Immunological testing for platelet functional activity in gestational thrombocytopenia
Aim. To investigate the value of immunological testing for platelet functional activity in patients with gestational thrombocytopenia in the setting of hemostatic system activation.Mysik O.L., Zainulina M.S., Baranov V.S., Polyakov A.S.
Materials and methods. This multicenter prospective study involved 299 pregnant women in the third trimester of pregnancy divided into the study group (n=249), including patients with thrombocytopenia and the control group (n=50) comprised of women with a healthy pregnancy and normal platelet counts. We used flow cytometry with FITC- and PE-labeled antibodies against GP IIb/IIIa receptors and P-selectin to determine the number of GP IIb/IIIa receptors and P-selectin expression on the platelet surface and conducted an association with the principle of ADP activation, which is used in standard induced aggregation.
Results. After ADP activation, the study group patients with thrombocytopenia had almost 40-fold higher P-selectin expression and significantly higher number of GP IIb/IIIa receptors on the platelet surface than women in the control group (p=0.0001; p=0.001). This indicates platelet hyper-aggregation, hemostatic system activation and suggests the feasibility of using acetylsalicylic-acid-containing drugs and low molecular weight heparins in this specific patient category to prevent obstetric complications.
Conclusion. The flow cytometry can be used as a new laboratory criterion for assessing platelet hyper-aggregation in pregnant women with thrombocytopenia. The findings also support the administration of acetylsalicylic acid and low molecular weight heparins in this patient category.
Keywords
Platelet activation is involved in many physiological and pathological processes, including hemostasis, angiogenesis, immune response, thrombosis, myocardial infarction, strokes, and other thromboembolic disorders. The mean range of the platelet count in pregnant women is 213,000–250,0×109/L [1]. Thrombocytopenia in pregnant women has been defined as a platelet count less than 150×109/L. In normal pregnancies, 7–12% of women present with mild thrombocytopenia, and up to 75% of them will not be associated with any pathology [2–4].
According to some data, 5–21% of cases of maternal thrombocytopenia are associated with activation of intravascular blood coagulation due to gestational complications, most often preeclampsia [5]. Platelet activation occurs under the influence of such factors as thrombin, collagen, and ADP. It is functionally manifested by a change in the shape and exposure of secondary receptors and proteins of the platelet cell surface, the key of which are GpIIb/IIIa and P-selectin [6]. Heterodimer GpIIb/IIIa, the most abundant platelet surface receptor, is activated due to signal transmission from adhesion receptors GPVI and GpIb/IX/V receptors associated with the G protein and ADP receptors (P2Y1 and P2Y12). In the process of Сa2+-dependent activation, the complex undergoes several changes contributing to platelet binding to fibrinogen [7–9]. P-selectin (CD 62 P) is a cell surface protein and the main component responsible for forming platelet-leukocyte aggregates and is expressed only by activated platelets [10].
The P-selectin expression on the platelet surface is the most informative in assessing platelets' functional activity, including in the setting of antiplatelet therapy. Adequate control of platelet functional activity currently remains an unsolved problem in clinical laboratory diagnostics [11], while the assessment of platelet aggregation activity is of great importance. Controlled anti-thrombotic prophylaxis and therapy in patients with activated intravascular coagulation can prevent placenta-associated pregnancy complications. To date, the primary, widely used, and available method for assessing platelet activity is the optical analysis of induced platelet aggregation using light transmission [12]. The method has many disadvantages, including that the blood sampling conditions and subsequent centrifugation activate platelets and distort the result. There are no clear criteria for inducers concentration, and the method does not allow reliable assessment of platelet hyper-aggregation [11, 12]. One of the modern immunological methods for analyzing platelet function is flow cytometry, a fluorescence-based assay that enables measurement of the structural components of blood cells using antibodies labeled with fluorescent dyes [6, 13–17]. Compared to other methods for assessing platelet functional activity, flow cytometry, perhaps, most adequately reflects the situation in vivo since it is based on a highly specific antigen-antibody reaction.
Cytometric analysis using various fluorescent dyes and specific antibodies can be used to assess indicators directly related to platelet hyper-aggregation, including platelet reactivity, circulating activated platelets, platelet-platelet aggregates, leukocyte-platelet aggregates, and expression and activation on the platelet surface receptors [12, 18, 19].
The present study aimed to investigate the clinical value of immunological testing for platelet functional activity in patients with gestational thrombocytopenia in the setting of hemostatic system activation.
Materials and methods
This prospective study involving 299 pregnant women in the third trimester of pregnancy was conducted at the V.F. Snegirev Maternity hospital №6 of the D.O. Ott Research Institute for Obstetrics, Gynecology, and Reproductology from 2013 to 2018. The study group (n=249) included patients with thrombocytopenia (platelet count <150×109/L). The control group (n=50) comprised women with normal platelet counts during a healthy pregnancy.
The inclusion criterium for the study group was thrombocytopenia (platelet count less than 150x109/L) in pregnant women aged 20–40 years in the third trimester of pregnancy (gestational age 28–30 weeks).
Exclusion criteria for the study group were pregnancy with immune thrombocytopenia (idiopathic thrombocytopenic purpura), congenital thrombocytopenia, hereditary and acquired coagulopathies, systemic autoimmune diseases, type 1 diabetes mellitus with generalized complications, and thrombocytopenia less than 50×109/L).
The inclusion criterium for the control group was healthy pregnancy in the third trimester of pregnancy with normal platelet counts.
Exclusion criteria from the control group were obstetric complications in the third trimester of pregnancy, including preeclampsia, placental insufficiency, somatic comorbidities, and chronic diseases.
All pregnant women included in the study were under observation and underwent complete clinical, laboratory, and anamnestic investigations, and received individual therapy.
Along with standard investigations (complete blood count, hemoglobin level, coagulation tests), all study participants were tested for induced platelet aggregation (with ADP at a concentration of 2 μM and 0.2 μM) and platelet aggregation activity by flow cytometry.
Cytometric research technology
To study platelet aggregation and expression of platelet surface antigens P-selectin and glycoprotein IIb/IIIa, blood samples were obtained by aseptic venipuncture and anticoagulated with 3.8% sodium citrate. The expression of platelet surface antigens P-selectin and glycoprotein IIb/IIIa were determined by flow cytometric analysis using fluorescently labeled monoclonal FITC anti-human CD61 and anti-CD62P-PE antibodies on a CYTOMICS FC 500 flow cytometer (Beckman Coulter, US).
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010 and IBM SPSS Statictics 23. Quantitative variables are presented as the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test. The statistical significance of between-group differences for continuous variables was tested with Mann–Whitney test. Categorical variables were summarized using counts and percentages. Categorical variables were compared using Fisher’s exact test. For binary outcomes, the odds ratio (OR) and the 95% confidence interval (95% CI) were calculated. The critical level of significance when the testing statistical hypothesis was considered at p <0.05.
Results
The mean age of patients in the study and control groups was 31.1 (27.8; 35.0) and 30.7 (28.0; 34.8) years with a non-significant difference between the groups (p=0.68); Median gestational age of thrombocytopenia detection in the study group was 23.1 (22.5; 29.0) weeks. Median gestational age at inclusion in the study did not differ significantly and was 28.8 (28.0; 30.0) weeks in the study group and 29.4 (28.5; 30.0) weeks in the control group (p=0.15 ). Pregnancy without the use of assisted reproductive technologies occurred in 225 (90.3%) women in the study group and 45 (90%) in the control group (p=1.00).
Eighty-three (33.3%) women in the study group and 19 (38%) in the control group were primigravidas. There were no statistically significant differences in parity between the groups (p=0.52). In both study and control groups, women had a history of reproductive losses, including missed miscarriage and spontaneous miscarriages. There are no significant differences between the groups (p> 0.05).
Detailed comparative characteristics of non-obstetric comorbidities and obstetric complications are presented in Table1.
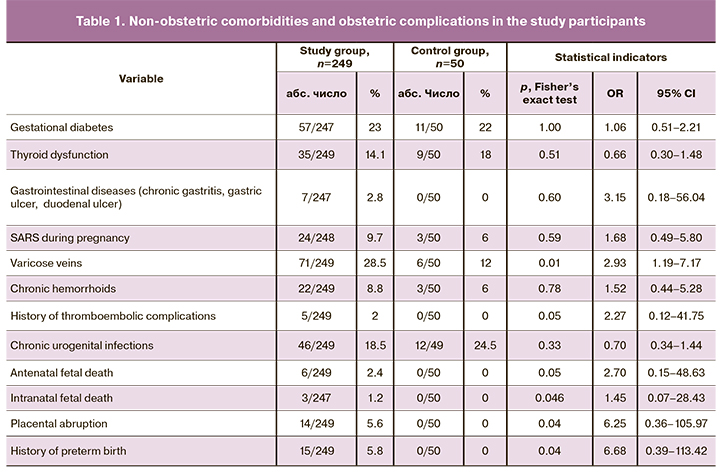
As can be seen from Table 1, pregnant women with thrombocytopenia were more likely to have varicose veins of the lower extremities [71 (28.5%)] than women with normal platelet count and healthy pregnancy [6 (12%)] (p=0.01). Placental abruption [14 (5.6%)] and preterm birth [15 (5.8%)] were registered only in pregnant women with thrombocytopenia (p <0.05). Women with healthy pregnancies also had no history of antenatal and intrapartum fetal death 0 (50), compared with women with thrombocytopenia (p <0.05). There were no statistically significant differences between the groups in the past gynecological history (p=0.32). History of thromboembolic complications was noted only in 5 (2%) women of the study group (p=0.05). Complete hemostatic profiles of both groups are presented in Table 2.
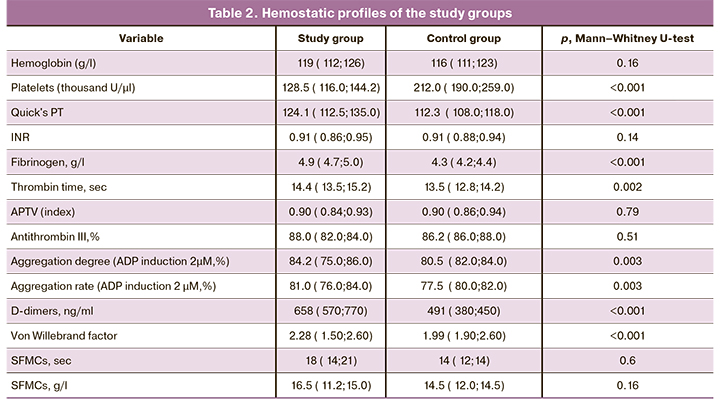
Analysis of coagulation parameters (Table 2) showed that the levels of prothrombin index (PTI), thrombin time, fibrinogen, D-dimer, and von Willebrand factor were significantly higher in pregnant women with thrombocytopenia than in women with a healthy pregnancy (p <0.05). There were no significant differences between the groups in activated partial thromboplastin time (APTT), antithrombin III, soluble fibrin-monomer complexes (SFMCs), and international normalized ratio (INR) (p> 0.05). All the numerical blood parameters presented in table 2 testify in favor of the activation of intravascular blood coagulation in the study group of pregnant women with thrombocytopenia, compared with the control group.
The level of platelet aggregation in the study group was significantly higher than in the control group and amounted to 84.2 (75.0; 86.0), in the control group [80.5 (82.0; 84.0)] (p=0.003) (Table 2). The rate of platelet aggregation in the study group [81.0 (76.0; 84.0)] was also higher than in the control group [77.5 (80.0; 82.0)] (p=0.003). Despite statistically significant differences between groups, the difference in numerical indicators was insignificant.
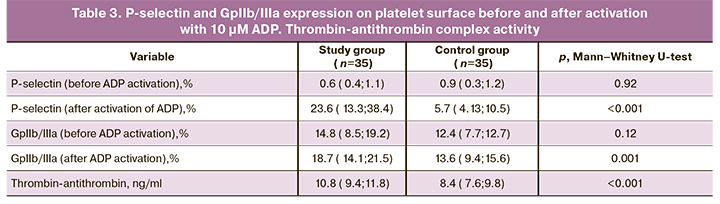
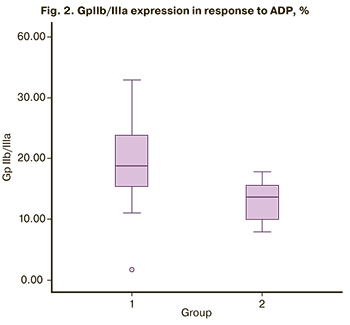
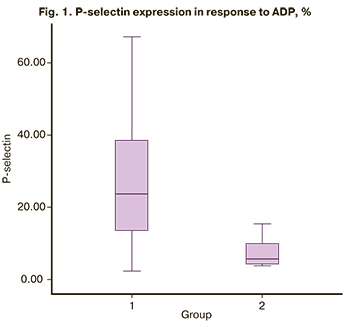 Flow cytometric analysis of platelet functional activity showed that the P-selectin expression before the activation with ADP was 0.6 (0.4; 1.1) and 0.9 (0.3; 1,2) in the study and control group, respectively. There were no significant differences between the groups (p = 0.92). After activation with 10 μM ADP, there was a statistically significant increase in P-selectin expression on the platelet surface in the study group [23.6 (13.3; 38.4)] compared with the control group [5.7 (4.1; 10.5)], (<0.001) (Table 3). The most pronounced expression of P-selectin after activation with 10 μM ADP was detected in pregnant women with thrombocytopenia (Figure 1), which proves the activation of intravascular blood coagulation and high platelet hyperaggregation. The number of GP IIb/IIIa on the platelet surface before ADP activation in the study group was 14.8 (8.5; 19.2) and 12.3 (7.7; 12.7) in the control group (Table 3). It should be noted that the number of GP IIb/IIIa receptors on the platelet surface in the absence of ADP was slightly higher in pregnant women with thrombocytopenia than in women with a healthy pregnancy (p=0.12). But there were no statistically significant differences between the groups. In response to ADP, there was a significant increase in the number of GP IIb/IIIa in the study group [18.7 (14.1; 24.5)] compared with the control group [13.6 (9.4; 15.6)] (Fig. 2). A significant increase in the number of GPIIb/IIIa on the platelet surface in pregnant women with thrombocytopenia suggests significant activation of the hemostatic system compared with the control group (p=0.001).
Flow cytometric analysis of platelet functional activity showed that the P-selectin expression before the activation with ADP was 0.6 (0.4; 1.1) and 0.9 (0.3; 1,2) in the study and control group, respectively. There were no significant differences between the groups (p = 0.92). After activation with 10 μM ADP, there was a statistically significant increase in P-selectin expression on the platelet surface in the study group [23.6 (13.3; 38.4)] compared with the control group [5.7 (4.1; 10.5)], (<0.001) (Table 3). The most pronounced expression of P-selectin after activation with 10 μM ADP was detected in pregnant women with thrombocytopenia (Figure 1), which proves the activation of intravascular blood coagulation and high platelet hyperaggregation. The number of GP IIb/IIIa on the platelet surface before ADP activation in the study group was 14.8 (8.5; 19.2) and 12.3 (7.7; 12.7) in the control group (Table 3). It should be noted that the number of GP IIb/IIIa receptors on the platelet surface in the absence of ADP was slightly higher in pregnant women with thrombocytopenia than in women with a healthy pregnancy (p=0.12). But there were no statistically significant differences between the groups. In response to ADP, there was a significant increase in the number of GP IIb/IIIa in the study group [18.7 (14.1; 24.5)] compared with the control group [13.6 (9.4; 15.6)] (Fig. 2). A significant increase in the number of GPIIb/IIIa on the platelet surface in pregnant women with thrombocytopenia suggests significant activation of the hemostatic system compared with the control group (p=0.001).
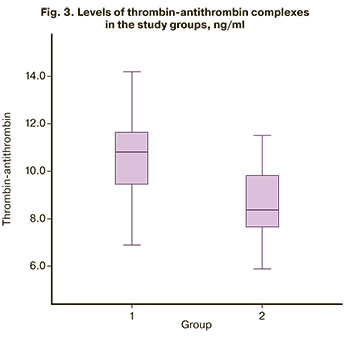
 A quantitative analysis of the thrombin-antithrombin complex (Figure 3) showed that in pregnant women with thrombocytopenia, this indicator was significantly higher [10.8 (9.4; 11.8)] than in women with healthy pregnancy [8.4 (7.6; 9.8) (p <0.001)] (Table 3). Therefore, all findings of this study confirm platelet hyperaggregation in pregnant women with thrombocytopenia, suggesting the need to use acetylsalicylic acid and low molecular weight heparins in this group of patients to prevent the development of obstetric, placenta-associated pregnancy complications.
A quantitative analysis of the thrombin-antithrombin complex (Figure 3) showed that in pregnant women with thrombocytopenia, this indicator was significantly higher [10.8 (9.4; 11.8)] than in women with healthy pregnancy [8.4 (7.6; 9.8) (p <0.001)] (Table 3). Therefore, all findings of this study confirm platelet hyperaggregation in pregnant women with thrombocytopenia, suggesting the need to use acetylsalicylic acid and low molecular weight heparins in this group of patients to prevent the development of obstetric, placenta-associated pregnancy complications.
Discussion
Activation of the hemostasis system during pregnancy is a risk factor for developing thrombotic complications during pregnancy, in childbirth, and postpartum. Women with congenital and acquired defects of the hemostatic system constitute a high-risk group for the development of hyper-aggregation and hypercoagulative states during pregnancy. This results in increased consumption of platelets and coagulation factors, disruption of implantation and placentation, deposition of fibrin and immune complexes on the syncytiotrophoblast membrane. All these processes are risk factors for placenta-associated complications of pregnancy, such as miscarriage, preeclampsia, antenatal fetal death, placental insufficiency, and placental abruption [20, 21]. Currently, the "gold standard" for the analysis of platelet activity is the optical method for assessing induced platelet aggregation, proposed by Born in the 60s of the last century. This method is appropriate for analyzing the lack of platelet functional activity but does not provide complete information about their hyperactivity [14]. A significant disadvantage of testing platelet aggregation by Born's method is also the duration of sample preparation, the effect of accelerations during centrifugation on platelets, the loss of the most active fraction of the platelet, as well as erythrocytes and leukocytes that significantly affect platelet activity, the difficulty of registering platelet activation by transmission in plasma [22]. The testing is laborious and cannot be performed at the patient's bedside. Optical aggregometers can detect only large platelet clots, consisting of more than 100 cells [22]. Therefore, many researchers prefer flow cytometry to study platelet characteristics. This method is used to determine patients’ immune status, immunophenotyping for hematological diseases, and other immune system parameters [12, 23].
 The current literature lacks sufficient coverage of the use of flow cytometry to determine GPIIb/IIIa and P-selectin receptors as markers of platelet activation and factors of individual cell sensitivity to antiplatelet drugs [18, 24, 25]. Hollander J.E. et al. studied the sensitivity and specificity of membrane-bound P selectin in diagnosing acute coronary pathology. They demonstrated its high specificity (71%) in detecting acute myocardial infarction in patients with typical clinical manifestations [26]. Sirotkina O.V. reported expression of membrane-bound GPIIb/IIIa receptors and P-selectin in response to ADP in patients with acute myocardial infarction. The number of GP IIb/IIIa in patients treated with acetylsalicylic acid and clopidogrel was less than 10%. The number of cells that bind to antibodies against P-selectin increased by 17% while taking aspirin and only by 5% when using clopidogrel, which indicates the beneficial effect of the drug in this category of patients [12]. Our study evaluated flow cytometry in pregnant women with thrombocytopenia in the third trimester of pregnancy to confirm platelet hyperaggregation. We analyzed the level of the glycoprotein receptor GP IIb/IIIa and the expression of P-selectin on the surface of inactive platelets and cells activated by incubation with 10 µm ADP. In pregnant women with thrombocytopenia, the amount of GP IIb/IIIa receptor significantly increased after activation of ADP, compared with the control group of women with normal platelet count and healthy pregnancy (p <0.05).
The current literature lacks sufficient coverage of the use of flow cytometry to determine GPIIb/IIIa and P-selectin receptors as markers of platelet activation and factors of individual cell sensitivity to antiplatelet drugs [18, 24, 25]. Hollander J.E. et al. studied the sensitivity and specificity of membrane-bound P selectin in diagnosing acute coronary pathology. They demonstrated its high specificity (71%) in detecting acute myocardial infarction in patients with typical clinical manifestations [26]. Sirotkina O.V. reported expression of membrane-bound GPIIb/IIIa receptors and P-selectin in response to ADP in patients with acute myocardial infarction. The number of GP IIb/IIIa in patients treated with acetylsalicylic acid and clopidogrel was less than 10%. The number of cells that bind to antibodies against P-selectin increased by 17% while taking aspirin and only by 5% when using clopidogrel, which indicates the beneficial effect of the drug in this category of patients [12]. Our study evaluated flow cytometry in pregnant women with thrombocytopenia in the third trimester of pregnancy to confirm platelet hyperaggregation. We analyzed the level of the glycoprotein receptor GP IIb/IIIa and the expression of P-selectin on the surface of inactive platelets and cells activated by incubation with 10 µm ADP. In pregnant women with thrombocytopenia, the amount of GP IIb/IIIa receptor significantly increased after activation of ADP, compared with the control group of women with normal platelet count and healthy pregnancy (p <0.05).
However, the most informative and visual concerning the assessment of the platelet functional activity was the expression of P-selectin on platelets surface. After activation with ADP, the number of cells expressing P-selectin increased almost 40 times in the group of pregnant women with thrombocytopenia and only five times in pregnant women with a normal platelet count (p <0.01). Thus, we can assume that the level of P-selectin adequately reflects platelet activity and can act as a marker of their hyperfunction. Flow cytometry can be used to confirm platelet hyper-aggregation.
Conclusion
New methods for assessing platelet functional activity and monitoring the response to antiplatelet therapy are essential for decision-making about the administration of acetylsalicylic acid and low molecular weight heparins, evaluating individual sensitivity to aspirin-containing drugs, and adjusting the drugs’ dosage during treatment. Analysis of platelet functional activity by flow cytometry showed high sensitivity and efficiency in pregnant women with thrombocytopenia. It allows confirmation of the high functional activity of platelets in this group of patients. In the future, it can be used as a laboratory criterion for assessing platelet hyper-aggregation and the optical analysis of induced platelet aggregation.
References
- Bowersox N.A., Ramus R.M. Тhrombocytopenia in pregnancy. Medscape. Drugs and Diseases. Obstetrics & Gynecology Updated: Sep 30, 2016. Available at: https://emedicine.medscape.com›article/272867-overview
- Burrows R.F., Kelton J.G. Fetal thrombocytopenia and its relation to maternal thrombocytopenia. N. Engl. J. Med. 1993; 329(20): 1463-6. https://dx.doi.org/10.1056/NEJM199311113292005.
- Boehlen F., Hohfeld P., Extermann P., Perneger T.V., de Moerloose P. Platelet count at term pregnancy: a reappraisal of the threshold. Obstet. Gynecol. 2000; 95(1): 29-33. https://dx.doi.org/10.1016/s0029-7844(99)00537-2.
- Петрухин В.А., Мареева М.Ю., Ковалева Л.Г., Мравян С.Р. Тромбоцитопении у беременных. Российский вестник акушера-гинеколога. 2011; 11(2): 20-6. [Petrukhin V.A., Mareeva M.Yu., Kovaleva L.G., Mravyan S.R. Thrombocytopenia in pregnant women. Russian Bulletin of Obstetrician-Gynecologist. 2011; 11(2): 20-6. (in Russian)].
- Practice Bulletin No. 166: Thrombocytopenia in pregnancy. Obstet. Gynecol. 2016; 128(3): e43-53. https://dx.doi.org/10.1097/aog.0000000000001641.
- Leytin V., Mody M., Semple J.W. Flow cytometric parameters for characterizing platelet activation by measuring P-selectin (CD62) expression: theoretical consideration and evaluation in thrombin-treated platelet populations. Biochem. Byophys. Res. Commun. 2000; 269(1): 85-90. https://dx.doi.org/10.1006/bbrc.2000.2255.
- Воронина Е.Н., Филипенко М.Л., Сергевичев Д.С. Мембранные рецепторы: функции и полиморфизм. Вестник ВОГиС. 2006; 10(3): 553-64. [Voronina E.N. Filipenko M.L., Sergevichev D.S. Membrane receptors: functions and polymorphism Bulletin of VOGiS. 2006; 10(3): 553-64. (in Russian)].
- Капустин С.И., Салтыкова Н.Б., Кобилянская В.А., Дрижун Ю.С. Особенности генетического полиморфизма компонентов системы гемостаза при различных клинических проявлениях венозного тромбоэмболизма. Вестник гематологии. 2009; 5(1): 16-24. [Kapustin S.I., Saltykova N.B., Kobilyanskaya V.A., Drizhun Yu.S. Features of genetic polymorphism of the components of the hemostasis system in various clinical manifestations of venous thromboembolism. Hematology Bulletin. 2009; 5(1): 16-24. (in Russian)].
- Кузник Б.И. Клеточные и молекулярные механизмы регуляции системы гемостаза в норме и патологии. Чита: Экспресс-издательство; 2010. 827c. [Kuznik B.I. Cellular and molecular mechanisms of regulation of the hemostasis system in health and disease. Chita: Express Publishing House, 2010; 827 p. (in Russian)].
- Berger G., Hartwell D.W., Wagner D.D. P-selectin and platelet clearance. Blood. 1998; 92(11): 4446-52. https://dx.doi.org/10.1182/blood.v92.11.4446.423k19_4446_4452.
- Bowbrick V.A., Mikhailidis D.P., Stansby G. Value of thromboelastography in the assessment of platelet function. Clin. Appl. Thromb. Hemost. 2003; 9(2):137-42. https:/dx.doi.org/10.1177/107602960300900208.
- Сироткина О.В., Боганькова Н.А., Ласковец А.Б., Кухарчик Г.А., Гайковая Л.Б., Вавилова Т.В. Иммунологические методы в оценке функциональной активности тромбоцитов у больных с сердечно-сосудистыми заболеваниями. Медицинская иммунология. 2010; 12(3): 213-8. [Sirotkina O.V., Bogankova N.A., Laskovets A.B. , G.A. Kukharchik , Gaykovaya L.B., Vavilova T.V. Immunological methods in assessing the functional activity of platelets in patients with cardiovascular diseases. Medical Immunology. 2010; 12(3): 213-8.(in Russian)].
- Andrews N.P., Gralnick H.R., Merryman P., Vail M., Quyyumi A.A. Mechanisms underlying the morning increase in platelet aggregation: A flow cytometry study. J. Am. Coll. Cardiol. 1996; 28(7): 1789-95.
- Andrioli G., Ortolani R., Fontana L., Gaino S., Bellavite P., Lechi C. et al. Study of platelet adhesion in patients with uncomplicated hypertension. J. Hypertens. 1996; 14(10): 1215-21. https://dx.doi.org/10.1097/00004872-199610000-00010.
- Michelson A.D., Barnard M.R. Krueger L.A., Frelinger A.L., Furman M.I. Evaluation of platelet function by flow cytometry. Methods. 2000; 21(3): 259-70. https://dx.doi.org/10.1006/meth.2000.1006.
- Sack U., Scheibe R., Wötzel M., Hammerschmidt S., Kuhn H., Emmrich F. et al. Multiplex analysis of cytokines in exhaled breath condensate. Cytometry Part A. 2006; 69A(3): 169-72. https://dx.doi.org/10.1002/cyto.a.20231.
- Undar L., Akkoc N., Alakavuklar M.N., Cehreli C., Undar L. Flow cytometric analysis of circadian changes in platelet activation using anti-GMP-140 monoclonal antibody. Chronobiol. Int. 1999; 16(3): 335-42. https://dx.doi.org/10.3109/07420529909116862.
- Storey R., Judge H., Heptinstall S. Inhibition of ADP-induced P-selectin expression and plateletleukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR-C69931MX but not aspirin. Thromb. Haemost. 2002; 88(9): 488-94. https://dx.doi.org/10.1055/s-0037-1613242.
- Еnjeti A.K., Lincz L.F., Seldon M. Detection and measurement of microparticles: an evolving research tool for vascular biology. Semin. Thromb. Hemost. 2007; 33(8): 771-9. https://dx.doi.org/10.1055/s-2007-1000369.
- Макацария А.Д., Бицадзе В.О. Антифосфолипидный синдром, генетические тромбофилии в патогенезе основных форм акушерской патологии. РМЖ. 2006; Специальный выпуск: 2-11. [Makatsaria A.D., Bitsadze V.O. Antiphospholipid syndrome, genetic thrombophilia in the pathogenesis of the main forms of obstetric pathology. Breast cancer; 2006: 2-11. Special issue. (in Russian)].
- Зайнулина М.С. Тромбофилия в акушерской практике: учебно- методическое пособие. Айламазян Э.К., Петрищев Н.Н., ред. СПб.: Издательство Н-Л; 2005. 46с. [Zainulina M.S. Thrombophilia in obstetric practice: training manual; ed. Ailamazyan E.K., Petrishchev N.N. SPb.: Publishing house of NL, 2005. 46 p. (in Russian)].
- Favaloro E.J., Mohammed S. Platelet function testing: auditing local practice and broader implications. Science. 2010; 23(1): 21-31. https://dx.doi.org/10.29074/ascls.23.1.21.
- Хайдуков С.В., Зурочка А.В. Вопросы современной проточной цитометрии. Клиническое применение. Челябинск; 2008. 195с. [Khaidukov S.V., Zurochka A.V. Questions of modern flow cytometry. Clinical application. Chelyabinsk, 2008; 195 p. (in Russian)].
- Barsky A.A., Arora R.R. Clopidogrel resistance: myth or reality? J. Cardiovasc. Pharmacol. Ther. 2006; 11(1): 47-53. https://dx.doi.org/10.1177/107424840601100104.
- Braun O.O., Johnell M., Varenhorst C., James S., Brandt J.T., Jakubowski J.A. et al. Greater reduction of platelet activation markers and plateletmonocyte aggregates by prasugrel compared to clopidogrel in stable coronary artery disease. Thromb. Haemost. 2008; 100(4): 626-33. https://dx.doi.org/10.1160/th08-05-0313.
- Hollander J.E., Mutreja M.R., Dalesandro M.R., Shofer F. Risk stratification of emergency department patients with acute coronary syndromes using P-selectin. J. Am. Coll. Cardiol. 1999; 34(1): 95-105. https://dx.doi.org/10.1016/s0735-1097(99)00175-8.s0735-1097(99)00175-8.
Received 23.10.2020
Accepted 09.11.2020
About the Authors
Marina S. Zainulina, Dr. Med. Sci., Professor of the Department of Obstetrics, Gynecology and Reproductology, I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of the Russian Federation; Chief Physician, Maternity hospital №6 named after prof. V.F. Snegirev. E-mail: zainulina@yandex.ru.ORCID: 0000-0002-2622-5000; Scopus Author ID: 37076359000; Researcher ID: B-5746-2018. 192014, Russia, St. Petersburg, Mayakovskaya str., 5.
Olga L. Mysik, obstetrician-gynecologist, Maternity hospital №6 named after prof. V.F. Snegirev. E-mail: olga_mysik88@mail.ru.
ORCID: 0000-0002-8895-6845. 192014, Russia, St. Petersburg, Mayakovskaya str., 5.
Vladislav S. Baranov, Dr. Med. Sci., Professor, Corresponding Member of RAS, Honored Scientist of the Russian Federation, Head of the Laboratory for Prenatal Diagnostics of Congenital and Hereditary Diseases, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology. E-mail: baranov@vb2475.spb.edu.
ORCID: 0000-0002-6518-1207; SPIN-код: 9196-7297; Scopus Author ID: 56057974100. 199034, Russia, St. Petersburg, Mendeleevskaya line, 3.
Aleksey S. Polyakov, Cand. Med. Sci., Head of the Hematology Department of the Clinic of the Department of Faculty Therapy named after S.P. Botkin, S.M. Kirov Military Medical Academy, Ministry of Defense of Russia. E-mail: doctorpolyakov@gmail.com. ORCID: 0000-0001-9238-8476;
Scopus Author ID: 56583551700; Researcher ID: M-4229-2016. 194044, Russia, St. Petersburg, Academician Lebedev str., 6, lit. A.
For citation: Mysik O.L., Zainulina M.S., Baranov V.S., Polyakov A.S. Immunological testing for platelet functional activity in gestational thrombocytopenia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 4: 76-83 (in Russian)
https://dx.doi.org/10.18565/aig.2021.4.76-83



