blastocyst formation. Mol. Reprod. Dev. 1996; 45(1): 31-7. https://dx.doi.org/10.1002/(SICI)1098-2795(199609)45:<31AID-MRD4>3.0.СО;2-T.
- Dai X., Wang Y., Yang H., Gao T., Yu C., Cao E. et al. AMH has no role in predicting oocyte quality in women with advanced age undergoing advanced age undergoing IVF/ICSI. Sci. Rep. 202o; 10(1): 19750. https://dx.doi.org/10.1038/s41598-020-76543-y.
- ESHRE Special Interest Group of Embryology, and Alpha Scientists in Reproductive Medicine, The Vienna consensus: report of an expert meeting on the development of art laboratory performance indicators. Reprod. Biomed. Onine. 2017; 35(5): 494-510. https://dx.doi.org/101016/j.rbmo.2017.06.015.
- Lin P.Y., Lin C.Y., Tsai N.C., Huang F.J., Chiang H.J., Lin Y.J. et al.Disposition of embryos from women who only produced morphologically poor embryos on day three. Biomed. J. 2002.; 45(1): 190-9. https://dx.doi.org/10.1016/j.bj.2021.01.001.
- Haas J., Meriano J., Bassil R., Barzilay E., Casper R.F. What is the optimal timing of embryo transfer when there are only one or two embryos at cleavage stage? Gynecol. Endocrinol. 2019; 35(8): 665-8. https://dx.doi.org/10.1080/09513590.2019.1580259.
- Lee S.H., Lee H.S., Lim CK., Park Y.S., Yang K.M., Park D. Comparison of the clinical outcomes of the day 4 and 5 of embryo transfer cycles. 2013; 40(3): 122-5. https://dx.doi.org/10.5653/cerm.2013.40.3.122.
Received 20.11.2023
Accepted 24.01.2024
About the Authors
Mariam T. Pogosyan, PhD student at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, mariam-pogosyan@yandex.ruTatyana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, t_nazarenko@oparina4.ru
Emil A. Gaysin, Embryologist, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, emil.g17@mail.ru



