Comparison of the diagnostic performance of MRI and MSCT in preoperative diagnosis of advanced ovarian cancer
Relevance. Ovarian cancer ranks ninth as the most common malignancy among women in the Russian Federation. Patients with ovarian cancer have a 5-year survival rate of 92% for a localized and 29% for advanced disease.Syrkashev E.M., Solopova A.E.
Aim. To compare the diagnostic performance of multislice spiral computed tomography (MSCT) and magnetic resonance imaging (MRI) in the detection of secondary changes in advanced ovarian carcinoma (III–IV).
Materials and methods. The study included 38 women with advanced ovarian carcinoma (AOC). Before the operation, they underwent MRI (20) and MSCT (20). The local spread and secondary changes in the abdominal cavity were analyzed. For each diagnostic modality, the diagnostic performance characteristics and odds ratios for any residual tumor tissue were calculated.
Results Stage IIIa/b, IIIc, and IV ovarian cancer (FIGO) were diagnosed in 18.4% (7), 47.4% (18), and 34.2% (13) of cases, respectively. High and low-grade serous carcinomas were detected in 94.7% (36) and 5.3% (2) of patients. MSCT had sensitivity, specificity, and accuracy of 0.67, 0.94, and 0.83 for detecting secondary changes, while MRI showed sensitivity, specificity, and accuracy of 0.84, 0.89, and 0.87. Complete, optimal and incomplete cytoreduction was achieved in 26 (68.4%), 7 (18.4%), and 5 (13.2%) cases, respectively. Two patients underwent life-saving surgery, which corresponds to the incidence of suboptimal cytoreduction (7.8%). The odds ratio for incomplete cytoreduction (any residual tumor tissue) with diffuse small intestine lesions (miliary dissemination) and its mesentery were 5.13 (95%CI 1.19; 22.10) and 5.92 (95%CI 1.09; 31.94), respectively. MRI had sensitivity, specificity, and accuracy of 0.73, 0.91, and 0.85 for detecting these secondary changes; corresponding characteristics for MSCT were 0.41, 1.0, and 0.77.
Conclusion. Diagnostic accuracy of MRI is superior to MSCT in detecting metastatic dissemination of ovarian cancer, thus allowing accurate staging of the disease and detecting secondary changes at most significant abdominal locations, which has implications for cytoreductive treatment.
Keywords
Most patients with ovarian cancer are diagnosed with advanced disease (AOC) that has spread throughout the abdominal cavity [1]. As a rule, such patients have peritoneal carcinomatosis, occurring once the cancer cells are successfully detached from the primary tumor and reach the peritoneal space carried by the peritoneal fluid [2]. The five-year survival rate is 92% for localized ovarian cancer and only 29% for patients with distant metastases. The location and prevalence of secondary changes are the most important prognostic factors that determine the possibility of successful cytoreductive surgery, i.e., removal of all visible secondary lesions. A residual tumor of no greater than 1.5–2.0 cm in diameter is considered an optimal tumor for debulking, whereas a residual tumor >2.0 cm is regarded as suboptimal for debulking [3]. If optimal primary cytoreduction cannot be achieved due to the extreme tumor load, neoadjuvant chemotherapy (NACT) followed by interval debulking surgery can be proposed [4].
Staging of AOC is performed according to the FIGO system, which provides accurate prognostic information and guidance on managing ovarian cancer [5]. Surgical staging is the reference method. Determination of unresectable tumors requires a careful assessment of the patent's performance status (according to the ECOG scale or Karnofsky's index), measurement of tumor markers’ levels, and preoperative chest, abdominal and pelvic MSCT. It should be noted that the sensitivity and specificity of MSCT depend not only on the size but also on the location of peritoneal implants, which can have a similar density to normal tissues [6]. In the absence of ascites, this problem is especially relevant.
Currently, multiparametric magnetic resonance imaging (MP-MRI) is supposed to allow a more accurate characterization of the tumor spread and the response to treatment [7]. MP-MRI is superior to MSCT in assessing peritoneal carcinomatosis with a sensitivity of 95%, a specificity of 70%, and an accuracy of 88%. The corresponding characteristics for MSCT are 55%, 86% and 63% [8, 9].
According to the consensus conference recommendations of the European Society of Gynecological Oncology (ESMO/ESGO), patients are not candidates for primary surgery if they have diffuse deep infiltration of the root of small bowel mesentery, diffuse carcinomatosis of the small bowel, stomach/ duodenum, pancreas, lung and brain metastases [7].
This study aimed to compare the diagnostic performance of MSCT and MRI in the detection of secondary changes in AOC in the most significant areas from the point of view of surgical intervention.
Materials and methods
This prospective study was based on diagnostic imaging, and surgical treatment results of 38 women with AOC stages III–IV (FIGO), who were managed at the Department of Innovative Oncology and Gynecology of the Institute of Gynecologic Oncology and Mammology, the V.I. Kulakov NMRC for OG&P.
The study inclusion criteria were ovarian cancer suspected by ultrasound (USG), stage ≥T3a/b (FIGO) of the disease, and no cytoreductive intervention history. The study's exclusion criteria were allergic reactions to the contrast agent, renal failure (creatinine clearance < 40 ml/min), and standard contraindications for MRI.
Before the primary cytoreduction, all patients underwent MP-MRI (20) with DW-MRI and DCE-MRI and MSCT (20) with oral and intravenous contrast medium no more than 15 days before surgery. All patients were operated on by the same team of surgeons. The results were verified by histopathological examination of the surgical specimens.
Reporting diagnostic imaging included the presence of secondary changes in the right and left lateral canals, the right and left subphrenic space, the perihepatic space (the falciform ligament and vesicular fossa), the presence of subcapsular and intraparenchymal deposits of the liver and spleen, omental bursa, hepatic hilum, greater omentum, superior mesenteric artery (and/ or celiac trunk), mesentery of the small intestine, retroperitoneal lymph nodes above the level of renal artery discharge, secondary changes in the anterior abdominal wall, presacral extraperitoneal spread, diffuse lesion of the small intestine, the involvement of the stomach, duodenum or pancreas. These findings were compared with intraoperative data. According to MSCT and MRI data, endpoints are values of sensitivity and specificity for detecting secondary changes in the respective locations.
Statistical analysis
Patient information was entered into MS Excel spreadsheets (Microsoft office Excel 2010). Statistical analysis was performed using the STATISTICA 12 software, StatSoft, Inc. (USA) and IBM SPSS Statistics 23 (USA).
The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. The statistical significance of between-group differences for continuous variables was tested with the Mann–Whitney test. Differences were considered significant at p <0.05. Sensitivity and specificity were calculated using two-by-two contingency tables. Specificity was defined as the proportion of patients without disease who had a negative test result. Sensitivity was defined as the proportion of patients with the disease who had a positive test result.
Results
The median age of the patients was 53.0 (45.0; 60.0), the CA-125 level was 388.0 (189.0; 936.5). Stages III/b, IIIc, and IV AOC (FIGO) were diagnosed in 18.4% (7), 47.4% (18), and 34.2% (13) cases, respectively. High and low-grade serous carcinomas were observed in 94.7% (36) and 5.3% (2) of patients, respectively.
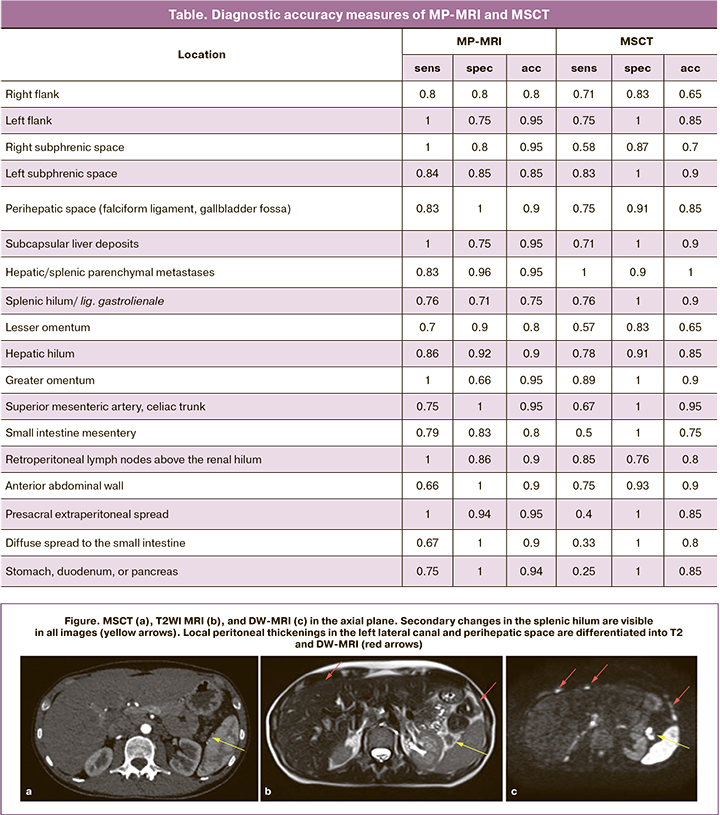
Diagnostic accuracy measures of MRI and MSCT calculated for each of the considered areas are shown in the table.
We also analyzed measured diffusion coefficients (MDC) in primary tumors and peritoneal deposits. The median MDC in primary tumors was 1.015×10-3 mm2/s (0.891×10-3; 1.122×10-3), which was statistically significantly higher than the MDC in the greater omentum and peritoneal deposits – 0.780×10-3 mm2/s (0.709; 0.814×10-3) and 0.743×10-3 mm2/s (0.673; 0.783×10-3), respectively (p <0.001).
Complete, optimal, and non-optimal cytoreduction was performed in 26 (68.4%), 7 (18.4%), and 5 (13.2%) cases, respectively. In 2 cases, surgery was performed for vital indications, which corresponds to the non-optimal cytoreduction frequency of 7.8%. In 3and 2 cases of suboptimal cytoreduction, MSCT and MRI were previously performed. The median age of patients who underwent suboptimal surgical treatment was 60.0 (57.0; 68.5), the CA-125 level was 748.0 (439.0; 973.5), the corresponding indicators in patients with complete and optimal by cytoreduction were 50.0 (43.0; 60.0) and 354.0 (180.0; 947.0). The odds ratio (OR) of performing incomplete cytoreduction (any residual tumor tissue) in diffuse lesions of the small intestine (miliary dissemination) and its mesentery was 5.13 (95% CI 1.19; 22.10) and 5.92 (95% CI 1.09; 31.94), respectively.
Discussion
Due to the high spatial resolution and speed of the procedure, MSCT is currently used as the primary imaging modality for abdominal structures. It should be noted that the contrast resolution of MSCT is relatively low, which makes it challenging to visualize small thickenings along the peritoneum and walls of hollow organs (Figure).
Sensitivity of MRI and CT for detecting secondary changes were 84% and 54%, respectively [10, 11]. The exact identification of the location and size of secondary changes according to MSCT data is 60%. The lowest sensitivity was observed in the presence of secondary changes in the small intestine (8–17%) [12].
Our data are consistent with the results of other studies. The mean sensitivity, specificity, and accuracy of MSCT for detecting secondary changes in each of the above-described locations were 0.67, 0.94, and 0.83; for MRI, it was 0.84, 0.89, and 0.87, respectively. There were significant differences in the diagnostic accuracy in detecting secondary changes that are considered unresectable according to the ESMO/ ESGO criteria (diffuse lesion of the small intestine and the small intestinal mesentery). The sensitivity of MSCT for detecting secondary changes in the small intestinal was twice lower than that of MRI (0.33 and 0.67, respectively). The differences in MRI and MSCT's diagnostic accuracy in detecting secondary changes in the superior mesenteric artery, celiac trunk, and intraparenchymal organs were less significant. Using MSCT for detecting changes in hollow organs' peritoneum (stomach, duodenum) and pelvis showed the lowest diagnostic accuracy.
Primary cytoreduction can significantly increase the survival of AOC patients. Appropriate patient selection based on preoperative diagnostic imaging allows avoiding suboptimal cytoreductive intervention in cases of extensive tumor spread. In the course of the study, we determined that one of the most critical risk factors for incomplete cytoreduction (i.e., the presence of any size of residual tumor tissue) was diffuse damage to the small intestine and its mesentery. In the presence of lesions of these locations, the odds ratio of performing incomplete cytoreduction was 5.13 (95% CI 1.19; 22.10) and 5.92 (95% CI 1.09; 31.94), respectively.
The MDC is based on water molecule diffusion, which reflects tissue microstructure. As the tumor progresses, changes in its microenvironment lead to increased proliferation of tumor cells, decreased differentiation, and an increase in the metastatic potential [13, 14]. This phenomenon is accompanied by an increase in cell density, which restricts the diffusion of water molecules in tumor foci relative to the primary tumor. In this case, the transformation of secondary tumor foci can lead to resistance to chemotherapy.
Conclusion
Diagnostic accuracy of MP-MRI is superior to MSCT in detecting metastatic dissemination of ovarian cancer, thus allowing accurate staging of the disease and detecting secondary changes at most significant abdominal locations, which has implications for cytoreductive treatment and the prognosis of the disease.
References
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. BMJ. 2018; 309(6970): 1689.
- Mikuła-Pietrasik J., Uruski P., Tykarski A., Książek K. The peritoneal ‘soil’ for a cancerous ‘seed’: a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell. Mol. Life Sci. 2018; 75(3): 509-25.
- Manning-Geist B.L., Hicks-Courant K., Gockley A.A., Clark R.M., Del Carmen M.G, Growdon W.B. et al. A novel classification of residual disease after interval debulking surgery for advanced-stage ovarian cancer to better distinguish oncologic outcome. Am. J. Obstet. Gynecol. 2019; 221(4): 326. e1-326. e7. https://dx.doi.org/10.1016/j.ajog.2019.05.006.
- Нечушкина В.М., Хохлова С.В. Практические рекомендации по лекарственному лечению рака яичников, первичного рака брюшины и рака маточных труб. Злокачественные опухоли. 2019; 9(9): 164-76. [Nechushkina V.M., Khokhlova S.V. Practical recommendations for drug treatment of ovarian cancer, primary peritoneal cancer and fallopian tube cancer. Malignant tumor. 2019; 9(9): 164-76. (in Russian)].
- Šišovská I., Minář L., Felsinger M., Anton M., Brednařiková M., Hausnerová J. et al. Current FIGO staging classification for cancer of ovary, fallopian tube and peritoneum. Ceska Gynekol. 2017; 82(3): 230-6.
- Javadi S., Ganeshan D.M., Qayyum A., Iyer R.B., Bhosale P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. AJR Am. J. Roentgenol. 2016; 206(6): 1351-60. https://dx.doi.org/10.2214/AJR.15.15199.
- Colombo N., Sessa C., du Bois A., Ledermann J., McCluggage W.G., McNeish I. et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019; 30(5): 672-705. https://dx.doi.org/10.1093/annonc/mdz062.
- Gadelhak B., Tawfik A.M., Saleh G.A., Batouty N.M., Sobh D.M., Hamdy O., Refky B. Extended abdominopelvic MRI versus CT at the time of adnexal mass characterization for assessing radiologic peritoneal cancer index (PCI) prior to cytoreductive surgery. Abdom. Radiol. (NY). 2019; 44(6): 2254-61. https://dx.doi.org/10.1007/s00261-019-01939-y.
- Low R.N., Barone R.M., Lucero J. Comparison of MRI and CT for predicting the peritoneal cancer index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann. Surg. Oncol. 2015; 22(5): 1708-15. https://dx.doi.org/10.1245/s10434-014-4041-7.
- Coakley F.V., Choi P.H., Gougoutas C.A., Pothuri B., Venkatraman E., Chi D. et al. Peritoneal metastases: detection with spiral CT in patie. Radiology. 2002; 223(2): 495-9. https://dx.doi.org/10.1148/radiol.2232011081.
- Low R.N., Barone R.M., Lacey C., Sigeti J.S., Alzate G.D., Sebrechts C.P. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997; 204(2): 513-20.
- Koh J.-L., Yan T.D., Glenn D., Morris D.L. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2009;16(2):327-33. https://dx.doi.org/10.1245/s10434-008-0234-2.
- Nakayama K., Nakayama N., Katagiri H., Miyazaki K. Mechanisms of ovarian cancer metastasis: biochemical pathways. Int. J. Mol. Sci. 2012; 13(9): 11705-17. https://dx.doi.org/10.3390/ijms130911705.
- van Baal J.O.A.M., van Noorden C.J.F., Nieuwland R., Van de Vijver K.K., Sturk A. et al. Development of peritoneal carcinomatosis in epithelial ovarian cancer: a review. J. Histochem. Cytochem. 2018; 66(2): 67-83. https://dx.doi.org/10.1369/0022155417742897.
Received 16.11.2020
Accepted 23.11.2020
About the Authors
Egor M. Syrkashev, MD, Researcher at the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: e_syrkashev@oparina4.ru.4 Oparina str., 117997, Moscow, Russia.
Alina E. Solopova, Dr.Med.Sci., Associate Professor, Leading Researcher at the Department of Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. E-mail: a_solopova@oparina4.ru. 4 Oparina str., 117997, Moscow, Russia.
For citation: Syrkashev E.M., Solopova A.E. Comparison of the diagnostic performance of MRI and MSCT in preoperative diagnosis of advanced ovarian cancer.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 137-142 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.137-142



