Algorithm for overcoming infertility secondary to ovarian endometriotic cyst: a view of a reproductive specialist and a surgeon
Aim. To investigate the demand and effectiveness of algorithms for the management of infertility using IVF and surgical methods in women with ovarian endometriotic cysts (OEC) on a case-by-case basis, taking into account the OEC manifestations and clinical presentations associated with age, ovarian reserve, and concomitant indications for IVF.Krasnopolskaya K.V., Popov A.A., Fedorov A.A., Ershova I.Yu.
Materials and methods. The study analyzed clinical spectrum OEC in 532 infertile women regarding the distribution of patients by age, ovarian reserve, and the presence of concomitant indications for IVF. The study compared the demand and effectiveness of three algorithms selected based on the OEC characteristics and clinical status affecting the reproductive potential.
Results. The most popular algorithm (80.3%) for the management of infertility in women with OEC was IVF’s initial administration, which resulted in a cumulative pregnancy rate (PR) of 86.7%. Algorithms with the initial use of surgery to create conditions for spontaneous pregnancy or to prepare for urgent IVF were in demand in 11.4% and 8.3% of cases, respectively. They had 93.4% and 36.4% cumulative PR, respectively.
Conclusion. The selection of the optimal algorithm for the management of infertility using IVF and surgical methods in women with OEC requires considering the age, ovarian reserve, and concomitant indications for IVF and the features of the detected OECs. The widespread use of cryopreservation technology allows the re-transfer of embryos without ovarian stimulation (thus reducing the program’s overall cost). It implies the possibility of continuing fertility treatment in cases of surgical menopause after OEC removal.
Keywords
Endometriosis is one of the most common gynecological diseases associated with infertility. While among fertile women endometriosis prevalence is 6–10%, it affects 35–50% of patients with infertility [1]. In about 1/3 of infertile women with endometriosis, it presents as an ovarian endometriotic cyst (OEC) (endometrioma). OEC may be the only manifestation of the disease or co-occur with other endometriotic lesions. Besides, infertility in patients with OEC can be associated with concomitant tubal and male factors infertility [2, 3].
Management of infertility in women with OEC requires optimal therapeutic algorithms regulating the use of surgical methods and IVF, taking into account the clinical situation, determined by the characteristics of patients’ reproductive status (associated with the age and state of the ovarian reserve) and the nature of endometriotic ovarian lesions [4–7].
The present study is aimed to investigate the demand and effectiveness of algorithms for the management of infertility using IVF and surgical methods in women with OEC on a case-by-case basis, taking into account the OEC manifestations and the characteristics of the clinical status associated with age, ovarian reserve, and concomitant indications for IVF.
Materials and methods
The study included 532 infertile patients with OEC who underwent infertility treatment at the Department of Reproduction of the MRRIOG in 2016–2019. When patients’ profiles were included in the study, OEC manifestations were characterized following the classification of K.V. Krasnopolskaya (Table 1) [8]. In addition to the OEC features given in this classification, the analysis included patients’ distribution according to their age, the state of the ovarian reserve, the presence/ absence of concomitant indications for IVF, and also excluded the possible malignant changes in the detected ovarian masses.
Age of OEC patients was classified as the «optimal» reproductive age (<35 years) and «late» reproductive age, which, in turn, were subdivided into three age categories(from 36 to 38, from 39 to 42 and over 42 years).
Changes in ovarian reserve were investigated by measuring the levels of anti-Müllerian hormone (AMH) and antral follicle count (AFC) of follicles measuring > 10 mm. The ovarian reserve was considered reduced at AMH values <0.3 ng/ml and/or AFC <4 in both ovaries [9].
Concomitant indications for IVF were associated with the presence of tubal and/or male factors infertility in OEC patients, established according to the medical history and diagnostic evaluation.
The risk of malignancy in the detected ovarian masses was assessed using ultrasound criteria based on the Simple Rules from the International Ovarian Tumor Analysis (IOTA) group [10, 11]. In difficult cases, patients’ examination included expert serial ultrasound monitoring of 2–3 cycles, color Doppler, magnetic resonance imaging, and computed tomography. Blood levels of tumor markers were also taken into account (mandatory were НЕ-4 + СА-125 with the calculation of the ROMA index; additionally – CA-19-9, cancer embryonic antigen, and alpha-fetoprotein).
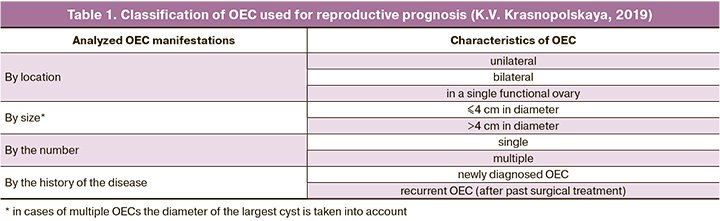
Based on initial evaluation and taking into account characteristics of OEC manifestations (Table 1), patient age, the state of ovarian reserve, and the presence/ absence of concomitant indications for IVF, it was decided to use one of three options for the basic infertility management algorithms, which regulate the use of IVF and surgical methods (Table 2). The study aimed at assessing the demand (frequency of use) of each of these three algorithms.
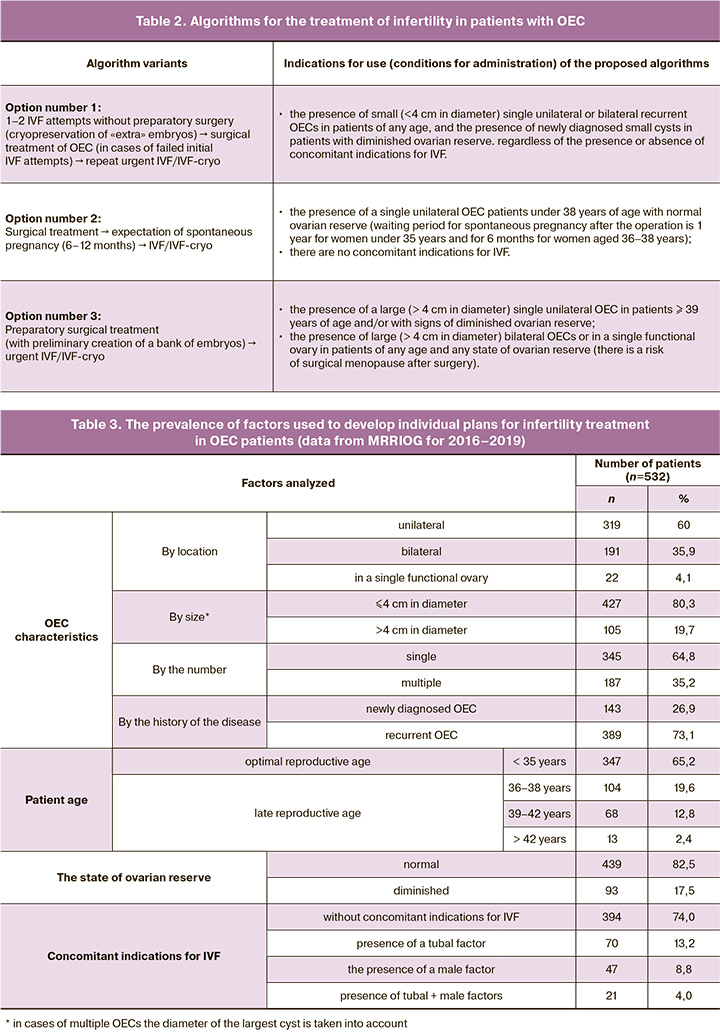
Surgery for OEC was performed based on the disease's symptoms, the size, and ovarian mass location using endoscopic or robotic-assisted techniques. During the surgical operation for OEC, other endometriotic lesions were removed. If there was an adhesive process, an appropriate correction was carried out to restore the anatomical relationships of the pelvic organs.
The IVF was performed according to the standard recommendations for managing patients at the pre- and post-transfer stages of the procedure [12]. Sixty percent of patients underwent ovarian stimulation with GnRH-antagonist-based ovarian stimulation protocols, and 40% had a protocol with a GnRH antagonist. Embryos received during the IVF program were preserved using cryotechnology (vitrification technique [13]) to ensure the possibility of embryo transfer in non-stimulated cycles (IVF-cryo). Cryopreservation was also used to create a bank of embryos before the preparatory surgery for OEC in cases of an increased risk of induction of surgical menopause (for example, if bilateral ovarian surgery was necessary, or in patients aged over 38 or those with an initially diminished ovarian reserve). Frozen-thawed embryo transfer was used in the postoperative period (IVF-cryo) in case of difficulties in obtaining fresh embryos.
The effectiveness of infertility treatment in patients with OEC using the proposed therapeutic algorithms (Table 2) was assessed by the cumulative pregnancy rate (CPR). The CPR was calculated:
- when using the therapeutic algorithm No. 1: according to the number of pregnancies resulting from the «starting» use of IVF and urgent IVF (administered after surgical preparation) with native or cryopreserved embryos.
- when using the therapeutic algorithm No. 2: by the number of pregnancies provided by surgical treatment (spontaneous pregnancies in the postoperative period) and IVF/IVF-cryo;
- when using the therapeutic algorithm No. 3: according to the number of pregnancies achieved due to urgent IVF after surgery using native and (in some patients) cryopreserved embryos.
Statistical analysis
Statistical analysis was performed using the Statistica-V.8.0 software. Categorical variables were compared by the Chi-square (χ2) test with the Yates correction for continuity and the two-tailed Fisher's exact test. Differences between the groups were considered statistically significant at p<0.05.
Results
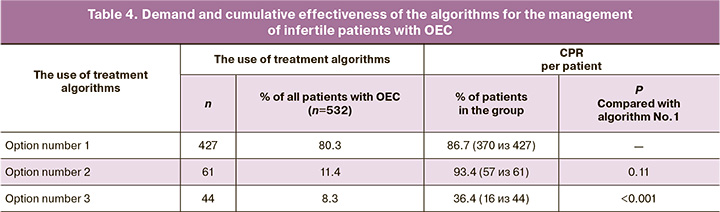
When assessing the prevalence among the observed patients with OEC of factors that should be taken into account for developing individual plans for infertility treatment, the following patterns were established (Table 3).
Unilateral OECs were detected in 319/532 (60%) patients (twice as likely in the left ovary). In comparison, bilateral ovarian lesions were diagnosed in 191/532 (35.9%) patients, and in 22/532 (4.1%) had a lesion of a single functional ovary. In terms of size, OECs measuring < 4 and > 4 cm in diameter were found in 427 (80.3%) and 105 (19.7%) patients, respectively. OECs were twice more likely to be single than multiple, comprising 345 (64.8%) and 187 (35.2%) patients, respectively. The analysis of the disease history (according to the anamnesis and archival medical case records) revealed that most of the OECs were recurrent 389 (73.1%), while only in 143 (26.9%) patients they were diagnosed for the first time.
An analysis of OEC patients’ age showed that 347/532 (65.2%) were aged <35, i.e., were in the «optimal» reproductive period. The rest of the patients were in the «late» reproductive period (104 (19.6%), 68 (12.8%), and 13 (2.4%) patients were 36–38, 39–42, and > 42 years old, respectively).
The ovarian reserve was satisfactory and diminished in 439 (82.5%) and 93 (17.5%) patients, respectively.
No concomitant indications for IVF were found in 394 (74%) patients. The remaining 138 (26%) patients had additional indications for IVF associated with the tubal factor [70 (13.2%)], male factor [47 (8.8%)], or a combination of these two factors of infertility [21 (4%)]. Most infertile patients with OEC [427/532 (86.7%)] were managed according to therapeutic algorithm No.1. Algorithms No.2 and No.3 were used in 61/532 (11.4%) and 44/532 (8.3%) patients, respectively (Table 4). At the same time, the CPR was the highest and not statistically different (p=0.11) among patients managed by therapeutic algorithms No.1 [370/427 (86.7%)] and No.2 [57/61 (93.4%)]. Among patients managed by therapeutic algorithm, No.3 CPR was only 16/44 (36.4%), which was statistically lower (p<0.001) than in the groups that used the therapeutic algorithms No.1 and No.2.
Discussion
The study findings suggest that infertile patients with OEC represent an extremely heterogeneous group in terms of manifestations of endometriotic ovarian lesions (taking into account the criteria of our classification), age, ovarian reserve, and the presence/absence of concomitant indications for IVF. Nevertheless, it can be stated that this group of patients is characterized by relatively small OECs [in 427 (80.3%) patients]. They were found as a single unilateral [in 345 (64.8%) patients] or bilateral [in 319 (60%) patients] OECs, and were recurrent according to the history of the disease in 389 (73.1%) patients. Also, infertile women with OEC were typically in «optimal» (<35 years) reproductive age [347 (65.2%) patients], normal ovarian reserve [439 (82.5%)], and had no concomitant indications for IVF [394 (74%)].
Our practice shows that to overcome infertility in women with OEC, the most demanded is therapeutic algorithm No.1, which suggests initial IVF use and its reapplication after OEC removal in patients with persistent infertility. This observation is explained by the fact that in most women, the endometrioma diameter does not exceed 4 cm, i.e., the size of the detected OECs does not require their mandatory preliminary removal. It can be noted here that, according to modern concepts, preserved small OECs (<4 cm) do not affect the effectiveness of IVF and generally have no apparent association with infertility [14, 15]. Therefore, all infertile women with this manifestation of ovarian endometriosis should be considered as patients with unexplained infertility, for which IVF is recommended without any preparatory surgery. In cases where the clinical situation allows using this therapeutic algorithm, the CPR can be very high values due to multiple repeated IVF attempts and frozen-thawed embryo transfers (in our work, we managed to provide CPR in 370 (86.7%) patients). The «embryonic» factor explains persistent infertility in individual patients undergoing multiple repeat IVF attempts after excision of small OECs. The deterioration of embryo quality can be associated with the late reproductive age. The decrease in the number of retrieved embryos may be attributed to the diminished ovarian reserve due to ovarian aging. A reduction in the follicular reserve may be linked to past OEC surgical treatment in patients with recurrent OEC and possible aneuploidies.
Therapeutic algorithm No.2 with initial surgical treatment of OEC aimed to restore natural fertility can be used relatively rarely (according to our observations, no more than 61/532 (11.4%) of all patients with OEC). This is because the use of such treatment is contraindicated in patients over 38 years of age with a diminished ovarian reserve and concomitant indications for IVF, associated, for example, with tubal or male factors infertility. Besides, surgery aimed at restoring natural fertility, in our opinion, is indicated only for large (> 4 cm) unilateral OECs. Small OECs are unlikely to be the cause of infertility, and therefore their surgical treatment to restore natural fertility has no theoretical foundation [6, 7]. It is noteworthy that the CPR resulted from using therapeutic algorithm No.2 was even higher than when using therapeutic algorithm No.1. This observation can be explained by the fact that therapeutic algorithm No.2 was used to manage patients under 38 years of age with normal ovarian reserve. It should also be noted that the surgical treatment of OEC used in therapeutic algorithm No.2 never resulted in a significant reduction of ovarian reserve since the interventions involved only one of the two ovaries. There is no doubt that these circumstances determined the best results of therapeutic algorithm No.2 in the treatment of infertility (in terms of CPR) in patients with OEC.
The therapeutic algorithm, including surgical treatment of OEC aimed to prepare for urgent IVF (algorithm No.3), also has minimal use (in our work, it was used only in 8.3% of all patients with OEC). Patients with OEC requiring the use of this algorithm for the management of infertility are characterized by the most unfavorable reproductive prognosis due to their age (≥39 years) and the state of the ovarian reserve (the initial presence of signs of its reduction in some patients). In these women, the risk of surgical menopause was increased due to the need to remove large bilateral ovarian cystic masses or in a single functional ovary. For this reason, in this group of patients with OEC, despite multiple repeat IVF attempts and the use of a bank of cryopreserved embryos created before the operation, the CPR was significantly lower than in the groups where the clinical situation allowed using therapeutic algorithms No.1 or No.2. It is noteworthy that about half of the patients managed by therapeutic algorithm No.3, who underwent OEC removal, required IVF with a frozen-thawed embryo (retrieved before surgical treatment) due to the lack of conditions for repeat stimulation due to a sharply reduced ovarian reserve. This observation confirms the opinion of specialists [5], who emphasize the importance of creating a frozen embryo bank before surgery associated with the risk of surgical menopause or in women with diminished ovarian reserve.
Conclusion
The choice of optimal algorithm for the management of infertility using IVF and surgery in women with OEC requires taking into account not only the age, the state of the ovarian reserve, and the presence of concomitant indications for IVF, but also the characteristics of OECs associated with symptoms, their size, number, location and whether it recurrent or not. Cryopreservation technologies should be used more widely since the supply of cryopreserved embryos allows for repeat embryo transfers without ovarian stimulation (which reduces the overall cost of the program) and makes it possible to continue fertility treatment in cases of surgical menopause after OEC removal.
The advances in assisted reproductive technologies in recent years have far exceeded those in reproductive surgery. Recently numerous new IVF centers that cannot perform surgical interventions for infertility have been opened. They are focused only on the use of assisted reproductive technologies (ART), which reduced the importance of surgical treatment of this form of infertility. Many studies now recognize the central role of ART, which, in our opinion, leads to a loss in the quality of surgery. Timely and appropriately performed surgery can achieve spontaneous pregnancy in 30–70% of cases. This means that one to two-thirds of patients does not need IVF.
This does not mean that surgery is the only way to treat infertility. It is especially necessary to reduce the number of the repeat (third, fourth, etc.) surgical interventions for this form of infertility, promptly consult patients with a reproductive specialist, and recommend ART. As in all medicine, there must be a «golden» balance between surgery and ART. This balance can be achieved in the context of a multidisciplinary team and a close relationship between surgeons and reproductive specialists. The council must develop a plan for achieving pregnancy and inform the patient about all possible ways to overcome infertility.
References
- Reid S., Condous G. Endometriomas and pelvic endometriosis. In: Guerriero S., Martins W., Alcazar J., eds. Managing ultrasonography in human reproduction. Springer, Cham; 2017: 123-36. https://dx.doi.org/10.1007/978-3-319-41037-1_7.
- Maggiore U.L.R., Gupta J.K., Ferrero S. Treatment of endometrioma for improving fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 81-5. https://dx.doi.org/10.1016/j.ejogrb.2016.02.035.
- Sönmezer M., Taşkin S. Fertility preservation in women with ovarian endometriosis. Womens Health. (Lond). 2015; 11(5): 625-31. https://dx.doi.org/10.2217/whe.15.49.
- Gelbaya T.A., Gordts T., D’Hooghe T.M., Gergolet M., Nardo L.G. Management of endometrioma prior to IVF: compliance with ESHRE guidelines. Reprod. Biomed. Online. 2010; 21(3): 325-30. https://dx.doi.org/10.1016/j.rbmo.2010.04.023.
- Kuroda K., Ikemoto Y., Ochiai A., Ozaki R., Matsumura Y., Nojiri S. et al.Combination treatment of preoperative embryo cryopreservation and endoscopic surgery (Surgery-ART Hybrid Therapy) in infertile women with diminished ovarian reserve and uterine myomas or ovarian endometriomas. J. Minim. Invasive Gynecol. 2019; 26(7): 1369-75. https://dx.doi.org/10.1016/j.jmig.2019.02.008.
- Lessey B.A., Gordts S., Donnez O., Somigliana E., Chapron C., Garcia-Velasco J.A. et al. Ovarian endometriosis and infertility: in vitro fertilization (IVF) or surgery as the first approach? Fertil. Steril. 2018; 110(7): 1218-26. https://dx.doi.org/10.1016/j.fertnstert.2018.10.003.
- Muzii L., Di Tucci C., Di Feliciantonio M., Verrelli L., Galati G., Benedetti Panici P. Infertility associated with ovarian endometriomas: surgery or in-vitro fertilization? J. In Vitro Fertil. 2018; 1(1): 1-3. https://dx.doi.org/10.36959/983/634.
- Краснопольская К.В. Лечение бесплодия при эндометриозе: взгляд репродуктолога. М.: МЕДпресс-информ; 2019. 112 c. [Krasnopolskaya K.V. Treatment of endometriosis-associated infertility: a view of the reproductologist. M.: MEDpress-inform; 2019. 112 p. (in Russian)].
- Tal R., Seifer D.B. Ovarian reserve testing: a user’s guide. Am. J. Obstet. Gynecol. 2017; 217(2): 129-40. https://dx.doi.org/10.1016/j.ajog.2017.02.027.
- Landolfo C., Froyman W., Bourne T., De Cock B., Testa A.C., Valentin L. et al. OC11.02: IOTA benign easy descriptors and ADNEX model integrated in a ‘traffic light system’ to guide the clinical management of adnexal pathology. Ultrasound Obstet. Gynecol. 2017; 50(Suppl. 1): 21-2. https://dx.doi.org/10.1002/uog.17623.
- Timmerman D., Van Calster B., Testa A., Savelli L., Fischerova D., Froyman W. et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis (IOTA) group. Am. J. Obstet. Gynecol. 2016; 214(4): 424-37. https://dx.doi.org/10.1016/j.ajog.2016.01.007.
- Краснопольская К.В., Назаренко Т.А. Клинические аспекты лечения бесплодия в браке. Диагностические и терапевтические программы с использованием методов восстановления естественной фертильности и вспомогательных репродуктивных технологий. Pуководство. М.: ГЭОТАР-Медиа; 2013. 376 c. [Krasnopolskaya K.V., Nazarenko T.A. Clinical aspects of treatment infertility in marriage. Diagnostic and therapeutic programs with usage of methods of reconstruction natural fertility and assisted reproductive technologies: guideline. Moscow: GEOTAR-Media; 2013. 376 p.(in Russian)].
- Краснопольская К.В., Сесина Н.И., Бадалян Г.В., Черкезов Я.А., Ивахненко В.Н., Назаренко Р.В. Медленное замораживание и витрификация эмбрионов. Сравнение эффективности. Проблемы репродукции. 2015; 21 (1): 48-53. [Krasnopolskaya K.V., Sesina N.I., Badalyan G.V., Cherkasov Ya. A., Ivahnenko V.N., Nazarenko R.V. Slow freezing and vitrification of embryos. Comparison of effectiveness. The problem of reproduction. 2015; 21 (1); 48-53. (in Russian)]. https://doi.org/10.17116/repro20152148-53.
- Ferrero S., Scala C., Tafi E., Racca A., Venturini P.L., Leone Roberty Maggiore U. Impact of large ovarian endometriomas on the response to superovulation for in vitro fertilization: a retrospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 213: 17-21. https://dx.doi.org/10.1016/j.ejogrb.2017.04.003.
- Vignali M., Mabrouk M., Ciocca E., Alabiso G., Barbasetti di Prun A., Gentilini D. et al. Surgical excision of ovarian endometriomas: does it truly impair ovarian reserve? Long-term anti-Müllerian hormone (AMH) changes after surgery. J. Obstet. Gynaecol. Res. 2015; 41(11): 1773-8. https://dx.doi.org/10.1111/jog.12830.
Received 29.04.2020
Accepted 29.05.2020
About the Authors
Ksenia V. Krasnopolskaya, Corr. Member of the RAS, Professor, Head of the Department of Reproduction, Moscow Regional Research Institute of Obstetrics and Gynecology. Tel.: +7(495) 980-40-28. E-mail: deti222@mail.ru. ORCID: 0000-0002-1275-9220.101000, Russia, Moscow, Pokrovka str., 22A.
Alexander A. Popov, Dr. Med. Sci., Professor, Head of the Department of Endoscopic Surgery, Moscow Regional Research Institute of Obstetrics and Gynecology.
Tel.: +7 (495) 625-73-32. E-mail: gyn_endoscopy@mail.ru. 101000, Russia, Moscow, Pokrovka str., 22A.
Anton A. Fedorov, Ph.D., Leading Researcher at the Department of Endoscopic Surgery, Moscow Regional Research Institute of Obstetrics and Gynecology.
Tel.: +7(495)625-73-32. E-mail: gyn_endoscopy@mail.ru. ORCID: 0000-0003-2590-5087. 101000, Russia, Moscow, Pokrovka str., 22A.
Irina Yu. Ershova, Ph.D., Researcher at the Department of Reproduction, Moscow Regional Research Institute of Obstetrics and Gynecology.
Tel.: +7(495)980-40-28. E-mail: deti222@mail.ru. ORCID: 0000-0001-9327-0656. 101000, Russia, Moscow, Pokrovka str., 22A.
For citation: Krasnopolskaya K.V., Popov A.A., Fedorov A.A., Ershova I.Yu. Algorithm for overcoming infertility secondary to ovarian endometriotic cyst: a view of a reproductive specialist and a surgeon.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 11: 78-84 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.78-84



