Для многих женщин такие менопаузальные расстройства, как приливы жара, нарушение сна, перепады настроения и сухость слизистой влагалища, могут нарушать естественный ход событий и качество жизни. Менопаузальная гормональная терапия (МГТ) (системная и локальная) эффективна и относительно безопасна. Тем не менее для женщин с личным анамнезом рака молочной железы (РМЖ) МГТ противопоказана. За последние два десятилетия накоплен определенный опыт использования различных терапевтических подходов в коррекции менопаузальных расстройств у данной группы женщин, который будет представлен в статье.
Коррекция вазомоторных симптомов
Для большинства женщин, выживших после РМЖ, вазомоторные симптомы (ВМС) являются основной проблемой. Гипергидроз и приливы часто возникают ночью и нарушают сон, способствуют развитию астении и расстройств эмоционального спектра. Такие простые меры, как поддержание психологического здоровья, переоценка стрессовых ситуаций, избегание приема горячих напитков и острой пищи могут ослабить интенсивность ВМС [1, 2]. Гормональная терапия [тамоксифен (ТАМ) и ингибитор ароматазы (ИА)], использующаяся для лечения РМЖ, часто сопровождается развитием ВМС, интенсивность которых может быть настолько сильной, что нередко пациентки вынуждены отказаться от приема гормон-депривационной терапии и пренебречь повышенным риском развития рецидива или контралатерального РМЖ.
Цимицифуга кистевидная (ЦК) и другие препараты растительного происхождения. Многим женщинам импонирует природное происхождение препаратов, использующихся для коррекции симптомов менопаузы. Наиболее часто с этой целью применяются препараты сои (Soy), цимицифуги (Cimicifuga racemosa) и красного клевера (Trifolium pretense). Nelson et al. выполнили метаанализ эффективности негормональной терапии экстрактов красного клевера при приливах и не выявили отличий от плацебо. Кроме того, так как эти препараты способны активировать рецепторы эстрогенов (ER), их следует считать относительно противопоказанными в условиях РМЖ. Гинестеин – изофлавон, способный стимулировать рост опухоли молочной железы и угнетать метаболизм ТАМ [3]. Напротив, экстракт ЦК не проявляет активности в отношении ER и может дополнительно оказывать некоторые антиэстрогенные воздействия на ткань молочной железы (табл. 1, 2) [4–6].

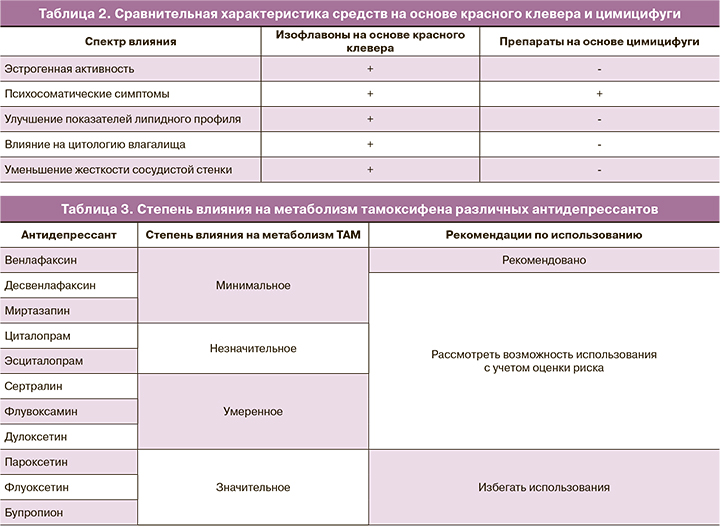
Einbond L.S. [4] продемонстрировал ингибирующее влияние ЦК на культуру клеток РМЖ человека, особенно сверхэкспрессирующих HER2. У пациенток не было диагностировано изменения маммографической плотности или толщины эндометрия при использовании ЦК более 6 месяцев [7]. В исследовании Obi et al. [5] использование ЦК было ассоциировано с меньшим риском РМЖ в сравнении с плацебо (OR 0,72; 95% CI 0,60–0,87).
Исследования по установлению эффективности ЦК представляют смешанные результаты вследствие неоднородной выборки, использования различных по составу фитопрепаратов. В частности, эффективность ЦК была продемонстрирована в четырех рандомизированных исследованиях [8]. Одно испытание показало эквивалентность его эффекта низкой дозе пластыря, содержащего эстрадиол. ЦК не влияет на метаболизм ТАМ. Таким образом, использование ЦК может быть разумным началом в терапии ВМС у пациенток с наличием РМЖ в анамнезе.
Серотонинергические препараты. Селективные ингибиторы обратного захвата серотонина (СИОЗС) и ингибиторы обратного захвата серотонина и норадреналина (СИОЗСН) были предложены в качестве альтернативы МГТ при коррекции ВМС.
Метаанализы [9, 10], объединенный анализ [11] и обзор, посвященный эффективности купирования ВМС у пациенток с диагностированным РМЖ в анамнезе [12], предоставляют доказательства эффективности СИОЗС и СИОЗСН при приливах легкой и/или умеренной степени тяжести. Статистически значимое уменьшение приливов было отмечено при использовании женщинами пароксетина, эсциталопрама, циталопрама, венлафаксина и десвенлафаксина. Уменьшение частоты приливов варьировало от 25 до 69%. Менее показательные результаты были получены с сертралином и флуоксетином [13, 14].
Для женщин, принимающих ТАМ, одновременный прием СИОЗС может привести к ингибированию фермента CYP2D6 (преобразует ТАМ до его наиболее активного метаболита – эндоксифена). Причем степень ингибирования данного фермента отличается среди разных препаратов из группы СИОЗС (табл. 3) [15].

Последствия ингибирования фермента CYP2D6 с последующим снижением эффективности ТАМ были продемонстрированы в эпидемиологическом исследовании (n=2430). На примере использования пароксетина в комбинации с ТАМ у пациенток отмечалось увеличение показателя смертности от РМЖ [15]. Следует отметить циркадный характер влияния некоторых психотропных препаратов. При превалировании ВМС в дневное время суток предпочтительнее назначать СИОЗС/СИОЗСН; при ВМС в ночное время суток более эффективен противоэпилептический препарат габапентин [16].
Габапентин. Противосудорожное и нейростабилизирующее действие препарата позволяет использовать его в ситуации нейропатической боли (например, в области хирургической раны), при мигрени, биполярном расстройстве и фибромиалгии. Многочисленные испытания с применением габапентина с целью коррекции приливов продемонстрировали его превосходство в сравнении с плацебо [17].
Клонидин – стимулятор α2-адренорецепторов, который в исследованиях по своему влиянию на ВМС превосходил плацебо. Однако часть женщин отказывались пролонгировать терапию данным препаратом вследствие ряда нежелательных явлений в виде головокружения, головной боли, запоров, сухости во рту. В исследованиях, сравнивающих эффективность клонидина и венлафаксина, было показано аналогичное влияние препаратов на приливы, однако действие последнего наступало быстрее и характеризовалось меньшим количеством побочных явлений [18].
Антагонисты рецепторов нейрокинина-3 (антагонист NK3-рецепторов, фезолинетант). Центры терморегуляции в головном мозге активируются рецепторами нейрокинина-3 и ингибируются эстрогенами. Дефицит эстрогенов способствует изменению гомеостаза этой системы и лежит в основе развития ВМС. Испытания антагониста NK3-рецепторов продемонстрировали снижение частоты ВМС на 93% от исходного уровня за 12-недельный период наблюдения. Уменьшалась и тяжесть приливов по сравнению с плацебо (-26,5 по сравнению с -12,2 с плацебо). Фезолинетант был эффективен с 1-го дня (начало действия эстрогенов регистрировали через 2–4 недели). Кроме того, отмечалось его положительное влияние на качество сна с минимальными побочными эффектами. Однако симптомы возобновлялись сразу после прекращения приема фезолинетанта. В настоящее время препарат находится в III фазе клинического исследования [19].
Менопаузальная гормональная терапия. В настоящее время использование системной МГТ у женщин с установленным диагнозом РМЖ противопоказано. Отрицательный опыт использования МГТ был сформирован после получения результатов ряда клинических испытаний, которые были проведены с целью изучения безопасности МГT у женщин с личным анамнезом РМЖ (табл. 4).
Эффект тиболона, по мнению некоторых авторов, может зависеть от рецепторного статуса опухоли и проводимой гормональной противоопухолевой терапии. Риск рецидива РМЖ в исследовании был высоким при использовании ИА (HR 2,42) или аналогов гонадотропин-рилизинг-гормона (HR 2,29). У женщин, имеющих опухоль, отрицательную по ER (HR 1,15), или принимающих ТАМ (HR 1,25) не было отмечено значительного повышения данного риска. Результаты исследований можно объяснить разным профилем антиэстрогеновых эффектов, которые могут проявляться в виде усиления, подавления или блокирования ER тиболоном [24].
У женщин с высоким риском развития наследственного РМЖ (например, наличие BRCA1, -2 мутации) текущие данные свидетельствуют о безопасности системной МГТ после риск-редуцирующей билатеральной сальпингоовариэктомии [25, 26].
Эстетрол – эстроген с ограниченными возможностями, который в настоящее время находится на стадии клинических испытаний [27]. Перспективность его использования основана на том, что эстетрол не стимулирует синтез глобулина, связывающего половые гормоны, или церулоплазмина, оказывает минимальное влияние на факторы свертывания крови и уровень триглицеридов (6,4% против 61%) по сравнению с эстрадиолом. Имеющиеся исследования подтверждают его антиэстрогенные свойства по отношению к некоторым тканям и потенциально менее агонистические эффекты на молочную железу [28]. Результаты предварительных испытаний демонстрируют более высокое соотношение польза/риск при приеме эстетрола в сравнении с эстрадиолом [27].
Тканеспецифический эстрогеновый комплекс. В состав препарата входят конъюгированные эстрогены 0,45 мг и селективный модулятор рецепторов эстрогена базедоксифен 20 мг. Базедоксифен предотвращает гиперпластические процессы в эндометрии и ткани молочной железы. Возможно, после проведения дополнительных испытаний откроются перспективные возможности использования препарата в коррекции ВМС у пациенток, имеющих РМЖ в анамнезе [17].
Согласно позиции NAMS (North Аmerican Menopause Society), 2020 [29], «использование системной МГТ у выживших после РМЖ не рекомендуется».
В объединенном решении BMS (British Menopause Society), IMS (International Menopause Society), EMAS (European Menopause and Andropause Society), RCOG (Royal College of Obstetricians and Gynaecologist), AMS (Australasian Menopause Society), рекомендации Комитета фармаконадзора, 2020 [30], указано, что «риск РМЖ следует рассматривать в контексте общих рисков, связанных с приемом МГТ, и преимуществ, включая коррекцию менопаузальных расстройств, улучшение качества жизни и долгосрочного влияния на состояние костной ткани и сердечно-сосудистой системы. В случае явных преимуществ и клинической необходимости решение о возможности использования МГТ у пациенток, имеющих РМЖ в анамнезе, следует принимать индивидуально при согласовании рекомендаций с онкологами». В настоящее время в Российской Федерации, согласно инструкциям к лекарственным препаратам МГТ, РМЖ является противопоказанием для их использования.
Оксибутинин применяется для лечения стрессового недержания мочи. Обладает антимускариновым, холинолитическим и спазмолитическим действием. Два исследования продемонстрировали превосходство оксибутинина в дозе от 2,5 до 15 мг в коррекции ВМС по сравнению с плацебо. В работе частота нежелательных явлений в виде сухости во рту достигала 50% и была основной причиной отказа от лечения [31].
Другие средства для коррекции ВМС: снижение веса и физические упражнения; модификация образа жизни и питания; акупунктура; когнитивно-поведенческая терапия; гипноз; релаксация, йога, тай-чи; снижение стресса на основе переключения внимания; блокада звездчатого ганглия; дыхательная гимнастика.
Коррекция генитоуринарного менопаузального синдрома (ГУМС)
Первоначально с пациенткой необходимо обсудить основные правила интимной гигиены (исключение мыла, использование моющих средств на водной основе и натуральных масел в качестве смазывающего материала), которые также эффективны в борьбе с урогенитальными расстройствами. Вследствие длительного течения ГУМС может сформироваться миалгический компонент проблемы (вагинизм), облегчить который можно с помощью физиотерапевтических процедур, расслабляющих мышцы тазового дна.
В когортном исследовании Dew J.E. 69 пациенток, имеющих РМЖ в анамнезе (из 1472), использовали локальные эстрогены. После проведения регрессионного анализа были получены обнадеживающие результаты (0,30 95% ДИ 0,20–1,58)). Применение микродоз эстрадиола в капсулах (4 мкг/cутки) не сопровождалось системной абсорбцией [32]. Однако препараты, содержащие микродозы эстрадиола, не зарегистрированы в России. Онкологи отрицательно относятся к назначению локальных эстрогенов из-за опасений абсорбции эстрогенов в системный кровоток с последующим увеличением риска рецидива РМЖ. Эстриол-содержащий препарат (30 мкг/cутки) для местного применения, имеющий регистрацию в России, в инструкции по применению имеет противопоказания: РМЖ (диагностированный, подозреваемый или в анамнезе).
Согласно NAMS, 2020 [29], «локальная терапия эстрогенами в микродозах остается эффективным вариантом лечения ГУМС с минимальной системной абсорбцией. Данный тип лечения может быть рассмотрен после первоначального применения негормональной терапии и после консультации с онкологом».
В недавно опубликованном Объединенном решении BMS, IMS, EMAS, RCOG, AMS, Рекомендациях Комитета фармаконадзора 2020 [30] указывается, что «локальные формы эстрогенов в микродозах не увеличивают риск РМЖ».
Оспемифен – селективный модулятор эстрогеновых рецепторов, предназначенный для коррекции диспареунии средней и тяжелой степени на фоне вульвовагинальной атрофии. Исследования на модели животных продемонстрировали его антиэстрогенное влияние на молочную железу. Требуется накопление опыта использования препарата у женщин, имеющих РМЖ в анамнезе. Препарат не имеет регистрации в Российской Федерации [33].
Дегидроэпиандростерон (ДГЭА). Широкий спектр биологической активности позволяет использовать ДГЭА с целью коррекции диспареунии средней/тяжелой степени и сексуальной дисфункции. Формы для локального применения одобрены FDA. Однако эффективность и безопасность на примере женщин с личным анамнезом РМЖ предстоит изучить в будущем [34].
Новым достижением в области лечения вульвовагинальной атрофии является использование твердотельного эрбиевого лазера. Фототермическое влияние лазера обеспечивает нагревание и сокращение коллагена в слизистых и коже с последующим подтягивающим эффектом. Минимальное термическое воздействие не сопровождается деструктивными изменениями тканей, поэтому сама процедура обычно безболезненна и комфортна для пациентки. На данный момент неизвестно, как долго сохраняется эффект лечения [35].
Остеопороз и рак молочной железы
Пациенты с РМЖ в анамнезе имеют высокий риск развития остеопороза вследствие наступления ранней менопаузы после химиотерапии и/или при использовании ИА. Сочетание дефицита витамина D с приемом ИА ассоциировано со значимой потерей минеральной плотности костей. Бисфосфонаты и деносумаб имеют весомую доказательную базу успешного лечения как остеопороза, так и костных метастазов. Стандартные дозы препаратов не только эффективно предотвращают потерю кости, но и низкоэнергетические переломы. Всем женщинам с диагнозом РМЖ, которым планируется назначение ИА или при наличии ранней менопаузы, должен быть проведен мониторинг состояния костной ткани. В случаях назначения препаратов группы ИА больным гормонозависимым РМЖ в постменопаузе (в т.ч. получающим овариальную супрессию) с целью профилактики остеопороза и снижения риска рецидива болезни рекомендуется назначить: бисфосфонаты (золедроновая кислота 4 мг внутривенно 1 раз в 6 месяцев) в течение 2–3 лет; витамин D3 800 МЕ / сут, внутрь ежедневно и кальций 1500 мг / сут, внутрь ежедневно; контроль минеральной плотности костей (денситометрия) 1 раз в год [36].
Заключение
Инновационные успехи ранней диагностики и современная противоопухолевая терапия обеспечивают постоянный прирост показателей выживаемости больных РМЖ. Многие из них имеют низкое качество жизни из-за постоянных ВМС, бессонницы и вульвовагинальной атрофии. За последние 15 лет накоплен опыт эффективной коррекции менопаузальных расстройств у пациенток с диагностированным РМЖ. Учитывая иммуногистохимические особенности РМЖ, негормональные методы лечения являются приоритетными инструментами в борьбе с менопаузальными расстройствами. Следует избегать системной МГТ, так как назначение последней предполагает более высокий риск рецидива заболевания. По мнению экспертов международных сообществ по менопаузе, локальная терапия ГУМС эстрогенами может проводиться только в микродозах и по веским показаниям. Однако в России на сегодняшний день микродозы эстрадиола не зарегистрированы. В инструкциях по применению препаратов эстриола, зарегистрированных в Российской Федерации, РМЖ указан как противопоказание к применению. Требуются долгосрочные исследования безопасности локальных эстриолсодержащих препаратов для применения у пациенток с РМЖ.



