Influence of heavy metals on the effectiveness of assisted reproductive technologies depending on the polymorphism of genes of the detoxification system
Aim. To analyze the relationship between the levels of heavy metals (cadmium, mercury, lead) in the blood, polymorphism of genes of the biotransformation system of xenobiotics and the outcomes of assisted reproductive technologies (ART) in infertile women.Syrkasheva A.G., Dolgushina N.V., Frankevich V.E., Donnikov A.E.
Materials and methods. 300 married couples underwent infertility treatment using ART. The levels of mercury, cadmium and lead in the blood of patients were detected using inductively coupled plasma mass spectrometry. The allelic variants of genes of the detoxification system were determined by real-time polymerase chain reaction with melting curve analysis.
Results. The blood levels of lead and mercury were significantly lower in patients with the absence of T allele of CYP1A1 gene and in patients with G allele of GSTP1 gene. Mercury level was also low in the absence of deletion of GSTT1 gene. The blood level of cadmium was not associated with genes polymorphism of xenobiotic biotransformation. The patients with absent T allele of CYP1A1 gene had low birth rate (р=0.0270), and low cumulative birth rate (р=0.0249): 51.6% in patients with T/T genotype, 38.1% in patients with T/C genotype and 20% in patients with С/С genotype. The combined index of CYP1A1*Pb in the blood significantly influenced fertilization of oocytes.
Conclusion. The obtained data showed that polymorphic variants of genes of the detoxification system play an important role in the accumulation of heavy metals in the body of patients, as well as in reduced rate of oocyte fertilization in ART cycles.
Keywords
The environment plays a great role in human health. According to data of the World Health Organization, at least 25% of all diseases in adults are associated with harmful environmental factors. However, the current tendencies such as overpopulation, pollution of water, air and soil, globalization, problems with waste disposal, increased demand for food, and industrial waste make the problem of the influence of environmental factors on health increasingly urgent.
Heavy metals are widely used in economic activity; they are able to enter the human body, pass through the histo-hematic barrier, accumulate in various tissues, and have a long elimination period (for example, cadmium is eliminated in 15 to 40 years).
The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) includes a dynamic list of hazardous substances most dangerous to human health, where heavy metals have been at the top for several decades [1].
The negative impact of heavy metals on reproductive health is also a concern of scientists and researchers. Increased exposure to heavy metals is associated with infertility, early reproductive losses, and premature births [2–4].
The susceptibility of an individuum to environmental factors depends on genetic features: the genes of xenobiotic biotransformation enzymes are characterized by significant population polymorphism, which leads to changes in the activity of antioxidant enzymes [4, 5]. Therefore, a study of the impact of heavy metals on female fertility, depending on the genetic features of the detoxification system, is an important target for scientific research.
The aim of this study was to analyze the relationship between the levels of heavy metals (cadmium, mercury, lead) in blood, genetic polymorphism of the xenobiotic biotransformation system, and the outcomes of assisted reproductive technologies (ART) in patients with infertility.
Material and methods
A cross-sectional study included 300 couples who chose ART in the period from 2017 to 2018, had no contraindications to ART, and signed an informed consent to participate in the study. The inclusion criteria were: the normal karyotype of both partners, the absence of pronounced pathozoospermia (100% teratozoospermia, absolute asthenozoospermia, all types of azoospermia), the woman's age from 18 to 39 years, the woman's body mass index (BMI) from 19 to 25 kg/m2. The exclusion criteria were: the use of donor gametes or surrogacy, and the retrieval of 3 or less oocytes during transvaginal ovarian drilling.
All couples included in the study were examined based on the Order of the Ministry of Health of the Russian Federation No. 107н dated August 30, 2012 “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications and Restrictions to their Application” [3].
Ovarian stimulation was performed according to the protocol with the use of gonadotropin-releasing hormone antagonists; the doses of gonadotropins were selected individually. The ovulation trigger was used in the presence of follicles 17 mm in diameter or more. Human chorionic gonadotropin 8,000-10,000 IU or gonadotropin-releasing hormone agonist 0.2 mg was used as an ovulation trigger. Luteal support and post-transfer period in all patients was performed according to the standard protocol [6].
Venous blood sampling was performed on the day of transvaginal drilling, after which the samples were cryopreserved at temperature t=-70°C. The levels of mercury (Hg), cadmium (Cd), and lead (Pb) were determined by inductively coupled plasma mass spectrometry; the laboratory had no access to the clinical characteristics of the patients. Determination of polymorphic loci of glutathione-S-transferase T1 (GSTT1, gene deletion), glutathione-S-transferase M1 (GSTM1, gene deletion), glutathione-S-transferase P1 (GSTP1, rs1695), superoxide dismutase (SOD2, rs4880), cytochromes P-450 (CYP1A1, rs4646903, rs1048943, rs1799814), glutathione peroxidase 1 (GPX1, rs1050450) epoxide hydrolase 1 (EPH1, rs1051740), N-acetyltransferase 2 (NAT2, rs1801280, rs179993, rs179930), sulfotransferase 1A1 (SULT1A1, rs9282861) were performed by real-time polymerase chain reaction and melting curve analysis using commercial test systems (“DNA-Technology LLC”, Russia).
Fertilization of oocytes was performed using in vitro insemination of oocytes (“classic” IVF, hereinafter – fertilization), or intracytoplasmic sperm injection (ICSI). Morphological assessment of oocytes was performed during fertilization. Embryo cultivation and transfer were performed according to standard techniques.
The outcomes of ART programs were assessed by fertilization rate (<90% or ≥90%), blastulation rate (<30% or ≥30%), pregnancy rate (PR), live birth rate, and cumulative live birth rate.
Statistical analysis
The statistical software package Statistica 10 (USA) was used for statistical analysis. Quantitative parameters are presented as medians (interquartile range). Qualitative parameters are presented as absolute and relative values of frequency (n, %). Statistical analysis was performed using χ2 test to compare categorical variables, the Kruskal–Wallis test and the Mann–Whitney U test to compare quantitative data. Regression analysis (logistic regression) was used to assess the effect of Hg and Pb levels in the blood on the outcomes of ART programs depending on genetic polymorphism of the xenobiotic biotransformation system. To assess the joint effect of genotype and Hg/Pb on the outcomes of ART programs, combined Hg/Pb and genotype indicators were used. The factors identified in the univariate analysis were studied as predictors.
Difference between statistical values was assumed to be statistically significant at p˂0.05.
The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russian Federation.
Results
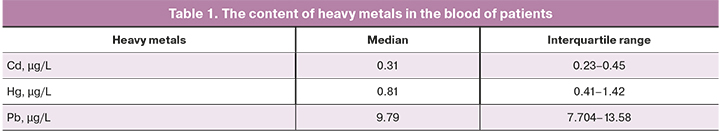
Patients included in the study were middle-aged, with normal BMI and ovarian reserve level. Median age was 31 (29–34) years, BMI – 21.6 (20.4–23.5) kg/m2, and anti-Müllerian hormone (AMH) – 3.2 (1.9–5.8) ng/ml. The concentrations of heavy metal in the patients are noted in Table 1.
We analyzed the clinical and anamnestic data of the patients and their relation to the level of heavy metals in the blood. The Cd level was significantly higher in smokers, and in patients with excessive body weight. The Hg level was significantly higher in patients of late reproductive age. The Pb and Hg blood levels were significantly lower in patients with the absence of the T allele of the CYP1A1 gene and in patients with the G allele of the GSTP1 gene. The Hg level was also lower in patients with the absence of GSTT1 gene deletion. The level of Cd was not associated with genetic polymorphism of the xenobiotic biotransformation system (Table 2).
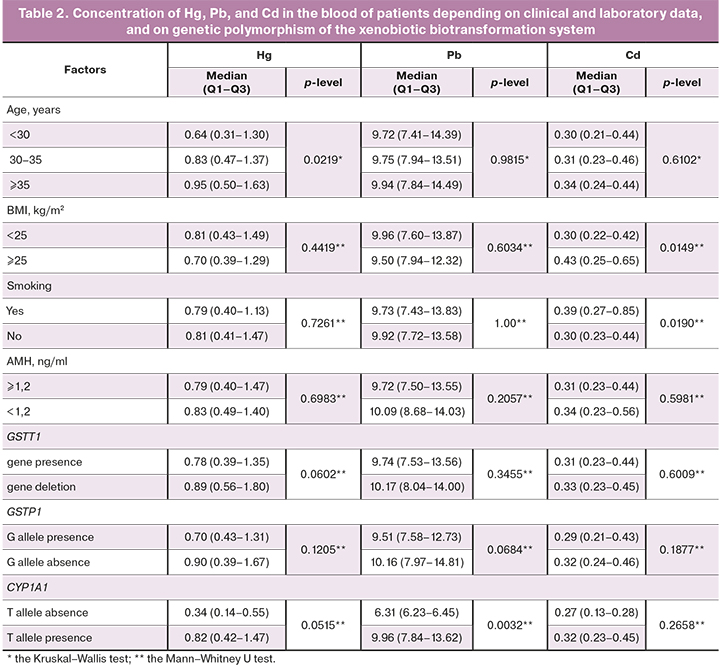
In different quartile subgroups of Cd, Hg, and Pb levels, comparable indicators of the clinical outcomes of ART- PR, labor, and cumulative live birth rates were observed. The Hg level was significantly higher in patients with low oocyte fertilization rate (p=0.0769).
In the analysis of the relationship between the concentration of heavy metals and genetic polymorphism of the xenobiotic biotransformation system, statistically significant differences were found for 3 variants: deletion of the GSTT1 gene, presence of the G allele of the GSTP1 gene, and presence of the T allele of the CYP1A1 gene (Table 2).
Patients with the absence of T allele of the CYP1A1 gene had a lower live birth rate (p=0.0270) and cumulative live birth rate (p=0.0249), which was 51.6% (113/219 patients) in patients with the T/T genotype, 38.1% (29/76 patients) in patients with the T/C genotype, and 20% (1/5 patients) in patients with the C/C genotype.
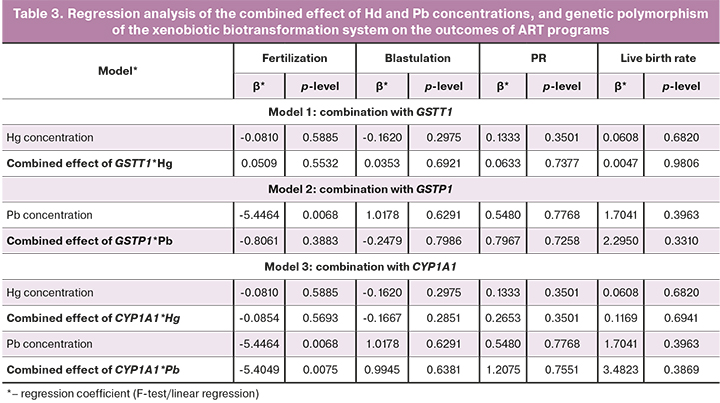
Combined effect of Hg and Pb concentrations, GSTT1 deletion, and the predominance of protective alleles in the GSTP1 and CYP1A1 genes on PR are shown in Table 3. Combined effect of CYP1A1*Pb had a significant impact on oocyte fertilization.
Discussion
According to our study, the blood level of Hg increased with age, and the level of Cd was higher in smokers, and in patients with excessive body weight.
Hg has a short half-life compared to other heavy metals (about 60–70 days). All people are exposed to Hg, but regular consumption of contaminated food (fish, shellfish) or contact with Hg vapor at work increase exposure to this metal. In order to minimize data errors, only patients without occupational hazards were included in the study. Thus, the study did not include data indicating an increase in Hg levels due to contact with this metal. The most likely cause of increased Hg levels in patients of late reproductive age is accumulation of this pollutant with age due to longer exposure.
The connection between increased Cd level and smoking has been confirmed by many researchers [7, 8]. During active and passive smoking, Cd enters the body with smoke. Correlation between increased Cd level and a patient's BMI have not been found in similar studies. A possible reason is a change in Cd metabolism (blood-parenchymal organs-blood) depending on the patient's metabolic features.
The most likely toxic effect of heavy metals is the induction of chronic oxidative stress and mitochondrial dysfunction [9]. Previous studies have shown the impact of even low concentrations of heavy metals on fertility [10]. However, negative effect of heavy metals on the body is also determined by genetic features of the detoxification system [11, 12].
Glutathione-S-transferases (GSTs) are a family of phase II detoxification enzymes that play a key role in protecting cells against exogenous toxic substances. This family of proteins catalyzes the conjugation of glutathione with various electrophilic and hydrophobic compounds. Human GST enzymes can be divided into 5 main classes: alpha, mu, pi, theta, and zeta. The genes encoding the synthesis of these enzymes are characterized by significant population polymorphism. Alternative splicing of the GSTT1 and GSTP1 genes can lead to a variety of transcript variants. The role of GST gene polymorphisms has been studied by many scientists. Polymorphisms of this gene increase the risk of cardiovascular [13], oncological [14], and immunological [15–17] diseases, and also affect pharmacodynamics of drugs [18].
The CYP1A1 gene encodes the cytochrome P450 1A1 enzyme, which belongs to the cytochrome P450 enzyme superfamily. Cytochrome P450 is a monooxygenase that catalyzes many reactions involved in the metabolism of xenobiotics and medications, synthesis of cholesterol, steroids, and other lipids. This protein is located in the endoplasmic reticulum, and its expression is induced by some polycyclic aromatic hydrocarbons (PAHs). The endogenous substrate of this protein is unknown; however, the carcinogenic compounds are synthesized in the process of PAH transformation, so polymorphic variants of this gene increase the risk of lung cancer in smokers [19]. This gene is also associated with the development of other oncological diseases [20, 21].
Our study revealed an association between detoxification gene polymorphism and the level of heavy metals in women, as well as the combined effect of heavy metals and gene polymorphisms, leading to a low rate of oocyte fertilization. A review of the scientific literature found no similar data in patients with infertility in ART cycles.
The GSTT1 gene deletion is associated with an increased blood level of Hg. The absence of the G allele in the GSTP1 gene is associated with an increased blood level of Cd. Presence of the T allele in the CYP1A1 gene is associated with increased levels of both Hg and Cd in blood.
The combined effect of the polymorphic variants of CYP1A1 and an increased level of Pb led to a decrease in the oocyte fertilization rate, but had no impact on the blastulation rate or on the clinical outcomes of ART (PR, live birth rate).
Fertilization of oocytes is a complex cascade of molecular events leading to the removal of block of meiosis II and the resumption of meiosis, and the formation of a zygote with a maternal and paternal pronuclei. The oocyte cytoplasm is reorganized, and the genes responsible for proliferation and differentiation are activated. Organelles are distributed between the different poles of the oocyte; this is necessary for the equal distribution of organelles among the blastomeres. The most likely pathogenetic mechanism of heavy metals that affect the oocyte fertilization is a disruption of intracellular calcium metabolism or damage to the cleavage spindle; however, this concept requires further study.
Conclusion
The results of our study indicate an important role of detoxification gene polymorphism in the accumulation of heavy metals, and its contribution to a decrease of the oocyte fertilization rate in ART cycles.
References
- Centers for Disease Control and Prevention. Fourth national report on human exposure to enviromental chemicals. Atlanta, GA : U.S. Department of Health and Human Services; 2009.
- Tolunay H.E., Şükür Y.E., Ozkavukcu S., Seval M.M., Ateş C., Türksoy V.A. et al. Heavy metal and trace element concentrations in blood and follicular fluid affect ART outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 198: 73-7. https://dx.doi.org/10.1016/j.ejogrb.2016.01.001.
- Сыркашева А.Г., Франкевич В.Е., Долгушина Н.В. Ассоциация между уровнем тяжелых металлов в организме женщин с бесплодием и исходами программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2020; 11: 124-30. [Syrkasheva A.G., Frankevich V.E., Dolgushina N.V. Association between heavy metals in infertile women and IVF outcomes. Akusherstvo i ginekologia/Obstetrics and Gynecology. 2020; 11: 124-30 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.124-130.
- Казанцева Е.В., Долгушина Н.В., Донников А.Е., Беднягин Л.А., Баранова Е.Е., Терешков П.П. Влияние пренатальной экспозиции бенз(а)пирена, стирола и формальдегида на массу тела при рождении в зависимости от полиморфизмов генов системы детоксикации. Акушерство и гинекология. 2016; 7: 68-78. [Kazantseva E.V., Dolgushina N.V., Donnikov A.E., Bednyagin L.A., Baranova E.E., Tereshkov P.P. Impact of prenatal exposition of benz(a)pyrene, styrene and formaldehyde on newborn weight depend on detoxification gene polymorphism. Akusherstvo i ginekologia/ Obstetrics and Gynecology. 2016; 7: 68-78 (in Russian)]. https://dx.doi.org/10.18565/aig.2016.7.68-78.
- Долгушина Н.В., Казанцева Е.В., Пивоварова Л.В. Влияние антропогенных химических веществ на массу тела новорожденных. Акушерство и гинекология. 2013; 12: 58-64. [Dolgushina N.V., Kazantseva E.V., Pivovarova L.V. Impact of antropogenic chemicals on the newborn weight. Akusherstvo i ginekologia/ Obstetrics and Gynecology. 2013; 12: 58-64 (in Russian)].
- Сыркашева А.Г., Долгушина Н.В., Макарова Н.П., Ковальская Е.В., Агаршева М.А. Исходы программ вспомогательных репродуктивных технологий у пациенток с дисморфизмами ооцитов. Акушерство и гинекология. 2015; 7: 56-62. [Syrkasheva A.G., Dolgushina N.V., Makarova N.P., Kovalskaya E.V., Agarsheva M.A. IVF outcomes in patients with oocyte dysmorphisms. Akusherstvo i ginekologia/ Obstetrics and Gynecology. 2015; 7: 56-62 (in Russian)].
- Rzymski P., Rzymski P., Tomczyk K., Niedzielski P., Jakubowski K., Poniedziałek B. et al. Metal status in human endometrium: relation to cigarette smoking and histological lesions. Environ. Res. 2014; 132: 328-33. https://dx.doi.org/10.1016/j.envres.2014.04.025.
- Pizzol D., Foresta C., Garolla A., Demurtas J., Trott M., Bertoldo A. et al. Pollutants and sperm quality: a systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021; 28(4): 4095-103. https://dx.doi.org/10.1007/s11356-020-11589-z.
- Carvalho L.V.B., Hacon S.S., Vega C.M., Vieira J.A., Larentis A.L., Mattos R.C.O.C. et al. Oxidative stress levels induced by mercury exposure in Amazon Juvenile Populations in Brazil. Int. J. Environ. Res. Public Health. 2019; 16(15): 2682. https://dx.doi.org/10.3390/ijerph16152682.
- Lee S., Min J.Y., Min K.B. Female infertility associated with blood lead and cadmium levels. Int. J. Environ. Res. Public Health. 2020; 17(5): 1794. https://dx.doi.org/10.3390/ijerph17051794.
- Lamichhane D.K., Leem J.H., Park C.S., Ha M., Ha E.H., Kim H.C. et al. Associations between prenatal lead exposure and birth outcomes: Modification by sex and GSTM1/GSTT1 polymorphism. Sci. Total Environ. 2018; 619-620: 176-84.
- Shojaeepour S., Fazeli M., Oghabian Z., Pourgholi L., Mandegary A. Oxidative stress in opium users after using lead-adulterated opium: The role of genetic polymorphism. Food Chem. Toxicol. 2018; 120: 571-7. https://dx.doi.org/10.1016/j.fct.2018.07.061.
- Su H., Cao Y., Li J., Zhu Y., Ma X. GST null polymorphisms may affect the risk of coronary artery disease: evidence from a meta-analysis. Thromb. J. 2020; 18: 20. https://dx.doi.org/10.1186/s12959-020-00234-x.
- Chatterjee A., Gupta S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018; 433: 33-42. https://dx.doi.org/10.1016/j.canlet.2018.06.028.
- Bowatte G., Lodge C.J., Perret J.L., Matheson M.C., Dharmage S.C. Interactions of GST polymorphisms in air pollution exposure and respiratory diseases and allergies. Curr. Allergy Asthma Rep. 2016; 16(12): 85. 1 https://dx.doi.org/0.1007/s11882-016-0664-z.
- Broekman M.M.T.J., Bos C., Te Morsche R.H.M., Hoentjen F., Roelofs H.M.J., Peters W.H.M. et al. GST Theta null genotype is associated with an increased risk for ulcerative colitis: a case-control study and meta-analysis of GST Mu and GST Theta polymorphisms in inflammatory bowel disease. J. Hum. Genet. 2014; 59(10): 575-80. https://dx.doi.org/10.1038/jhg.2014.77.
- Du Y., Zhang H., Xu Y., Ding Y., Chen X., Mei Z. et al. Association among genetic polymorphisms of GSTP1, HO-1, and SOD-3 and chronic obstructive pulmonary disease susceptibility. Int. J. Chron. Obstruct. Pulmon. Dis. 2019; 14: 2081-8. https://dx.doi.org/10.2147/COPD.S213364.
- Nishikawa T., Yamaguchi H., Ikawa K., Nakayama K., Higashi E., Miyahara E. et al. Influence of GST polymorphisms on busulfan pharmacokinetics in Japanese children. Pediatr. Int. 2019; 61(6): 558-65. https://dx.doi.org/10.1111/ped.13859.
- Hussein A.G., Pasha H.F., El-Shahat H.M., Gad D.M., Toam M.M. CYP1A1 gene polymorphisms and smoking status as modifier factors for lung cancer risk. Gene. 2014; 541(1): 26-30. https://dx.doi.org/10.1016/j.gene.2014.03.003.
- Hidaka A., Sasazuki S., Matsuo K., Ito H., Charvat H., Sawada N. et al. CYP1A1, GSTM1 and GSTT1 genetic polymorphisms and gastric cancer risk among Japanese: A nested case-control study within a large-scale population-based prospective study. Int. J. Cancer. 2016; 139(4): 759-68. https://dx.doi.org/10.1002/ijc.30130.
- Wongpratate M., Ishida W., Phuthong S., Natphopsuk S., Ishida T. Genetic polymorphisms of the human cytochrome P450 1A1 (CYP1A1) and cervical cancer susceptibility among Northeast Thai women. Asian Pac. J. Cancer Prev. 2020; 21(1): 243-8. https://dx.doi.org/10.31557/APJCP.2020.21.1.243.
Received 12.03.2021
Accepted 07.06.2021
About the Authors
Anastasia G. Syrkasheva, PhD, Senior Researcher of the IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. E-mail: a_syrkasheva@oparina4.ru. ORCID: 0000-0002-7150-2230. 4 Oparina str., 117997, Moscow, Russia.Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department of Research Administration, V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. E-mail: n_dolgushina@oparina4.ru. ORCID: 0000-0003-1116-138X. 4 Oparina str., 117997, Moscow, Russia.
Vladimir E. Frankevich, PhD, Head of the Department of Systems Biology in Reproduction, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. Tel.: +7(495)438-07-88. E-mail: v_frankevich@oparina4.ru. ORCID: 0000-0002-9780-4579.
4 Oparina str., 117997, Moscow, Russia.
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. E-mail: donnikov@oparina4.ru. ORCID: 0000-0003-3504-2406. 4 Oparina str., 117997, Moscow, Russia.
For citation: Syrkasheva A.G., Dolgushina N.V., Frankevich V.E., Donnikov A.E. Influence of heavy metals on the effectiveness of assisted reproductive technologies depending on the polymorphism of genes of the detoxification system.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 95-101 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.95-101



