Influence of controlled mechanical microvibration on the oocyte fertilization and embryo during the first five days of development
Objective. To evaluate the influence of controlled mechanical microvibration on oocyte fertilization rate and embryo during the first five days of development.Romanov A.Yu., Romanov E.A., Makarova N.P., Dolgushina N.V.
Materials and methods. The quality of 952 embryos obtained from 166 patients was evaluated in the microvibration group. The quality of 3369 embryos obtained from 757 patients was assessed in the control group. In the microvibration group, the incubator was placed on the ArisTT180-s platform (K&S Advanced Systems Ltd, Israel) in the active vibration mode with a frequency of 40 Hz for 30 seconds with intervals of 30 minutes.
Results. The number of embryos scored as grades one and five (according to Gardner’s classification), with grades AA and BA increased during the cultivation under the conditions of controlled mechanical microvibration. The rate of embryo cryopreservation was 1.22 times higher in the microvibration group in comparison with the control group. The average number of cryopreserved embryos was 1 (0 – 3) in patients of the microvibration group and
0 (0 – 2) in patients of the control group (p=0.003).
Conclusion. Controlled mechanical microvibration can be used in oocyte fertilization and cultivation of embryos to increase the quality of embryos on the fifth day of cultivation and to improve the possibility of obtaining embryos suitable for cryopreservation.
Keywords
The choice of parameters for cultivating human embryos plays an important role in the development of assisted reproductive technologies (ART) in Russia and abroad [1–4]. When an embryo is cultivated, the conditions of the embryological laboratory are different from the natural ones. These conditions include pH and temperature fluctuations, the effect of atmospheric oxygen, natural and artificial light [5]. In recent years, there have been significant advances in the development of embryology, including those related to the optimization of routine conditions for embryo cultivation [6–8]. However, a number of conditions in vitro are still considerably different from those in vivo [9–12].
The dynamic interaction of the embryo and its microenvironment can be provided by the contractions in the wall of the fallopian tube together with the contractions of the villi of the mucous membrane [13–15]. Moreover, these processes are aimed at ensuring the diffusion of nutrients. All of the above has a significant impact on the preimplantation development of the embryo [16, 17].
The combination of routine conditions for embryo cultivation and cultivation systems with controlled mechanical microvibration (CMMV) can become a new approach to improving the effectiveness of ART [16, 18, 19].
The aim of the study was to evaluate the influence of CMMV on oocyte fertilization rate and preimplantation embryo development.
Materials and Methods
The study included 923 couples aged 18-45 years; all patients were examined according to the Order of the Ministry of Health of the Russian Federation dated August, 30, 2012 No. 107n “On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use” [20]. The quality of 952 embryos (1119 oocyte cumulus complexes) obtained from 166 patients was evaluated in the microvibration group. The quality of 3369 embryos (3886 oocyte cumulus complexes) obtained from 757 patients was assessed in the control group. Unfertilized or abnormally fertilized oocytes were not taken into account during the analysis (except the assessment of fertilization rate). Patients with donor oocytes were not included in the study.
The patients who were included in the study did not differ in age (34 (31-39) versus 35 (32-41) years, p=0.69) and body mass index (23.2 (3.8) versus 23.3 (4.3) kg/m2 in the microvibration group and in the control group, respectively).
All patients signed a voluntary informed consent to participate in the research. The study was approved by the local institutional review board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Ovarian stimulation was performed using a gonadotropin-releasing hormone (GnRH) antagonist with medications of recombinant follicle stimulating hormone (FSH) or human menopausal gonadotropin. In order to prevent a premature peak of luteinizing hormone (LH), GnRH antagonist was administered when the leading follicle reached a diameter of 14 mm. The trigger of ovulation was injected if the leading follicle was greater than or equal to 19 mm. Human chorionic gonadotropin (hCG) at a dose of 8,000-10,000 IU or a combination of hCG with a GnRH agonist was used for ovulation triggering. Transvaginal ultrasound-guided puncture of follicles was performed 36 hours after ovulation triggering [21].
The embryos were cultured in separate drops of Irvine CSC culture medium (Fujifilm, USA) of equal volume (25 μl) for 5 days in a mixed solution N2/O2/CO2 (89/5/6%, respectively). When the embryos were cultivated under the conditions of CMMV, MINC® Benchtop Mini Incubator (COOK, USA) was placed on the ArisTT180-s platform (K&S Advanced Systems Ltd, Israel) in an active vibration mode at a frequency of 40 Hz for 30 seconds with intervals of 30 minutes.
There was an assessment of the rate of oocyte fertilization, the quality of embryos on the fifth day of development according to Gardner’s classification [22], the frequency of cryopreservation and the number of cryopreserved embryos.
There was an assessment of the rate of oocyte fertilization, the quality of embryos on the fifth day of development according to Gardner’s classification [22], the rate of cryopreservation and the number of cryopreserved embryos.
Statistical analysis
The GraphPad Prism statistical software package (GraphPad Software, USA) was used for statistical analysis and plotting. The D’Agostino-Pearson test was used to determine the normality of the distribution. The mean value and standard deviation were calculated for parametric data, and t-test was used. The median and interquartile range were calculated for nonparametric quantitative data, and the Mann-Whitney test was used. The absolute value and percentage were calculated for qualitative data, the Fisher’s exact test and the odds ratio (OR) with a 95% confidence interval (CI) were used. Differences were considered statistically significant at p <0.05.
Results
The rate of fertilization in the microvibration group was 85.1% (952/1119) versus 86.7% (3369/3886) in the control group (p=0.17; OR=0.87, 95% CI: 0.72–1.06).
In order to evaluate the influence of CMMV on the development of embryos during the first five days of cultivation, we analyzed the quality of all embryos on the fifth day of development in these groups. The following characteristics were identified during the detailed analysis. Morula formation was observed in 66.6% (634/952) of cases in the microvibration group and in 64.3% (2166/3369) of cases in the control group (p=0.20; OR=1.11, 95% CI: 0.95–1.29). Blastocyst formation was noted in 43.1% of cases in the microvibration group and in 41.9% in the control group (p=0.51; OR=1.05, 95% CI: 0.91–1.22).
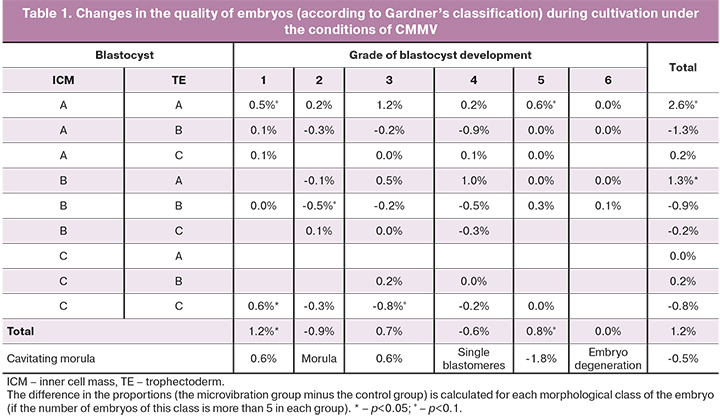
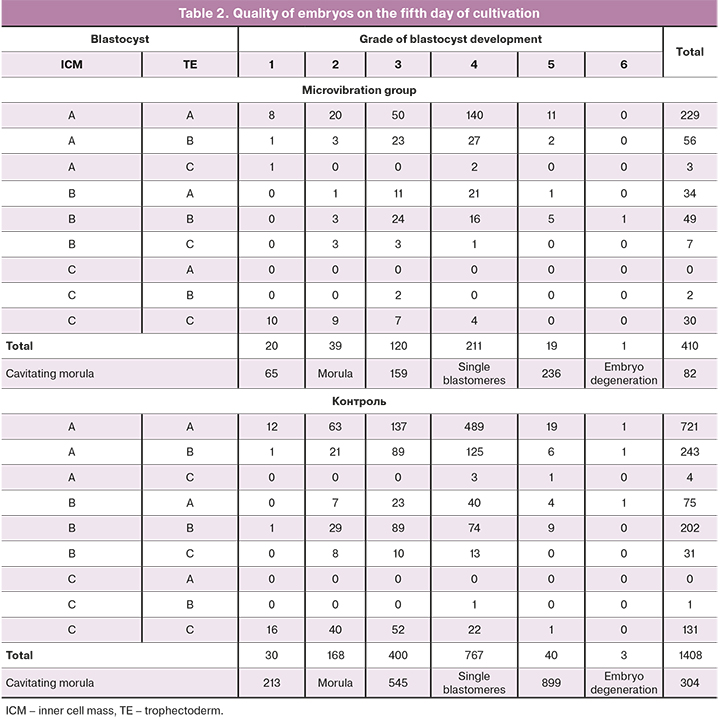
When embryos were cultivated under the conditions of CMMV (Table 1), the number of embryos scored as grade one increased by 1.2% (p=0.004; OR=2.39, 95% CI: 1.35–4.23) and the number of embryos scored as grade five increased by 0.8% (p=0.08; OR=1.70, 95% CI: 0.98 – 2.94) (Table 2). Therefore, it can be assumed that microvibration has a positive effect on rapidly (timely) developing embryos and a negative effect on low-quality embryos. The evaluation of the quality of the inner cell mass and trophectoderm showed that the number of embryos with grade AA was 2.6% higher in the microvibration group (p=0.09; OR=1.16, 95% CI: 0.98–1.38) and the number embryos with grade BA was 1.3% higher (p=0.03; OR=1.63, 95% CI: 1.08–2.46) than in the control group (Table 2).
After embryo transfer into the uterine cavity, cryopreservation of one or more embryos was performed in 98 (59.0%) patients of the microvibration group and 367 (48.5%) patients of the control group (p=0.02). Odds ratio of embryo cryopreservation depending on CMMV was 1.53 (95% CI: 1.09–2.15). The average number of cryopreserved embryos was 1 (0-3) in patients in the microvibration group and 0 (0-2) in patients in the control group (p=0.003). The average number of cryopreserved embryos in patients who underwent embryo cryopreservation was 3 (1-4) in the microvibration group and 2 (1-4) in the control group (p=0.052).
Discussion
In this study, we conducted a comparative analysis of the assessment of the rate of oocyte fertilization and embryo development during the first five days of cultivation depending on the cultivation conditions. The patients who were included in the study did not differ in age, body mass index, clinical data and medical history.
In the literature, there are contradictory data on the effect of CMMV on the fertilization of human oocytes during cultivation in ART programs [23–25]. The average rate of fertilization did not differ in the comparison groups in the general cohort of patients who were included in the study. Therefore, it is important to continue studying the influence of cultivation systems on the rate of fertilization in patients with a low rate of fertilization and insufficient number of obtained oocytes.
In order to assess the influence of CMMV on the development of embryos during the first five days of cultivation, the quality of all embryos on the fifth day of development in these groups was analyzed. The rate of embryo development to the stage of morula and blastocyst did not differ in the comparison groups. Our data are consistent with the findings obtained by Hur Y.S. et al. (2013) [26], whereas the studies of Isachenko E. et al. (2010) and Isachenko V. et al. (2017) showed that microvibration led to a significant improvement in the quality of embryos [16, 27]. The number of embryos scored as grades one and five and embryos with grades AA and BA increased during the cultivation under the conditions of controlled mechanical microvibration. Thus, it can be assumed that microvibration has a positive effect on rapidly (timely) developing embryos and a negative effect on low-quality embryos.
Taking into consideration the low effectiveness of ART per embryo transfer, it is extremely important to have an opportunity for cryopreservation of embryos of a high morphological class for subsequent cryopreservation in ART programs. The rate of embryo cryopreservation was 1.22 times higher in the microvibration group in comparison with the control group. The average number of cryopreserved embryos was also higher in the microvibration group. Therefore, CMMV used in embryo cultivation may improve the effectiveness of ART programs due to an increase in the number of cryopreserved embryos.
Conclusion
Controlled mechanical microvibration does not affect the rate of oocyte fertilization in ART programs; however, it may improve the conditions for the cultivation of human embryos and increase the effectiveness of the embryological stage in the ART programs. Still, further studies with a prospective design and evaluation of long-term outcomes are necessary for the routine application of this technique.
References
- Шафеи Р.А., Сыркашева А.Г., Романов А.Ю., Макарова Н.П., Долгушина Н.В., Семенова М.Л. Хетчинг бластоцисты у человека. Онтогенез. 2017; 48(1): 8-20. [Shafei R.A., Syrkasheva A.G., Romanov A.Y., Makarova N.P., Dolgushina N. V., Semenova M.L. Blastocyst hatching in humans. Oncogenez. 2017; 48(1): 8-20 (in Russian)].
- Романов А.Ю., Ковальская Е.В., Макарова Н.П., Сыркашева А.Г., Долгушина Н.В. Использование цейтраферной съемки для оценки качества эмбрионов человека в программах экстракорпорального оплодотворения. Цитология. 2017; 59(7): 462-6. [Romanov A.Yu., Kovalskaya E.V., Makarova N.P., Syrkasheva A.G., Dolgushina N.V. Embryo quality assessment by evaluation of morphokinetics of the human embryos in assisted reproduction. Cytology. 2017; 59(7):462-6. (in Russian)].
- Ибрагимова Э.О., Долгушина Н.В., Сыркашева А.Г., Романов А.Ю., Языкова О.И., Макарова Н.П. Роль вспомогательного хетчинга в программах лечения бесплодия методами вспомогательных репродуктивных технологий: обзор литературы. Гинекология. 2016; 18(2): 44-7. [Ibragimova E.O., Dolgushina N.V., Syrkasheva A.G., Romanov A.Yu., Yazikova O.I., Makarova N.P. The role of assisted hatching in in vitro fertilization cycles: a literature review. Gynekology. 2016; 18(2): 44-7. [(in Russian)].
- Долгушина Н.В., Ибрагимова Э.О., Романов А.Ю., Макарова Н.П., Довгань А.А., Сыркашева А.Г., Калинина Е.А. Роль проназного хетчинга в повышении эффективности программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2018; 3: 70-5. https://dx.doi.org/10.18565/aig.2018.3.70-74 [Dolgushina N.V., Ibragimova E.O., Romanov A.Yu., Makarova N.P., Dovgan A.A., Syrkasheva A.G. et al. Role of pronase hatching in enhancing the effectiveness of assisted reproductive technology programs. Obstetrics and gynecology. 2018; 3: 70-5. (in Russian)].
- Ковальская Е.В., Сыркашева А.Г., Романов А.Ю., Макарова Н.П., Долгушина Н.В. Современные представления о компактизации эмбрионов человека в условиях in vitro. Технологии живых систем. 2017; 1: 25-35. [Kovalskaya E.V., Syrkasheva A.G., Romanov A.Yu., Makarova N.P., Dolgushina N.V. Modern views on the compaction of the human embryo in vitro. Living systems technologies. 2017; 1: 25-35. (in Russian)].
- Biggers J.D., Summers M.C. Choosing a culture medium: making informed choices. Fertil. Steril. 2008; 90(3): 473-83. https://dx.doi.org/10.1016/j.fertnstert.2008.08.010.
- Loutradis D., Drakakis P., Kallianidis K., Sofikitis N., Kallipolitis G., Milingos S. et al. Biological factors in culture media affecting in vitro fertilization, preimplantation embryo development, and implantation. Ann. N. Y. Acad. Sci. 2000; 900: 325-35. https://dx.doi.org/10.1111/j.1749-6632.2000.tb06245.x.
- Chronopoulou E., Harper J.C. IVF culture media: past, present and future. Hum. Reprod. Update. 2015; 21(1): 39-55. https://dx.doi.org/10.1093/humupd/dmu040.
- Brison D.R., Houghton F.D., Falconer D., Roberts S.A., Hawkhead J., Humpherson P.G. et al. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum. Reprod. 2004; 19(10): 2319-24. https://dx.doi.org/10.1093/humrep/deh409.
- Thompson J.G. Culture without the petri-dish. Theriogenology. 2007; 67(1): 16-20. https://dx.doi.org/10.1016/j.theriogenology.2006.09.016.
- Gardner D.K., Lane M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod. Fertil. Dev. 2005; 17(3): 361-70. https://dx.doi.org/10.1071/rd04103.
- Isachenko V., Maettner R., Sterzik K., Strehler E., Kreinberg R., Hancke K. et al. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod. Biomed. Online. 2011; 22(6): 536-44. 1 https://dx.doi.org/0.1016/j.rbmo.2011.02.006.
- Muglia U., Motta P.M. A new morpho-functional classification of the Fallopian tube based on its three-dimensional myoarchitecture. Histol. Histopathol. 2001; 16(1): 227-37. https://dx.doi.org/10.14670/HH-16.227.
- Lyons R.A., Djahanbakhch O., Mahmood T., Saridogan E., Sattar S., Sheaff M.T. et al. Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum. Reprod. 2002; 17(3): 584-8. https://dx.doi.org/10.1093/humrep/17.3.584.
- Lyons R.A., Saridogan E., Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update. 2006; 12(4): 363-72. https://dx.doi.org/10.1093/humupd/dml012.
- Isachenko E., Maettner R., Isachenko V., Roth S., Kreienberg R., Sterzik K. Mechanical agitation during the in vitro culture of human pre-implantation embryosdrastically increases the pregnancy rate. Clin. Lab. 2010; 56(11-12): 569-76.
- Matsuura K., Hayashi N., Kuroda Y., Takiue C., Hirata R., Takenami M. et al. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod. Biomed. Online. 2010; 20(3): 358-64. https://dx.doi.org/10.1016/j.rbmo.2009.12.002.
- Romanov A.Y., Silachev D.N., Makarova N.P., Dolgushina N.V. Effect of Mechanical microvibration on the quality of human embryos during in vitro culturing and outcomes of assisted reproduction technologies. Bull. Exp. Biol. Med. 2018; 165(4): 544-7. https://dx.doi.org/10.1007/s10517-018-4211-x.
- Романов А.Ю., Силачев Д.Н., Макарова Н.П., Долгушина Н.В. Влияние механической микровибрации на качество эмбрионов человека при культивировании in vitro и исходы программ вспомогательных репродуктивных технологий. Клеточные технологии в биологии и медицине. 2018; 2: 86-90. [Romanov A.Y., Silachev D.N., Makarova N.P., Dolgushina N. V. Effect of Mechanical Microvibration on the Quality of Human Embryos during In Vitro Culturing and Outcomes of Assisted Reproduction Technologies. Bull Exp Biol Med. 2018; 2: 86-90. (in Russian)].
- Министерство здравоохранения Российской Федерации. Приказ Минздрава России от 30.08.2012 N 107н (ред. от 11.06.2015) "О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению". [Ministry of Health of the Russian Federation. Order of the Ministry of Health of Russia of 30.08.2012 N 107n (as amended on 11.06.2015) "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use." (in Russian)].
- Романов А.Ю., Эльдаров Ч.М., Фролова А.М., Макарова Н.П., Бобров М.Ю., Долгушина Н.В. Влияние контролируемой механической микровибрации на метаболомный профиль сред культивирования эмбрионов человека пятых суток развития. Акушерство и гинекология. 2020; 11: 131-8. [Romanov A.Yu., Eldarov Ch.M., Frolova A.M., Makarova N.P., Bobrov M.Yu., Dolgushina N.V. Influence of controlled mechanical microvibration on embryo metabolomic profile. Obstetrics and Gynecology. 2020; 11: 131-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.131-138.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- López-Teijón M., Castelló C., Asensio M., Fernández P., Farreras A., Rovira S. et al. Improvement of fertilization rates of in vitro cultured human embryos by exposure to sound vibrations. J. Fertil. In Vitro IVF Worldw. Reprod. Med. Genet. Stem Cell Biol. 2015; 3: 160. https://dx.doi.org/10.4172/2375-4508.1000160.
- El-Danasouri I., Sandi-Monroy N.L., Winkle T., Ott K., Krebs C., Maas D.H.A. et al. Micro-vibration culture of human embryos improves pregnancy and implantation rates. Fertil. Steril. 2014; 102(3): e217. https://dx.doi.org/10.1016/j.fertnstert.2014.07.732.
- Yang S.H., Yoon S.H., Jung J.H., Lim J.H., Ko Y. Improvement of embryonic development and clinical outcomes of germinal vesicle stage oocytes using a microvibration culture system. Syst. Biol. Reprod. Med. 2019; 65(4): 333-41. https://dx.doi.org/10.1080/19396368.2019.1602681.
- Hur Y.S., Park J.H., Ryu E.K., Park S.J., Lee J.H., Lee S.H. et al. Effect of micro-vibration culture system on embryo development. J. Assist. Reprod. Genet. 2013; 30(6): 835-41. https://dx.doi.org/10.1007/s10815-013-0007-0.
- Isachenko V., Sterzik K., Maettner R., Isachenko E., Todorov P., Rahimi G. et al. In vitro microvibration increases implantation rate after embryonic cell transplantation. Cell Transplant. 2017; 26(5): 789-94. https://dx.doi.org/10.3727/096368916X693428.
Received 19.02.2021
Accepted 02.06.2021
About the Authors
Andrey Yu. Romanov, postgraduate student, researcher of R&D Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(903)158-94-00. E-mail: romanov1553@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Evgeniy A. Romanov, embryologist of IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Healthcare of Russian Federation. E-mail: e_romanov@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya P. Makarova, Dr. Bio. Sci., Leading Researcher of IVF Department, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. E-mail: np_makarova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department of Research Administration, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. E-mail: n_dolgushina@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Romanov A.Yu., Romanov E.A., Makarova N.P., Dolgushina N.V. Influence of controlled mechanical microvibration on the oocyte fertilization and embryo during the first five days of development.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 7: 152-157 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.152-157



