Urine steroid profile in pregnant women with isthmic-cervical insufficiency and hyperandrogenism
Objective: To evaluate the features of urine steroid profile and quantification of urinary steroids in pregnant women with isthmic-cervical insufficiency (ICI) and hyperandrogenism (HA). Materials and methods: A prospective cohort controlled study was conducted from 2014 to 2019 in Maternity hospital No. 1, a branch of the City Clinical Hospital No. 67 named after L.A. Vorokhobov (Moscow). The study included 98 women. The main group consisted of 63 women with ICI. The control group included 35 women without ICI. HA was assessed by determination of the steroid profile and quantification of urinary steroids using high-performance liquid chromatography. Statistical data processing and analysis was performed using Microsoft Excel 2007, Statistica 12.0. Specific characteristics of urine steroid profile in pregnant women with isthmic-cervical insufficiency and hyperandrogenism were evaluated. Results: The women with ICI, regardless of the presence or absence of HA, were characterized by monomarkers of the steroid profile of urine – higher levels of androsterone (abs.) (p<0.001), total 17-ketosteroids (17-CS) (p=0.02), van de Calseide discriminants (p<0.001), delta-5-pregnandiol (p=0.03), but low levels of 16-alpha-Hydroxy-Et (p<0.001), 16-alpha-Hydroxy-An (p<0.001) and 16-alpha-hydroxy-DHEA (p=0.02); a combination of markers – van de Kalseide discriminant, 16-alpha-Hydroxy-An, allo-Pd, cholesterol and estrone (OR=19.5). In women with ICI associatade with HA are characterized by the markers: higher values of An, abs. (p=0.001), van de Calseide (p=0.002), Et/Ch (p=0.0003) and estrone (p=0.03) discriminants, and lower ones – 11-Keto-Et, abs. (p=0.005) and % of the sum of 17-KS (p=0.001), 16-alpha-Hydroxy-Et (p=0.04). An association between ICI and HA was established (OR=3.48, 95% CI 1.37–8.84). A trend of greater association of GA of ovarian origin with ICI was revealed in comparison with mixed origin (OR=1.62 (95% CI 0.17–15.72). Conclusion: HA may lead to changes in metabolic processes, that are manifested by urine steroid profile, since urine composition reflects a general metabolic state and contains metabolites. Based on the stratification data, it is possible to identify specific metabolic patterns that indicate an association between GA and the potential risk of developing ICI. Authors’ contributions: Levakov S.A. – design of the study, study manager; Mokh A.V. – design of the study, data collection and analysis, article writing. Conflicts of interest: The authors declare that they have no conflict of interest to declare. Funding: The study was conducted without any sponsorship. Ethical Approval: This study was approved by the local Ethics Committee of I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University) on January 20, 2022. Patient Consent for Publication: The patients signed informed consent for publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Mokh A.V., Levakov S.A. Urine steroid profile in pregnant women with isthmic-cervical insufficiency and hyperandrogenism. Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2023; (9): 72-81 (in Russian) https://dx.doi.org/10.18565/aig.2023.167Mokh A.V., Levakov S.A.
Keywords
The problem of miscarriage goes beyond obstetrics and gynecology and requires deep knowledge in other areas of medicine, in particular, endocrinology. Insufficient understanding of the etiopathogenesis of miscarriage explains the difficulties of its prediction, prevention, untimely diagnosis, and treatment failures. Isthmic-cervical insufficiency (ICI) is recognized as a potential precursor of preterm birth [1–5], that is detected in 0.5% of the general obstetrical population [6], and is 1% of the total number of all pregnancies [7], 8% [2] or 20% of spontaneous abortions in the second trimester [6, 7]. Hyperandrogenia (HA) is recognized as one of risk factors for miscarriage [8].
Despite the fact, that association between ICI and HA previously was not thoroughly studied in Russia, there are several mechanisms of relationship between these two conditions. First, androgens may influence collagen metabolism in connective tissue, that may affect cervical resistance to dilatation [2]. Second, it is known that HA may induce inflammatory response, which may have impact on the function and structure of the cervix [4]. Moreover, HA is often associated with polycystic ovary syndrome, that may indirectly influence on the risk of developing ICI [5].
At the same time, urine steroid profile in women is being studied for more than 10 years to diagnose HA [9]. In 2008 [10], it was shown that clinical manifestations of HA combine with predominant androgen metabolites of ovarian origin over metabolites of adrenal origin in urine steroid profile, the absence of isolated adrenal HA. Assessment of urine steroid profile in terms of informativeness competes with the low-dose dexamethasone suppression test.
In 2009 [11], the significance of hormone-depended endocrine disorders in the pathogenesis of refractory acne in women was convincingly proved and was based on urine steroid profile analysis (androsterone (An), etiocholanolone (Et), dehydroepiadrosterone (DHEA), 11-ketoandrosterone (11-Keto-An), 11-ketoethiocholanolone (11-Keto-Et), 11β-ketohydroxyandrosterone (11-OH-Keto-An), 11-13 hydroxyethiocholanolone (11-OH-Keto-Et), (Pt), cholesterol (Ch), and their ratio), which was more informative than the assessment of the blood hormone levels.
Taking into account that the characteristics of metabolism of steroid hormones in women of reproductive age with HA (non-classical form of congenital adrenal dysfunction with 21‑hydroxylase deficiency, polycystic ovary syndrome, HA syndrome in the presence of obesity, idiopathic HA) have been sufficiently studied [12], this topic remains essential [13, 14]. Asymptomatic ICI and untimely diagnosis explain the delay in preventive and therapeutic measures. Assessment of urine steroid profile appears to be an informative method for early differential diagnosis of the genesis of HA during pregnancy, that is necessary for the selection of the most effective measures for ICI prevention and timely correction.
The purpose of this study was to evaluate the characteristics of urine steroid profile and quantification of urinary steroids in pregnant women with ICI and HA.
Materials and methods
A prospective cohort controlled study was conducted from 2014 to 2019 in maternity hospital No.1, a branch of City Clinical Hospital No. 67 named after L.A. Vorokhobov (Moscow). The study included 98 women with singleton pregnancy: 63 women with isthmic-cervical insufficiency (ICI) (the main group), 35 women without ICI (the control group). HA was assessed by determination of the steroid profile and quantification of urinary steroids using high-performance liquid chromatography (gas chromatograph Agilent 6850, Agilent Technologies, USA).
Statistical analysis
Statistical data processing and analysis was performed using Microsoft Excel 2007, Statistica 12.0; StatSoft; TIBCO Software. For normally distributed variables, the quantitative data were described as the arithmetic mean (M) and standard deviation (SD). The qualitative variables were presented in absolute (n) and relative (%) values. The significance of differences (p) were assed using Student’s t-test for normal distribution of variables and homogeneity of variances, and Mann–Whitney U-test was used for abnormality of distribution. The level of significance (p) less than 0.05 was considered as critical. Based on the studied factors, the significance of differences in the outcomes were assessed using the chi-square (χ2) test, for the number of participants under study less than 10 the chi-square (χ2) test was used with Yates correction. The strength of association between the outcome and exposure factor was evaluated by measuring the odds ratio (OR) with 95% confidence interval (95% CI).
Logistic regression was used for binary classification (to split data into two classes) – machine learning algorithm to predict the probability that a data point belonged to a certain class. The intercept term (B0), standard error, the significance of p-value of the Wald test was assessed. The ratio of disagreement was calculated as the ratio of the number of correctly classified observations to the number of incorrectly classified observations. The ratio of disagreement greater than 1 indicated that it was better to use constructed classification than random forest classification. ROC curve was constructed and the area under the curve (AUC) was determined for quality assessment of the resulting models. AUC value reflected the quality of the model – the value of 8.0 and above indicated a very good quality of the mathematical model.
Results
Urine steroid profile indicated the presence of HA in 32/63 (50.79%) women with ICI (among them, in 26/31 (81.25%) women HA was of combined genesis, in 6/31(18.75%) women it was of ovarian origin. In 22.86% women in the control group (among them, in 7/8 (87.5%) women HA was of combined genesis, in 1/27 (12.5%) it was of ovarian origin.
It was noted that in some women without HA, both in the group with ICI and in the control group, normal levels of androgens reached the upper limits; and in women with ICI, low levels of excretion of pregnanediol and other steroids in pregnancy were noted, that reflected threatened miscarriage (Fig. 1).
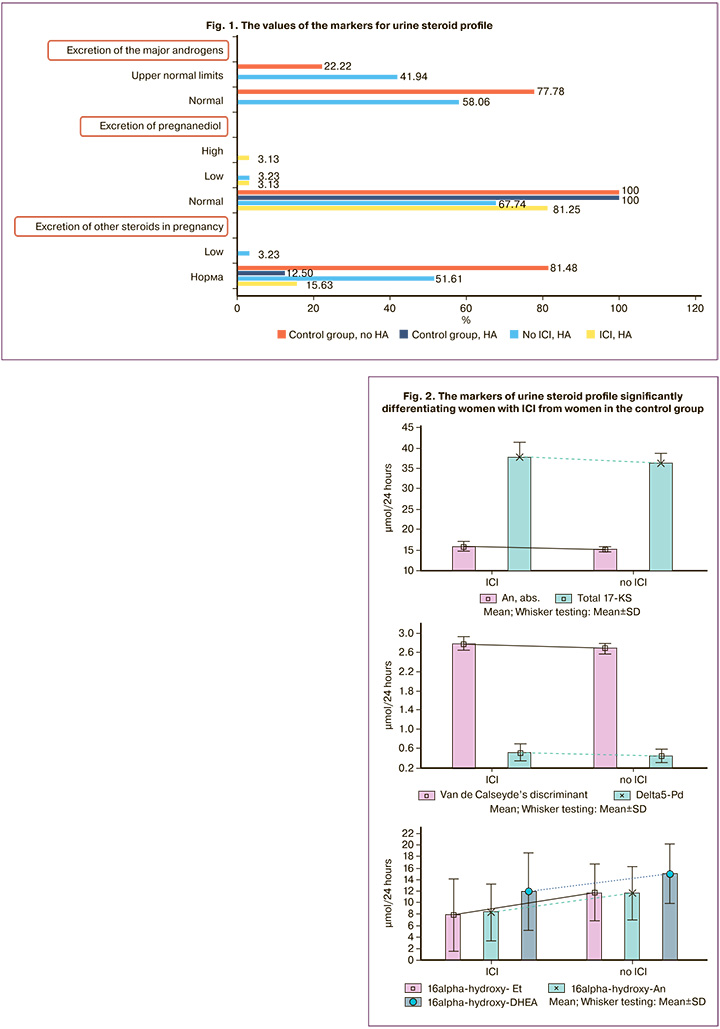
In the absence of ICI, the values of androgens conformed to the upper normal limits in 6/35 (17.14%) women. The comparison of all 22 markers of urine steroid profile identified significant markers for differentiation between the woman with ICI and without ICI in the control group, despite the presence of HA or no HA (Table. 1)
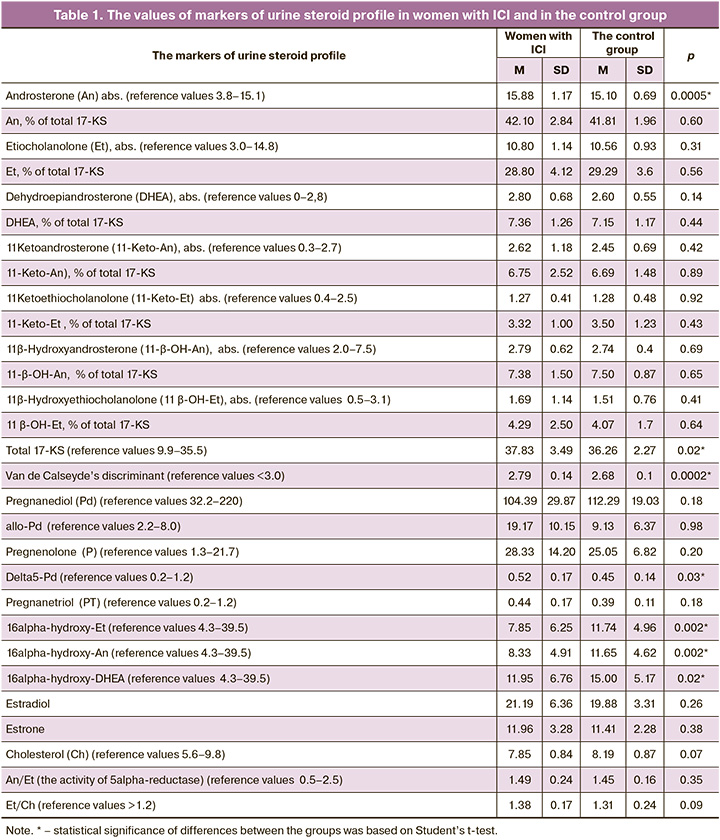
The women with ICI had higher levels of androsterone (abs.) (р<0.001), total 17 KS (р=0.02), Van de Calseyde’s discriminant (р<0.001), delta5-Pregnanediol (р=0.03), but lower levels of 16alpha-hydroxy-Et (р<0.001), 16alpha-hydroxy-An (р<0.001) and 16alpha-hydroxy-DHEA (р=0.02) versus the women in the control group (Fig. 2).
Despite the presence or no HA, the characteristics of urine steroid profile in women with ICI were: 1) high values of androngen markers, both of predominantly ovarian origin and combined genesis (androsterone) (abs.), total 17 KS, Van de Calseyde’s discriminant (the parameter that indicated probability of polycystic ovary syndrome (PCOS), which was calculated by the formula 0.09×(An+Et+11-OH-An+An/Et)); 2) high levels of delta5-pregnanediol, progestin metabolites, that probably reflect the compensatory mechanisms in woman’s body to maintain pregnancy; 3) low level of androgen metabolites, both ovarian origin and mixed genesis (16alpha-hydroxy-Et, 16alpha-hydroxy-An, 16alpha-hydroxy-DHEA (Table 2).
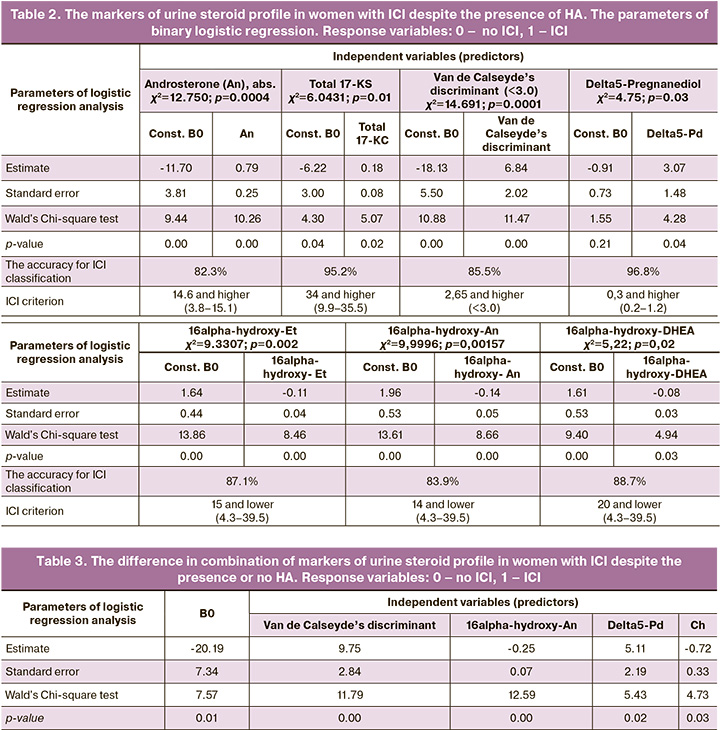
Table 2 shows the difference in the values of the markers of urine steroid profile with accuracy of 80% in women with ICI and in the control group despite the presence or no HA. It should be noted that all threshold values matched the values of normal ranges, and were not only beyond HA criterion, but also reached the upper limits.
The difference in combination of the markers of uterine steroid profile was found in women with ICI despite the presence or no HA (Table 3).
The combination of the markers partially differed from monomarkers as is shown above. Also, the combination was formed by the markers, the levels of which could be significantly different in women with ICI and in the control group (Van de Calseyde’s discriminant, 16alpha-hydroxy-An, allo-Pd), but could be comparable between the groups (cholesterol and estrone).
The total accuracy for prediction of differentiation of women with or without ICI based on a set of markers of urine steroid profile was 82.47% (OR=19,5; Log-OR=2.97): with the presence of ICI – 87,1%, and no ICI – 74.29%. The ROC curve that characterizes the accuracy of differentiation is shown in Figure 3.
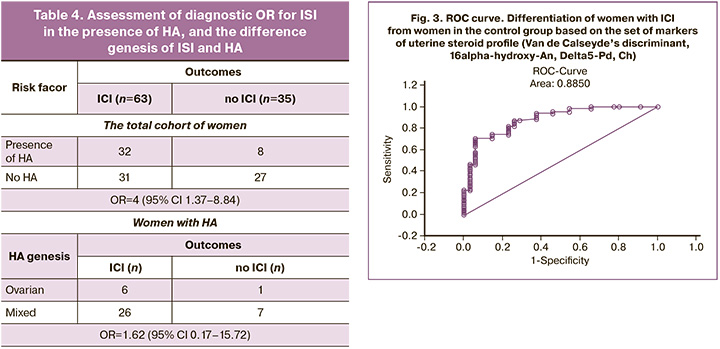
Thus, uterine steroid profile based on mono- or a set of markers was an objective method for diagnostic assessment of ICI despite the presence or no HA.
This study found association between ICI and HA (χ2=6.159, р=0.14; OR=3.48, 95% CI 1.37–8.84) (Table 4).
Despite the fact that the number of women with ICI and HA of different genesis was comparable (χ2=0.011, р=0.92), HA of ovarian origin seemed to be more associated with ICI. We identified the markers that significantly differentiate the women with ICI either associated with HA or not (Table 5).
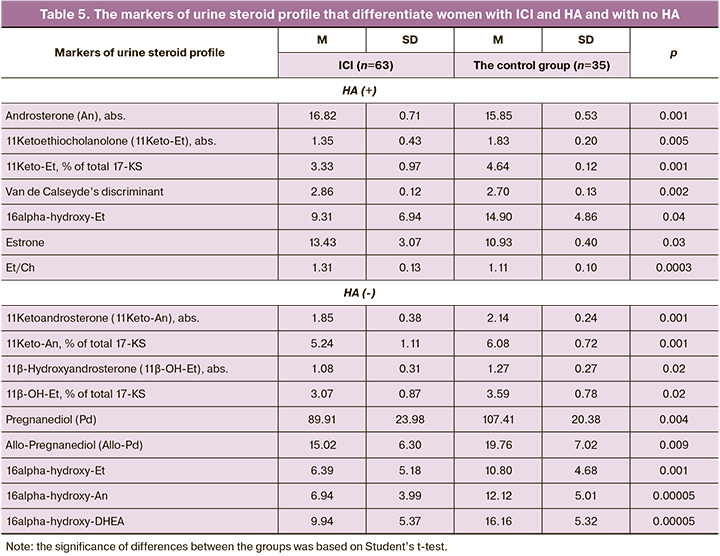
In fact, despite a wide range of 29 markers of urine steroid profile, significant differences in androgen levels (6 of 7) were between women with ICI and in the control group: in the presence of HA, the values of An, Van de Calseyde’s discriminant, Et/Ch and Estrone were high (р<0.05), and the values of 11-Keto-Et, abs. and % of total 17-KS, 16-alpha hydroxyl Et were low. In the absence of HA, 7 markers of 9 were androgens. Significantly low levels (р<0.05) of 11-Keto-An, abs. and % of total 17-KS, 11β-ОН-Et, abs/, % of total 17-KS, 16alpha-hydroxy-Et, 16alpha-hydroxy-An and 16alpha-hydroxy-DHEA were in women with ICI versus the control group (р<0.05). It should be noted that only in women without HA, ICI was accompanied by significantly low values of Pd (89.91±23.98 and 107.41±20.38 µmol/24h, р=0.004) and allo-Pd (15.02±6.30 and 19.76±7.02 µmol/24h, р=0.009), and these values reflected a threatened miscarriage. In women with HA in the presence of ICI and in the control group, the levels of Pd (118.87±28.39 and 117.53±17.91 µmol/24h, р=0.9) and allo-Pd (23.32±11.60 and 17.01±2.72 µmol/24h, р=0.14) were comparable.
Taking into account association beеween HA and ICI, the values of each marker (monomarker) of urine steroid profile that differentiated women with HA in the main group from women in the control group, were calculated (Table 6).
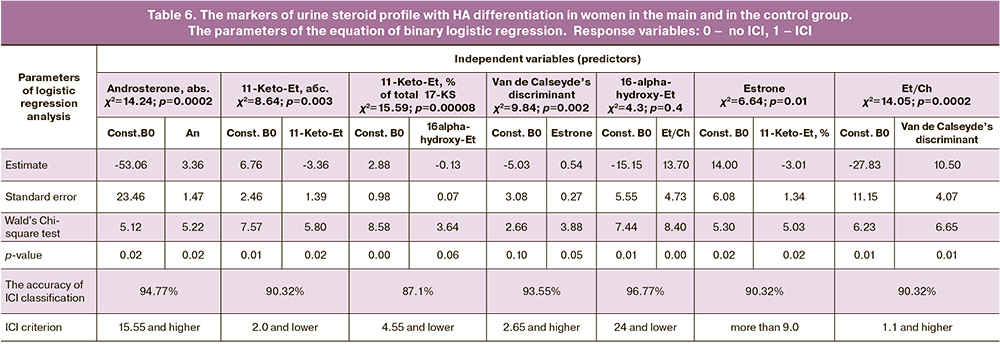
The combination of the markers of uterine steroid profile differentiating women with HA and without HA in the main group and in the control group was determined. In the presence of HA, differentiation between women with ICI and the control group was defined by the combination of only “androgen” markers (Table 7); An abs., DHEA (% of total 17-KS) and Et/Chol (the accuracy for ICI was 96.77%, and 100% for the control group (χ2=35.329, p<0.001)).
In the absence of HA, differentiation of women with ICI was determined by the combination of the markers – androgen metabolites: 16alpha-hydroxy-DHEA, 11-Keto-An, % of total 17-KS, abs. values of 16alpha-hydroxy-Et and 11β-ОН-Et) (the accuracy for ICI in women in the main group was 80.65% and 85.19% in the control group (χ2=33.75, p<0.001; OR=23.96; Log-OR=3.18) (Table 8).
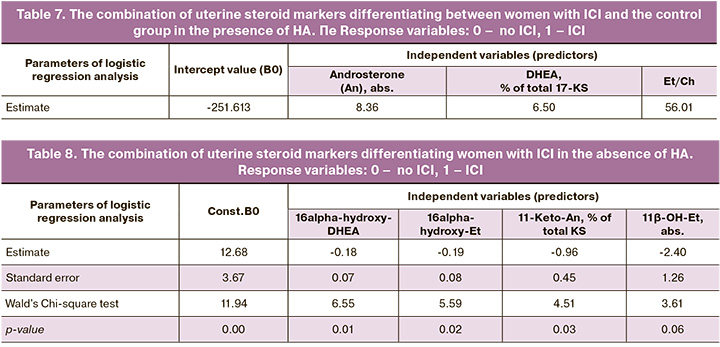
Differentiation of women with ICI, either associated or not associated with HA, which was based on the set of markers of uterine steroid profile was confirmed by ROC curves (Fig. 5).
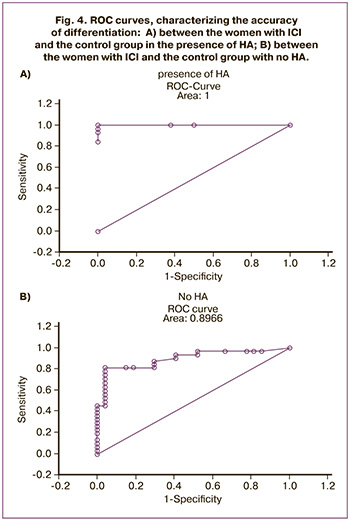
Thus, the assessment of uterine steroid profile was performed by differential diagnosis of ICI, either associated or not associated with HA.
Discussion
Modern measures for prediction of preterm birth and pathogenetic approaches for correction of ICI do not always take into account the presence of HA and, moreover, its genesis, and this fact justified the conduction of this study.
Assessment of uterine steroid profile is an informative method for diagnosis of HA and its genesis in pregnancy in addition to standard techniques for timely prevention and treatment of ICI. Modern methods for correction of ICI [15, 16] imply conservative, pharmacological and surgical approaches. However, the presence of HA is not taken into account as well as in treating a miscarriage in general. In some studies, there are indications for using progesterone for treatment of ICI associated with HA [17]. The use of vaginal progesterone is as effective as cerclage for prevention of preterm birth and improvements in perinatal outcomes in women with singleton pregnancy, spontaneous preterm birth in history, and sonographic markers of cervical shortening in the second trimester of pregnancy [18].
Assessment of the characteristics of uterine steroid profile and quantification of uterine steroids showed the presence of HA both in women with ICI (50.79%), and in in women in the control group (22.86%). HA of mixed genesis was predominant (in 81.25% and 87.5% of women, respectively). It was found that despite the presence or no HA, ICI is characterized both by certain monomarkers of uterine steroid profile (higher levels of androsterone (abs.), total KS, van de Calseide’s discriminant, delta5-pregnanediol, but lower levels of 16alpha-hydroxy-Et, 16alpha-hydroxy-An and 16alpha-hydroxy-DHEA) and their combination (van de Calseide’s discriminant, 16alpha-hydroxy-An, Allo-Pd, cholesterol and estrone).
The women with ICI associated with HA have higher levels of An (abs.), van de Calseide’s discriminant, Et/Ch and estrone, but lower levels of 11-Keto-Et (abs.), and % оf total 17-KS, 16alpha-hydroxy-Et. It should be noted that the differentiating levels of markers matched the upper normal limits, but not the reference range of HA. It was found that significantly lower levels of Pd and allo-Pd objectively were the diagnostic criteria for threatened miscarriage in women with ICI and without HA, but not in the presence of HA.
Conclusion
HA may lead to changes in metabolic processes, that are manifested by urine steroid profile, since urine composition reflects a general metabolic state and contains metabolites. Based on the stratification data, it is possible to identify specific metabolic patterns that indicate an association between GA and the potential risk of developing ICI.
The study of urinary steroid profile in combination with stratification of metabolites is an important tool for determination of association between HA and the risk of ICI, and provides understanding of the metabolic processes underlying this association.
References
- Информационное письмо МАРС по клиническим рекомендациям «Истмико-цервикальная недостаточность»: Направлены письмом Минздрава России от 28 декабря 2018 года №15-4/10/2-7991. М.: Редакция журнала StatusPraesens; 2019: 52с. [MARS Informational letter on clinical recommendations» Isthmic-cervical insufficiency»: Sent by letter of the Ministry of health of the Russian Federation dated 28 December 2018 no 15-4/10/2-7991. Moscow: StatusPraesens. 2019. 52p. (in Russian)].
- Brown R., Gagnon R., Delisle M.F. No. 373-cervical insufficiency and cervical cerclage. J. Obstet. Gynaecol. Can. 2019; 41(2): 233-47.https://dx.doi.org/10.1016/j.jogc.2018.08.009.
- ACOG Practice Bulletin No.142: Cerclage for the management of cervical insufficiency. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2014; 123(2, Pt 1): 372-9. https://dx.doi.org/10.1097/01.AOG.0000443276.68274.cc.
- South Australian Perinatal Practice Guidelines. Cervical Insufficiency and Cerclage. 2017. 13р.
- Diagnosis and Management of Cervical Insufficiency GLM0055. Maternity Guidelines Group Christchurch Women’s Hospital. New Zealand. 2017. 8 р.
- Thakur M., Mahajan K. Cervical incompetence. [Updated 2019 Dec 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK525954
- Crum C.P., Lee K.R., Nucci M.R., Granter S.R., Howitt B.E., Parast M.M. et al. Diagnostic gynecologic and obstetric pathology. Elsevier; 2017. 1296p.
- Шалина Р.И. Коррекция истмико-цервикальной недостаточности. Акушерство и гинекология. Новости, мнения, обучение. 2015; 1(7): 40-3. [Shalina R.I. Correction of isthmic-cervical insufficiency. Obstetrics and Gynecology: News, Opinions, Training. 2015; 1(7): 40-3. (in Russian)].
- Шафигуллина З.Р., Великанова Л.И., Ворохобина Н.В., Лисицын А.А., Кухианидзе Е.А., Стрельникова Е.А., Поваров В.Г., Тейлор Н.Ф. Диагностическое значение стероидных профилей биологических жидкостей у больных синдромом Иценко–Кушинга. Проблемы эндокринологии. 2015; 61(4): 4-8. [Shafigullina Z.R., Velikanova L.I., Vorohobina N.V., Lisicyn A.A., Kuhianidze E.A., Strelnikova E.G., Povarov V.G., Talor N.F. The diagnostical importance of steroid profiles of biological fluids of patients with Cushing’s syndrome. Problems of Endocrinology. 2015; 61(4): 4-8.(in Russian)]. https://dx.doi.org/10.14341/probl20156144-8.
- Орлов Е.Н., Николаев Н.Н., Антипов Е.М., Чмож Л.А., Макаров О.В. Диагностическое значение стероидных профилей мочи. Проблемы эндокринологии. 1995;41(2): 35-9. [Orlov Y.N., Nikolayev N.N.,Antipov Y.M., Chmozh L.A., Makarov O.V. Diagnostic value of urinary steroidal profiles. Problems of Endocrinology. 1995; 41(2): 35-9. (in Russian)].https://dx.doi.org/10.14341/probl11373.
- Подтетенов А.Д., Братчикова Т.В., Орлов Е.Н. Стероидные гормоны и их роль в течении беременности и родов. М.; 2000. [Podtetenov A.D., Bratchikova T.V., Orlov E.N. Steroid hormones and their role during pregnancy and childbirth. Moscow; 2000 (in Russian)].
- Ворохобина Н.В., Татаринова М.В., Великанова Л.И., Серебрякова И.П., Малеваная Е.В., Галахова Р.К. Особенности метаболизма стероидных гормонов у женщин репродуктивного возраста с различными формами гиперандрогении. Вестник Северо-Западного государственного медицинского университета им. И.И. Мечникова. 2016; 8(3): 42-9. [Vorokhobina N.V., Tatarinova M.V., Velikanova L.I., Serebryakova I.P., Malevanaya E.V., Galakhova R.K. Features of steroid hormone metabolism in fertile age females of with various forms of hyperandrogenism. Herald of North Western State Medical University named after I.I. Mechnikov. 2016; 8(3): 42-9 (in Russian)].
- Главнова О.Б., Ворохобина Н.В., Великанова Л.И., Ярмолинская М.И., Малеваная Е.В., Стрельникова Е.Г., Баландина К.А. Метаболомика стероидных гормонов по данным газовой хромато-масс-спектрометрии у женщин с различными фенотипами синдрома поликистозных яичников с нормальной массой тела. Медицинский вестник Юга России. 2022; 13(3): 107-17. [Glavnova O.B., Vorokhobina N.V., Velikanova L.I., Yarmolinskaya M.I., Malevanaya E.V., Strelnikova E.G., Balandina K.A. Gas chromatography-mass spectrometry based steroid metabolomics in women with different phenotypes of polycystic ovarian syndrome and normal body weight. Medical Bulletin of the South of Russia. 2022; 13(3): 107-17. (in Russian)]. https://dx.doi.org/10.21886/2219-8075-2022-13-3-107-117.
- Круско О.В., Рашидова М.А., Бричагина А.С., Шарифулин Э.М., Беленькая Л.В. Особенности функционального состояния гипофизарно-овариально-надпочечниковой системы и процессов ПОЛ–АОЗ у женщин с синдромом поликистозных яичников в раннем репродуктивном периоде. Acta Biomedica Scientifica. 2020; 5(6): 20-6. [Krusko O.V., Rashidova M.A., Brichagina A.S., Sharifulin E.M., Belenkaya L.V. Features of the Functional State of the Hypophysis-Ovarian System and Processes of Lipid Peroxidation – Antioxidant Protection in Women with Hyperandrogenism of Ovary Genesis in the Early Reproductive Period. Acta Biomedica Scientifica. 2020; 5(6): 20-6. (in Russian)]. https://dx.doi.org/10.29413/ABS.2020-5.6.2.
- Medley N., Vogel J.P., Care A., Alfirevic Z. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2018; 11(11): CD012505.https://dx.doi.org/10.1002/14651858.CD012505.pub2.
- Withanawasam N., Tara S. The shortened cervix in pregnancy: Investigation and current management recommendations for primary caregivers. Aust. J. Gen. Pract. 2019; 48(3): 121-3. https://dx.doi.org/10.31128/AJGP-09-18-4708.
- Астраханцева М.М., Бреусенко Л.Е., Лебедев Е.В., Плеханова Е.Р., Савельева Г.М., Шалина Р.И. Истмико-цервикальная недостаточность. Диагностика и коррекция. Российский вестник акушера-гинеколога. 2016; 16(2): 83-8. [Astrakhantseva M.M., Breusenko L.E., Lebedev E.V., Plekhanova E.R., Savelyeva G.M., Shalina R.I. Isthmicocervical insufficiency: Diagnosis and correction. Russian Bulletin of Obstetrician-Gynecologist. 2016; 16(2): 83-8. (in Russian)]. https://dx.doi.org/10.17116/rosakush201616283-88.
- Conde-Agudelo A., Romero R., Da Fonseca E., O'Brien J.M., Cetingoz E., Creasy G.W. et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am. J. Obstet. Gynecol. 2018; 219(1): 10-25. https://dx.doi.org/10.1016/j.ajog.2018.03.028.
Received 04.07.2023
Accepted 14.09.2023
About the Authors
Sergey A. Levakov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology of the N.V. Sklifosovsky Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), levakoff@yandex.ru , 119991, Russia, Moscow, Trubetskaya str., 8-2.Artur V. Mokh, Head of the Maternity Department, M.P. Konchalovsky City Clinical Hospital, Perinatal Center, mohar2205@mail.ru, 124489, Russia, Moscow, Zelenograd, Kashtanovaya alleya, 2-1.



