Adaptation of the fetus to sequential cardiotocographic examinations and the development of closed loops of self-regulation
Loginov V.V., Davydov D.G., Prikhodko A.M., Baev O.R., Kan N.E., Tyutyunnik V.L., Romanov A.Yu., Degtyarev D.N.
Objective: To study changes in fetal heart rate variability (HRV) during antenatal cardiotocographic (CTG) monitoring.
Materials and methods: This study included data from 13,520 CTG recordings obtained from 3,380 pregnant women. The analysis was performed using clustering methods and statistical analysis of HRV.
Results: The initial increase in HRV was identified as a response to the stimulus, whereas subsequent reductions reflected the development of self-regulatory mechanisms. Strategy-type habituation (STH) correlated with the maturity of fetal regulatory systems. In some cases, HRV reduction was associated with dysregulation and neonatal complications.
Conclusion: HRV dynamics can serve as an objective and quantitative criterion for assessing fetal physiological states and predicting potential deviations. Antenatal CTG monitoring revealed a consistent pattern of fetal HRV changes, which may indicate adaptive processes. The initial HRV increase is a response to the stimulus, whereas the subsequent reduction reflects the formation of self-regulatory mechanisms. The key parameter is STH, which enables the assessment of fetal regulatory system maturity. Furthermore, it has been established that HRV reduction under certain conditions may be linked to dysregulation and neonatal complications. The study results suggest that HRV dynamics can be utilized as a quantitative criterion for assessing fetal physiological states.
Authors' contributions: Loginov V.V. – review of relevant publications, conception and design of the study, collection and processing of material, drafting of the manuscript; Davydov D.G. – collection and processing of material, data analysis; Prikhodko A.M., Kan N.E., Tyutyunnik V.L. – review of relevant publications, drafting of the manuscript; Baev O.R., Romanov A.Yu., Degtyarev D.N. – conception and design of the study, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the research project “Search for new methods of objective quantitative assessment of cognitive functions during normal and pathological development of the fetus”, conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation in 2019–2020.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Loginov V.V., Davydov D.G., Prikhodko A.M., Baev O.R., Kan N.E., Tyutyunnik V.L., Romanov A.Yu., Degtyarev D.N. Adaptation of the fetus to sequential cardiotocographic examinations and the development of closed loops of self-regulation.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 69-78 (in Russian)
https://dx.doi.org/10.18565/aig.2025.73
Keywords
When recording antenatal cardiotocography (CTG), fetuses show an increase in heart rate variability (HRV) in response to the procedure, which can be considered an adaptive response. The subsequent decrease in HRV probably reflects the stabilization of fetal regulatory mechanisms, formation of stable neuronal circuits, and transition to a more economical mode of cardiovascular function. This decrease may be associated with the development of a habituation-like strategy (STH) that provides an optimal response to repeated stimuli and reduces the energy expenditure of the organism. Also, the HRV profile during the adaptation period reliably reflects the fetal condition at the time of CTG registration according to the fetal condition indicator (FCI)1 and is an objective predictor of the outcome of labor [1, 2]
The main criterion of satisfactory fetal condition in both cases is an increase in heart rate variability – the initial deviation of basal heart rate (HR) by habituation type, which has received a special name – strategy type habituation (STH), as an indicator of a fundamental, evolutionarily conditioned and genetically fixed learning mechanism [3].
In this regard, STH is recognized as an objective and quantitative indicator of physiologically normal development of fetal regulatory systems, and a decrease in variability associated with low FCI (dishabituation) as an indicator of the development of various complications in the early neonatal period [1, 4]
In our opinion, STH reflects the process of stabilization of the basal HR level, providing the supply of necessary plastic and morphogenetic factors. Adaptation according to the type "impact–reaction" is manifested only in the formed hierarchical system of regulation, working within the physiological norm. In this case, the "active" character of learning is associated with the selection of stimuli by the significance and fixation of information in memory, which forms adaptive mechanisms. A decreased response to the CTG procedure in the first adaptation period is associated with the recognition of stimuli, their significance, and formation of specific neuronal networks. From examination to examination, STH reflects the successful maturation of cognitive processes, such as memory and the analysis of repetitive information, which ensures fetal self-regulation during early development. However, the adequate "reactive" nature of adaptation of the "impact-response" type is manifested when the entire complex, polyfunctional, and multilevel hierarchical system of adaptability is formed, its individual components are synchronized in functionality, tuned, and working within the physiological norm, and biological expediency consists mainly of maintaining dynamic equilibrium in the organism. Such representation leaves out of the broad discussion the "active" character of learning, which is realized through the mechanisms of selection of stimuli by significance at a particular moment of time and fixation of pragmatic information in memory. Without this, it is impossible to imagine the coding of adaptability as a phylogenetically verified sequence of realization of biological expediencies as the main driving force of organism development, including the formation of the adaptation system as a separate global function. It follows that the functional significance of the decreased response to the CTG procedure in the first adaptation period is related to the recognition of stimuli, determination of their significance, formation of specific neural networks, and fixation of pragmatic information on memory. Whereas STH from examination to examination is an indicator of successful maturation of such cognitive processes as access to memory, search for relevant information, comparison of repeated information with the "neural model of the stimulus" formed in the first examination in search of objective indicators of coordination of self-regulating mechanisms of different levels into a holistic adaptive activity at the early stages of the organism's life cycle and choice of behavioral attitude [5]
The aim of this study was to retrospectively analyze STH in healthy fetuses (whose development most clearly reflects the active character of adaptability formation) during CTG.
Materials and methods
The main PARADOX format database was created from the memory of eight UNIKOS2 devices, which were stored in a binary format.
The total data volume was 1.8 Gb. Using a specially developed application with the help of the standard CTG analysis program Fetal, data were extracted for the following parameters: RR intervals (in milliseconds) and a total of 47,000 CTG records containing numerical values in text format. In the RR interval dataset, records with system errors, duplicates, those with a recording duration of less than 2300 values (approximately 15 min), and observations with more than 20% missing values from the entire recording length were removed. To maintain the data processing speed, the length of all observations was limited to 5000 intervals (approximately 33 min of CTG recording). The total number of records was 43520, obtained from 30150 pregnant women aged 14–55 years, with gestational ages of 30 and 41 weeks from 20.07.2011 to 26.02.2020 (Fig. 1).
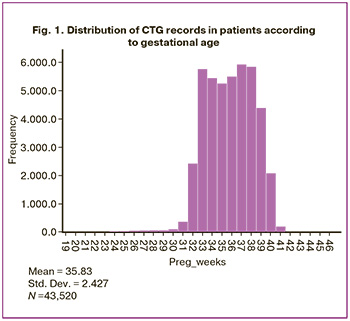
The data of all patients included in the study and their newborns were uploaded from the Electronic Medical Record Medialog with the diagnoses of healthy fetuses, according to the ICD-10 code. The records contained the child's year of birth and the full name of the mother, which made it possible to link this set with the list of CTG records.
CTG registration was performed using a computerized cardiotachograph UNIKOS, which includes ultrasonic sensors and signal processing based on the Doppler effect. The initial data were successive measurements of the RR interval HR of the fetus. Additionally, the following parameters were used: mother's age, gestational age, and an automatically calculated fetal condition index. The data were processed using the IBM SPSS 25 statistical software package and the Orange 3.21 graphical interface for applied analysis algorithms in the Python language.
To study STH in sequential CTGs, records made sequentially at least four times at an interval of no more than 60 days were selected. In total, 13520 CTG records were obtained from 3380 pregnant women.
The difference between the successive pairs of conjugate RR intervals was then calculated. The results were standardized by calculating individual z-scores (the difference from the individual average for the CTG record, expressed in fractions of the standard deviation). Then, median smoothing was performed with a moving window equal to 5 RR intervals and normalization of values by logarithmic transformation, as a result of which all HRV values were reduced to a scale from -3 to +3 with an average value for the CTG record equal to 0.
In the CTG records, five ranges of the initial RR interval sequences were analyzed: 10, 20, 30, 50, and 100 first HRV measurements.
Fetuses that reacted similarly to the start of the CTG procedure were identified via cluster analysis using the k-means method. The average HRV values of the first 20 RR intervals in four consecutive CTG procedures were used as variables. The number of clusters was determined using the Bayesian Schwarz criterion (BIC) and Akaike information criterion (AIC).
A separate dataset (19 CTGs) was created using recording fragments collected after the fourth examination, which involved three consecutive cycles of switching the CTG sensor on and off. Each recording fragment was separated by a period of sensor shutdown, denoted by a long sequence of zeros. In accordance with this, each original record was divided into three separate records. The beginning of the recorded fragments was determined as the end of the zeros sequence. Each record was encoded in accordance with the order of the location: 1, 2, and 3. The resulting dataset consisted of 57 observations, consisting of three groups corresponding to the first, second, and third inclusion of the ultrasonic emitter of the UNIKOS device. HRV was calculated as the standard deviation for each of the 10 consecutive RR intervals, with a shift of one unit (from the 1st to 10th, from the 2nd to 11th, etc.).
Results
The selection of the relevant epoch for HRV analysis was performed visually and based on data processing across five ranges of initial RR interval sequences: the first 10, 20, 30, 50, and 100 HRV measurements. Figure 2A presents a graphical depiction of the dynamics of HRV mean values (8132 observations – control group) in healthy fetuses during the first CTG registration (analysis epoch 50). It is evident that the first ultrasound signal activation caused a clear increase in HRV relative to the baseline level of the CTG recording. Subsequently, a gradual decrease in this indicator was observed, which persisted until the 31st RR interval. It is also visible that the monotonic decline is disrupted starting from the 23rd RR interval, and further HRV changes indicate stabilization of the baseline CTG level.
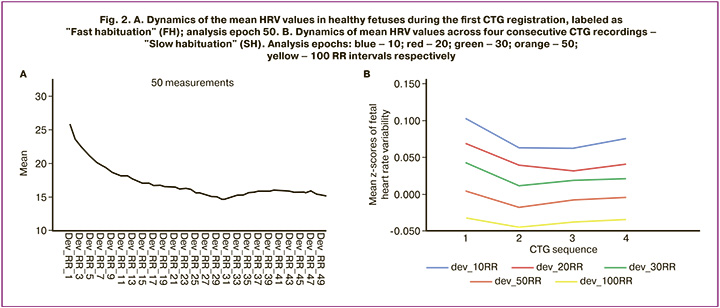
Figure 2B shows the mean HRV values across four consecutive CTG recordings at different epoch durations: blue – 10, red – 20, green – 30, orange – 50, yellow – 100 RR intervals. It is apparent that the longest HRV decline (habituation), albeit with some disruption of monotonicity (see Fig. 2B, red), is characteristic of 20 RR-interval analysis epochs. Additionally, when the epoch duration decreased (blue line), HRV stabilized after the second CTG registration, whereas increasing it led to dishabituation. Thus, it was established that the 20 RR-interval analysis epoch was the most promising for further research.
The dynamics of the mean values of the first 20 fetal heartbeats across the four consecutive CTG procedures are shown in Figure 3A. There was a decrease in HRV over three consecutive CTG examinations, with a significant reduction in the mean values of this indicator between the first and third CTG examinations (Student's t-test, p≤0.01). The subsequent increase in HRV from the third to fourth examination could indicate the presence of opposing trends within the overall observation sample.
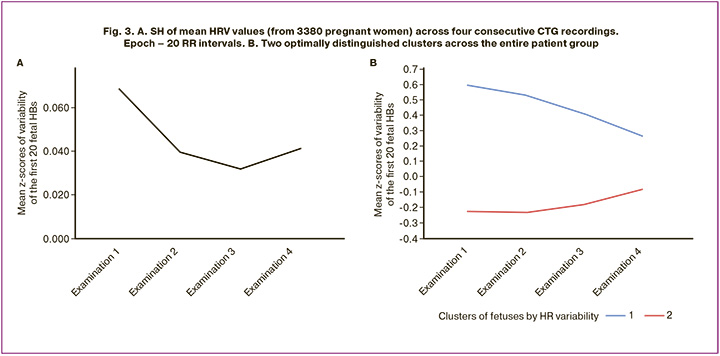
Figure 3B presents the results of clustering fetal responses to ultrasound signals during consecutive CTG examinations using k-means cluster analysis. The number of clusters was determined using the Schwarz Bayesian and Akaike information criteria. The optimal solution was to identify two clusters. In the first cluster (Fig. 3B, blue line) included 1218 fetuses (36%), and the second (Fig. 3B, red line) – 2162 (64%).
Analysis of the gestational age showed that the first cluster (Fig. 3B, blue line) comprised relatively early fetuses, from 30 to 36 weeks, whereas the others (Fig. 3B, red line) ranged from 34 to 41 weeks respectively. Comparing this with stages of fetal cardiovascular system development: at week 5 – autonomous heartbeat, week 18 – parasympathetic innervation, week 27 – sympathetic innervation, the stage analyzed in this work represents a period of fine regulatory adjustment of the overall functional system.
The study findings suggest that fetuses from different groups perceive the same ultrasound exposure differently. The fetuses in the first cluster (Fig. 3B, blue line) were in a state of excessive residual sympathetic influence. Ultrasound exposure further enhanced HRV. However, by the fourth CTG examination, the realization of FH and SH approaches near-normal levels. The fetuses in the second cluster (Fig. 3B, red line) are in a state of residual excessive parasympathetic influence. Dishabituation was observed, with a gradual increase in the initially decreased HRV. Ultrasound stimulation stimulates the formation of a more powerful synaptic pool to optimize energy expenditure during higher-level functional regulation. Despite this, both perception types were adaptive.
Thus, the results are related to at least four variants of neural activity reorganization. The first reorganization is a classic response to a novel stimulus with uncertain significance, involving ultrasound radiation as a stressful impact (the "What is it" reaction according to I.P. Pavlov), including mechanisms of the ascending reticular activating system, mechanisms for determining stimulus novelty, engagement of the limbic system of the brain, decision-making regarding the significance of the event, and mechanisms for fixing pragmatic information in memory (Fig. 2A). The second reorganization occurs when the stimulus is no longer new and is at least indifferent and not dangerous. This reorganization also begins with the ascending reticular activating system but then activates pathways to various memory levels, mechanisms for comparing incoming information with the "Nerve model of the stimulus" formed during previous exposure (Fig. 3A, between the 1st and 2nd CTG examinations) and decision making (Fig. 3A, between the 2nd and 3rd CTG examinations). The third reorganization concerns the formation of a higher-level regulatory system that ensures the operation of the cardiovascular system by synchronizing the separately functioning sympathetic and parasympathetic systems (Fig. 3B). The fourth reorganization occurred after four prior CTG recordings, when all issues of novelty and significance were resolved, and a stable perception of ultrasound exposure as negative and disruptive to perceiving potentially more important information was formed. As a result of this mismatch, another important biological reaction – the avoidance reaction (AR) with negative emotional experiences, attempts to withdraw from the stimulus, and perceptual protection – inevitably develops (Fig. 4).
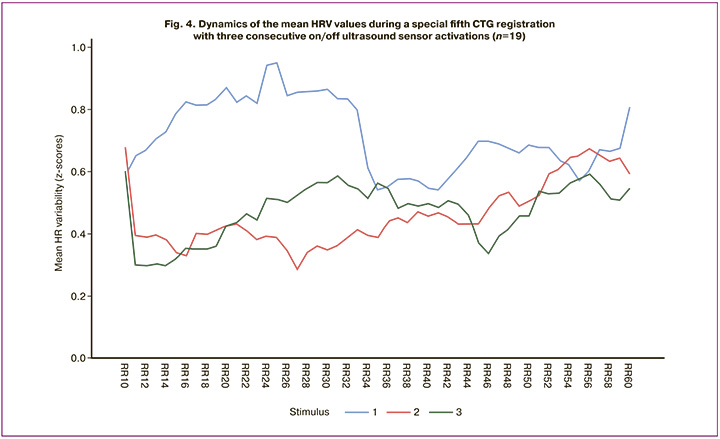
The figure shows a gradual increase in mean HRV in response to the first ultrasound sensor activation (dishabituation) during the first analysis epoch (Fig. 4, blue line), continuing up to the 25th RR interval, after which a slow, non-monotonic decrease (SH) was observed. During the second (Fig. 4, red line) and third (Fig. 4, green line) ON/OFF activations, there was a sharp reduction in the latent period of SH (diminishing at the second RR interval) and a slow, non-monotonic dishabituation until the end of the analysis epoch. The increase in the latent period of SH during the first ultrasound activation and the sharp reduction in subsequent activations indicate that, in this case, the dominant reorganization of neural activity is not the "ascending reticular activating system," but the Peypitz circle (the limbic system) – the emotional-motivational sphere. Negative experiences accumulated during previous CTG recordings upon subsequent ultrasound activation evoke a powerful excitation of various subfields, primarily the associative and motor cortex of the brain, with transmission of current information to effector structures for implementing withdrawal reactions.
Discussion
The findings of this study cannot be fully understood through the traditional "reflex arc" model: receptor → interneuron → effector. The reconfiguration of the fetal neuronal activity appears to be more "active" than merely "reactive." Furthermore, the invariance of STH becomes evident, where a seemingly similar reduction in reaction conceals the involvement of diverse brain structures.
The study's paradigm plays a significant role in interpreting (revealing the invariance of) the STH study's results, especially when studying the fetus, as the use of a wide range of already tested standard conditions (protocol) of examination and STH indicators for different effects is unacceptable. For example, one can note the following paradigms:
* paradigm of recording STH to stress exposure with rhythmic stimulation [6, 7];
* paradigm of recording STH with paired stimuli and a change in the inter-stimulus interval (study of the refractory period);
* various options for recording STH with a change in the probability of a significant stimulus appearing against the background of insignificant "noisy" stimulation;
* recording STH by the time of looking at the image [8];
* fading of galvanic skin response (GSR) [9]
* recording STH during functional magnetic resonance imaging [10], etc.
More than half a century has passed since the development of a special paradigm for studying STH in fetuses [4]. At the end of the last century, in experiments using vibro-acoustic stimulation (VAS) as an "adequate" stimulus on healthy fetuses (32-37 weeks of gestation), a regular monotonous decrease in behavioral reactions such as blinking frequency, turning the head towards the stimulus, opening the mouth, sticking out the tongue, moving the cheeks, moving the hands towards the head, and stretching the legs was observed. These studies demonstrated the relationship between STH and behavioral responses not only in cases of gross morphological pathology of the central nervous system (CNS) but also in terms of features such as gestational age, method of conception (natural or in vitro fertilization), and delivery (natural or cesarean section). In addition, altered STH patterns were described with a decrease in the partial pressure of oxygen, smoking, drug use, and alcoholism of the mother [4]. Based on the fact that each behavioral response of the fetus can be a regulatory manifestation of the CNS, the authors described the ontogenesis of fetal behavior, observing the development of each movement with increasing gestational age. It has been shown that the profile of eye motor activity is most sensitive to changes in the CNS, whereas other behavioral responses exhibit integrative activity [11–15]. Differences in the reorganization of ongoing activity depending on the state of the fetus clearly indicate a direct regulatory function of the CNS. In addition, these results to a certain extent indicated the possibility of determining the degree of localization of functional disorders of the brain in utero [12, 16, 17]. It follows that by assessing the behavioral reactions of the fetus during pregnancy, it is possible to identify with considerable certainty in utero the degree of neurophysiological development or pathological lesions if they are localized caudally to the pons in the medulla oblongata. However, if the deviations were localized in the hemispheres of the brain, no behavioral anomalies were detected in the fetuses during the intrauterine period [12, 18]. This gave the authors reason to judge that today, it remains impossible to assess in utero higher cognitive functions in the CNS of the fetus, including the functions of the cerebral hemispheres.
Some inconclusiveness of the results obtained in the paradigm with VAS can be explained by at least three circumstances:
* Subjectivity in the assessment of behavioral reactions
* conditionally quantitative assessments of STH;
* modest sample sizes.
Thus, all paradigms involving multiple repetitions of the same stimulus during the same STH examination seem to be of limited use for studying fetal STH.
Our study shows that Habituation to a Novel Environment paradigm, often used in rodent STH studies as an indicator of the natural tendency to seek and analyze novelty, is suitable for studying STH in the developing brain. Rodents are placed in a new environment, and STH is determined by the total distance traveled or the amount of time during which they actively explore the environment [19]. The animal can be tested in one or more sessions (i.e., intra-session or inter-session STH can be recorded) under fully controlled conditions [19, 20]. Another important finding, which often attracts attention in the above-mentioned works, are the various signs of maturity (in fetuses after 28–30 weeks of gestation) of the structures of the “brain stem”, in which the reticular formation (RF) is recognized as an integrating link. The RF receives information from all sensory organs and internal organs, evaluates its significance, filters it by this feature, and transmits it to the limbic system and the cerebral cortex. It regulates the level of excitability and tone of various parts of the CNS, including the cerebral cortex, and plays an important role in perception, emotions, sleep, wakefulness, vegetative functions, purposeful movements, as well as in the formation of unconditional reflexes and more complex holistic reactions of the body. The main structures of the RF include the lateral and paramedian reticular nuclei, the reticular nucleus of the pontine tegmentum, the reticular gigantocellular nucleus, the reticular parvocellular nucleus, the ventral and lateral reticular nuclei, the locus coeruleus and the trigeminal nuclei (caudal, interpolar and oral nuclei), the inferior and medial vestibular nuclei, the medial part of the lateral nucleus, the raphe nuclei, the nucleus of the solitary tract, the commissural nucleus, the Edinger-Westphal nucleus and the dorsal nucleus of the vagus nerve, the reticular nucleus of the thalamus, the salivary nuclei, and the respiratory and vasomotor centers of the medulla oblongata. Since the RF includes vital centers (damage to the respiratory and vasomotor centers leads to immediate death), the "sentinel structures" of the organization of the central response to stress are also located nearby. From this, we can conclude that the RF is the central structure of the ancient brain and is adapted to the formation and maintenance of individual unconditional adaptive mechanisms.
Our study showed that RF is actively involved in a healthy fetus in the period from the 30th to the 41st week of gestation.
* in the implementation of STH, as a fundamental learning process, including activation through the "ascending activation system of the brainstem,” assessment of the novelty and significance of the ultrasound signal through the sanctioning "limbic system,” comparison of newly obtained information with previous experience through long-term memory and various fields of the associative cortex of the brain, and fixation of pragmatic information in short-term memory (FH; Fig. 2A).
* in memorization (SH; Fig. 3A);
* in synchronization of sympathetic and parasympathetic influences in the regulation of the cardiovascular system (Fig. 3A);
* in the formation of different types of effector response within the RI (Fig. 4).
Currently, the dominant idea is that the formation of a hierarchical self-regulating adaptive system, as a global function of the organism and a driving force in fetal development, occurs in three stages. At the molecular-genetic level, mechanochemical and histogenetic processes of brain morphogenesis are involved, which are realized according to the cyclic principle. The cycle is triggered by the synthesis of specific genetic signals that can lead to a change not only in the phenotype of cells but also in their size, which, in turn, changes the biomechanical status of the neuroepithelial plate as a whole. As a result, the contact polarization of cells and the work of mechano-dependent ion channels are described not only in the cells of the nervous system but also in most animal and plant tissues. A local shift in the extracellular ion composition is a source of new positional information for neuroblasts. It is read by cells that change their histogenetic activity, and closed loop self-regulation (CLS) is triggered again. Reading of specific biomechanical tension, in which the cell is included, occurs by discrete-morphogenetic systemic quanta of development, so that "changes in the spatial organization of the brain are a consequence of morphological implementation of mechanochemical marking of the processes of morphogenesis through the expression of specific genes." CLS during the neuroendocrine period of fetal brain development is triggered by a change in the concentration of at least one biologically active compound. Each of the glands included in the endocrine system affects different organs of the system and, in turn, is affected by these organs. At high hormone concentrations, the activity of the gland is weakened because of its effect on the corresponding receptors of the hormone-producing gland. In a number of glands, regulation is established through the hypothalamus and anterior pituitary gland, especially during stress reactions. The resulting reaction acquires a generalized course and spreads to other systems and organs. The physiological significance of hormones lies in the regulation of generalized activation of various functions of the body. There is no selective regulation of any homeostatic constant during this period of fetal development, since all CLS are interrelated. The homeostatic constants of this period have a particularly wide reaction norm with an unstable basal level, react slowly to deviations, and slowly normalize the basal level of the constant for the perception of a new stimulus. At each specific moment, homeostatic regulation aims to achieve the optimal level of the constant that has deviated maximally from its average value and not at fine-tuning vital support systems. In this regard, regulatory mechanisms in the neuroendocrine period of fetal brain development before the appearance of a formed blood-brain barrier (BBB) resemble the work and "programming language" of a long-forgotten analog computer; and it can be assumed that the main function of the BBB is to reformat the functions of signaling molecules and turn on the "program of morpho-functional assembly of a higher level" capable of responding in millisecond intervals.
During the first two stages of fetal development, STH is studied exclusively in the paradigm of observing changes in the behavior of an object in response to a recurring stimulus, which allows for a significant subjective component in the results of the study. In this regard, of particular interest is the recently published work of an international group of scientists from the Netherlands and Czech Republic [3], who identified 258 evolutionarily determined genes in different animal species. They described the biological functions that trigger and maintain these genes, and identified CLS and drugs that can alleviate STH deficiency. In addition, they compiled a catalog of STH genes, summarized modern paradigms for studying STH, and obtained reliable data indicating the important role of STH in diagnosing diseases, choosing therapies, and assessing their effectiveness. The authors showed that many of the identified genes demonstrated overlap between different species and different types of STH, significantly overlapping with genes involved in different forms of learning and memorization, but rarely coinciding with the results of studying STH in a behavioral paradigm. The third stage of fetal development, which begins after the maturation of the BBB, may well be designated as the period of formation of "digital" high-speed CLS, since:
* the rate of "saltatory" conduction along myelinated fibers increases sharply compared to unmyelinated fibers.
* a semiconductor type of action potential conduction in the synapse is formed (binary type of information transfer: "all" – 1; "nothing" – 0);
* the integrative activity of neurons as "summators" of information from brain structures of different specializations increases.
* specialized CLS develops as a result of assessing the novelty and significance of individual "memory cells
This leads to a new objective and quantitative psychophysiological level for assessing the state of the regulatory systems of the fetus, which we have not come across. In scientific and practical terms, the results obtained can be used in the development of improvements and additions to the CTG examination of pregnant women, especially in questionable cases. In medical practice, the obtained results can be used as normative data in an objective quantitative assessment of the successful development of the fetus in the first two stages and the prognosis of a favorable pregnancy course in the third stage.
Conclusion
The results of this study demonstrate that STH is an objective indicator of the maturity of fetal regulatory systems. Analysis of HRV during successive CTGs revealed consistent changes that were indicative of the adaptation process. It was found that the initial increase in HRV is a response to an external stimulus, whereas the subsequent decrease reflects the development of stable self-regulation mechanisms.
Differences in HRV dynamics among fetuses in different groups suggest heterogeneity in adaptive processes, potentially related to gestational age and individual neurophysiological development characteristics. These observed patterns position STH as a promising tool for assessing fetal conditions, including its potential for predicting perinatal complications.
The study findings can be applied in practical obstetric and perinatal medicine to enhance fetal monitoring accuracy. Utilizing HRV analysis algorithms in CTG examinations can improve diagnostic precision and personalize pregnancy management approaches. Further research in this area will refine antenatal monitoring methods and broaden the possibilities of objectively assessing the physiological state of the fetus.
____________________
1 The program is designed for automated mathematical analysis of antenatal cardiotocograms to determine the intrauterine condition of the fetus. The program calculates an integral fetal condition index (FCI) with values ranging from 0 to 4: healthy fetus (FCI value within 0.0-1.05); initial abnormalities (FCI value within 1.06-2.0); severe abnormalities (FCI value within 2.01-3.0); severe abnormalities (FCI value within 3.01-4.0).
2 UNIKOS – intranatal fetal monitor with automatic CTG analysis "Unikos".
References
- Логинов В.В., Давыдов Д.Г., Приходько А.М., Баев О.Р., Дегтярев Д.Н. Особенности адаптации плода к кардио-токографическому исследованию как критерий оценки его состояния. Акушерство и гинекология. 2021; 3: 138-44. [Loginov V.V., Davydov D.G., Prikhod'ko A.M., Baev O.R., Degtyarev D.N. Fetal adaptation to cardiotocography as a criterion of fetal condition. Obstetrics and Gynecology. 2021; (3): 138-44 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.138-44
- Приходько А.М., Романов А.Ю., Тысячный О.В., Гапаева М.Д., Баев О.Р. Современные принципы кардиотокографии в родах. Медицинский совет. 2020; 3: 90-7. [Prikhod’ko A.M., Romanov A.Yu., Tysyachnyy O.V., Gapayeva M.D., Bayev O.R. Modern principles of cardiotocography in childbirth. Medical Council. 2020; (3): 90-7 (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2020-3-90-97
- Blok L.E.R., Boon M., Reijmersdal B. van, Höffler K.D., Fenckova M., Schenck A. Genetics, molecular control and clinical relevance of habituation learning. Neurosci. Biobehav. Rev. 2022; 143: 104883. https://dx.doi.org/10.1016/j.neubiorev.2022.104883
- Leader L.R. The potential value of habituation in the fetus. In: Reissland N., Kisilevsky B.S., eds. Fetal Development. Research on brain and behavior, environmental influences, and emerging technologies. Springer; 2016: 189-209. https://dx.doi.org/10.1007/978-3-319-22023-9_11
- Соколов Е.Н. Очерки по психофизиологии сознания. М.: МГУ; 2010. 255 с. [Sokolov E.N. The psychophysiology of consciousness. Moscow: Moscow State University; 2010. 255 p. (in Russian)].
- Ogo K., Kanenishi K., Mori N., AboEllail M.A.M., Hata T. Change in fetal behavior in response to vibroacoustic stimulation. J. Perinat. Med. 2019; 47(5): 558-63. https://dx.doi.org/10.1515/jpm-2018-0344
- Das R., Jana N., Arora N., Sengupta S. Ultrasound assessment of fetal hearing response to vibroacoustic stimulation. J. Matern. Neonatal Med. 2020; 33(14): 2326-32. https://dx.doi.org/10.1080/14767058.2018.1548600
- Chard M., Roulin J.L., Bouvard M. Visual habituation paradigm with adults with profound intellectual and multiple disabilities: a new way for cognitive assessment? J. Appl. Res. Intellect. Disabil. 2014; 27(5): 481-8. https://dx.doi.org/10.1111/jar.12079
- Hudac C.M., DesChamps T.D., Arnett A.B., Cairney B.E., Ma R., Webb S.J. et al. Early enhanced processing and delayed habituation to deviance sounds in autism spectrum disorder. Brain Cogn. 2018; 123: 110-9. https://dx.doi.org/10.1016/j.bandc.2018.03.004
- Plichta M.M., Grimm O., Morgen K., Mier D., Sauer C., Haddad L. et al. Amygdala habituation: a reliable fMRI phenotype. Neuroimage. 2014; 103: 383-90. https://dx.doi.org/10.1016/j.neuroimage.2014.09.059
- Woitek R., Kasprian G., Lindner C., Stuhr F., Weber M., Schöpf V. et al. Fetal eye movements on magnetic resonance imaging. PLOS One. 2013; 8(10): e77439. https://dx.doi.org/10.1371/journal.pone.0077439
- Nakahara K., Morokuma S., Kato K. Recent topics in fetal behavioral assessment. Donald Sch. J. Ultrasound Obstet. Gynecol. 2021; 15 (3): 240-244. https://dx.doi.org/10.5005/jp-journals-10009-1703
- Donovan T., Dunn K., Penman A., Young R.J., Reid V.M. Fetal eye movements in response to a visual stimulus. Brain Behav. 2020; 10(8): e01676. https://dx.doi.org/10.1002/brb3.1676
- Maehara K., Morokuma S., Nakahara K., Okawa H., Kato K. A study on the association between eye movements and regular mouthing movements (RMMs) in normal fetuses between 24 to 39 weeks of gestation. PLOS One. 2020; 15(5): e0233909. https://dx.doi.org/10.1371/journal.pone.0233909
- Ronga I., Poles K., Pace C., Fantoni M., Luppino J., Gaglioti P. et al. At first sight: fetal eye movements reveal a preference for face‐like configurations from 26 weeks of gestation. Dev. Sci. 2025; 28(2): e13597 https://dx.doi.org/10.1111/desc.13597
- Haddad N., Govindan R.B., Vairavan S., Siegel E., Temple J., Preissl H. et al. Correlation between fetal brain activity patterns and behavioral states: an exploratory fetal magnetoencephalography study. Exp. Neurol. 2011; 228(2): 200-205. https://dx.doi.org/10.1016/j.expneurol.2011.01.003
- Einspieler C., Prayer D., Marschik P.B. Fetal movements: the origin of human behaviour. Dev. Med. Child Neurol. 2021; 63(10): 1142-8. https://dx.doi.org/10.1111/dmcn.14918
- Ji L., Majbri A., Hendrix C.L., Thomason M.E. Fetal behavior during MRI changes with age and relates to network dynamics. Hum. Brain Mapp. 2023; 44(4): 1683-94. https://dx.doi.org/10.1002/hbm.26167
- Bolivar V.J., Manley K., Messer A. Early exploratory behavior abnormalities in R6/1 Huntington’s disease transgenic mice. Brain Res. 2004; 1005(1-2): 29-35. https://dx.doi.org/10.1016/j.brainres.2004.01.021
- Bolivar V.J. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol. Learn. Mem. 2009; 92(2): 206-14. https://dx.doi.org/10.1016/j.nlm.2009.02.002
Received 13.03.2025
Accepted 28.05.2025
About the Authors
Viktor V. Loginov, PhD (Bio), Head of the Laboratory of Neurophysiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)316-13-75, v_loginov@oparina4.ru, https://orcid.org/0000-0002-4929-8763Denis G. Davydov, PhD (Psychology), Senior Researcher at the Laboratory of Neurophysiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)120-85-22, d_davydov@oparina4.ru,
https://orcid.org/0000-0003-3747-7403
Andrey M. Prikhodko, Dr. Med. Sci., physician at the 1st Maternity Department, Teaching Assistant at the Department of Obstetrics and Gynecology, Researcher at the Innovative Technologies Department of Obstetrics Institute, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-30-47, a_prikhodko@oparina4.ru, https://orcid.org/0000-0002-6615-2360
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor at the Department of Obstetrics, Gynecology, Perinatology, and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2, +7(495)438-11-88,
o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, kan-med@mail.ru, https://orcid.org/0000-0001-5087-5946
Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, tioutiounnik@mail.ru,
https://orcid.org/0000-0002-5830-5099
Andrey Yu. Romanov, PhD, Head of the Department of Planning and Support of Scientific Projects, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)158-94-00, romanov1553@yandex.ru,
https://orcid.org/0000-0003-1821-8684
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Head of the Department of Neonatology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, 119991, Russia, Moscow, Trubetskaya str., 8-2; Deputy Director, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., glav_neolog@yahoo.com, d_degtiarev@oparina4.ru, https://orcid.org/0000-0001-8975-2425



