Improvement of management tactics for pregnancy in patients at false-positive risk for fetal chromosomal abnormalities
Objective. To assess the risks of pregnancy complications in patients with false-positive results (FPRs) of screening for fetal chromosomal abnormalities.Yarygina T.A., Bataeva R.S., Gus A.I.
Subjects and methods. Combined screening was carried out to determine the risk of fetal chromosomal abnormalities in 2500 patients. The course of pregnancy and its outcomes were analyzed in 1618 cases without fetal chromosomal and structural abnormalities.
Results. In accordance with the screening results, the investigators identified 2 groups: 1) FPRs of screening
(n = 55); 2) its low-risk results (n = 1563). Groups 1 and 2 displayed a statistically significant difference in the incidence of the following complications: spontaneous abortions before 22 weeks of gestation (9.1% and 0.7%) (Relative risk (RR), 12.9); preterm birth (23.6% and 3.97%) (RR 5.9); birth of a low-weight fetus (16.36% and 3.67%) (RR 4.1); perinatal death (3.6% and 0.06%) (RR 113.7), respectively.
Conclusion. Patients with FPRs of screening are a high-risk group for pregnancy complications.
Keywords
Combined screening with assessment of the risk for fetal chromosomal abnormalities is one of the mandatory stages of prenatal clinical care in the Russian Federation and in other countries [1-3].
Combination of the demographic, anthropometric characteristics of the mother, her general and obstetric history, ultrasound markers and biochemical screening parameters (free β-subunit of human chorionic gonadotropin (b-hCG) and pregnancy-associated protein A (PAPP-A) [3, 4] can help detect 80-96% of cases of trisomy 21 (Down syndrome), 92% of trisomy 18 (Edwards syndrome) and 100% of trisomy (Patau syndrome) [4]. According to an official audit, the effectiveness of prenatal detection of trisomy 21 in the Russian Federation in 2017 was 84%. [5]
However, in a number of patients, invasive genetic diagnosis does not confirm the presence of a chromosomal pathology in the fetus; the rate of false positive results (FPR) of screening algorithms according to literature is 2.5 – 5.0% [4].
As far back as the last millennium, it was noted that the frequency of preterm birth, pre-eclampsia (PE), antenatal death, and small-for-gestational age (SGA) newborns was 3.5 times higher in patients with FPR of maternal screening than in those with normal test results [6]. Recent studies conducted in a number of countries around the world (Portugal, India, USA, Iran) have confirmed the results obtained previously [7–10]. However, a meta-analysis of Pylypjuk et al. [11], which included 68 515 patients and confirmed an increase in the frequency of PE and antenatal fetal death after FPR, did not reveal a similar relationship with SGA and premature birth.
In almost all publications, a strict correlation was found between the degree of deviation from normative biochemical screening parameters and further complicated course of pregnancy [7–10]. It is well known that a decrease in the levels of PAPP-A and placental growth factor (PLGF) in the first trimester of pregnancy is correlated with the subsequent development of placental dysfunction and the associated insufficient fetal growth [12, 13]. Currently, in the Russian Federation, a study of the PLGF level is being introduced into everyday clinical practice, while the determination of the PAPP-A level is carried out by every pregnant woman in most countries of the world [3, 14, 15].
The recommendations of the Royal College of Obstetricians and Gynecologists of Great Britain (RCOG) on the diagnosis and management of cases of SGA fetuses have identified a decrease in the level of PAPP-A as a “large” risk factor [16]. However, a meta-analysis of Zhong et al. [17], which included 103 studies (n = 432 621), showed low prognostic value of any isolated biochemical marker, recommending the use of combined algorithms to form a risk group for obstetric complications. One of the algorithms is the Fetal Medicine Foundation algorithm (The Fetal Medicine Foundation, FMF, UK) introduced into the system of early prenatal screening of the Russian Federation, which makes it possible to conduct simultaneous calculation of the risks for chromosomal abnormalities and SGA births [17, 18].
According to an official audit [5] carried out in 2017 in our country, screening coverage reached 80% of the total number of pregnant women: the number of examined was 1,126,662 patients, of which 20,280 pregnant women were assigned to the risk group for chromosomal abnormalities of the fetus (1.8% from the number of examined). Chromosomal abnormalities and congenital malformations of the fetus were confirmed in 5997 (29.57%) cases [5]. An analysis of the subsequent course of pregnancy and outcomes in patients with FPR screening has not yet been conducted.
The aim of this study was to assess the likelihood of development of pregnancy complications in patients with FPR of screening for fetal chromosomal abnormalities.
Materials and Methods
Sample size of 52 people was calculated as there was an assumed frequency of SGA birth in the control group equal to 5% and a three-fold increase in the frequency of these complications in the group with FPR of screening for fetal chromosomal abnormalities, for the values of alpha risk 5% and beta risk 20% (study power 80%).
In a prospective observational cohort study in outpatient clinic Fetal Medicine Center (Moscow, Russia) for the period of 2015 to 2017, we examined 2500 patients at their 11+1 to 13+6 weeks’ gestation.
Inclusion criteria were singleton pregnancy, a live fetus at the time of the study.
Exclusion criteria were multiple pregnancy, congenital malformations and fetal chromosomal abnormalities. Informed consent for the study was obtained from each patient.
Combined screening was performed in full accordance with the FMF algorithm [3, 4, 19]. For each pregnant woman, a doctor filled in a questionnaire according to the general, family, and obstetric history, including the age of the pregnant woman, race, parity (number of pregnancies lasting 24 weeks or more), a history of SGA births in multiparas, and the method of conception (natural conception / stimulation of ovulation without in vitro fertilization (IVF) / in vitro fertilization with the date and type of procedure), smoking, the presence of diabetes mellitus (DM) and its type, systemic lupus erythematosus, antiphospholipid syndrome (APS), a family history of PE.
The current weight (in kg) and height (in cm) of the patient were measured with the calculation of the body mass index. Blood pressure (BP) was measured according to international rules [17] by an automatic blood pressure monitor (OMRON Healthcare Europe B.V. Hoofddorp, The Netherlands). Determination of the level of PAPP-A and b-hCG in the serum of pregnant women was carried out on a DELFIA Xpress system analyzer (PerkinElmer Life and Analytical Sciences, USA).
Ultrasound investigation (ultrasound) was carried out by FMF certified specialists using the Voluson E8 Expert ultrasound system (GE Healthcare, USA) using 4D intracavitary multifrequency probe (5–13 MHz) and 4D transabdominal convex multifrequency probe (2–8 MHz).
The gestational age was determined by the crown-rump length of the fetus; nuchal translucency thickness, fetal heart rate, nasal bones, parameters of blood flow through the tricuspid valve and ductus venous, and the detection of congenital malformations were identified as well. The pulsation index (PI) in the uterine arteries on both sides was determined by transvaginal access according to the rules of FMF [19].
Conversion of the absolute values of biochemical and biophysical markers into multiples of medians (MoM), taking into account the maternal characteristics, was carried out by the Astraia Software Version 2.8 (Germany).
Based on the obtained results, the individual risk for fetal chromosomal abnormalities (Down, Edwards, and Patau syndromes) was calculated with a cutoff threshold of ≤1: 100 and the risk of SGA with a cutoff threshold of ≤1: 150 [20].
In cases of high risk for chromosomal abnormalities, an increase in nuchal translucency thickness ≥ the 99th percentile, the identification of congenital malformations of the fetus, patients underwent invasive genetic diagnosis.
Data on pregnancy outcomes were obtained from outpatient records and as a result of telephone interviews.
The main outcome studied was the weight of the newborn less than 10 percentile values for gestational age [21] with preterm and term delivery.
Additional outcomes studied were spontaneous abortion or fetal death before 22 weeks of gestation, premature birth (up to 37 weeks of pregnancy), placental abruption, hospitalization of the newborn in the intensive care unit, perinatal death, including antenatal death of the fetus after 22 weeks of pregnancy and death of the newborn up to 28 days of life.
Statistical analysis was performed using the MedCalc software (Mariakerke, Belgium) and XLSTAT of Microsoft Excel 2010 for Windows 10 (Microsoft Corporation, USA). To assess differences between groups by quantitative variables with a normal distribution (age of patients, body mass index), Student t-test was used for independent samples, with a deviation from the normal distribution of quantitative variables (biophysical and biochemical screening markers), the Mann – Whitney U test was used. The Kolmogorov-Smirnov test was used to verify the shape of the distribution, Fisher’s exact test was used to assess the differences between groups by qualitative characteristics.
Relative risk (RR) was calculated to assess the likelihood of complications and adverse pregnancy outcomes in patients with FPR for chromosomal abnormalities according to the results of combined screening in the first trimester of pregnancy compared with patients at low risk.
The criterion of statistical significance of the obtained results was considered as generally accepted in medical statistics, p < 0.05.
Results
We excluded 484 patients from the present study due to the lack of detailed information on the course and outcome of pregnancy (n = 325), the presence of congenital malformations in the fetus and chromosomal abnormalities (n = 159). Based on the results of combined screening, 2076 patients were divided into study groups:
- 1st group (n = 55): cases with FPR of screening for fetal chromosomal abnormalities combined with a high (subgroup 1a (n = 32)) or low (subgroup 1b (n = 23)) risk of SGA;
- 2nd group (n = 1563): cases with a low risk for chromosomal abnormalities and SGA (control group)
Cases with a low risk for chromosomal abnormalities and a high risk for SGA birth (n = 458) were not included in a further comparative analysis of this study.
When analyzing the clinical and epidemiological characteristics (Table 1), only the number of patients with APS in subgroup 1a was statistically significantly higher than that in the control group (p = 0.01). Also in subgroup 1a, there were large percentage of pregnant women with chronic arterial hypertension, systemic lupus erythematosus, nicotine addiction, and complicated obstetrical history, however, a statistically significant difference with the indices between groups was not achieved.
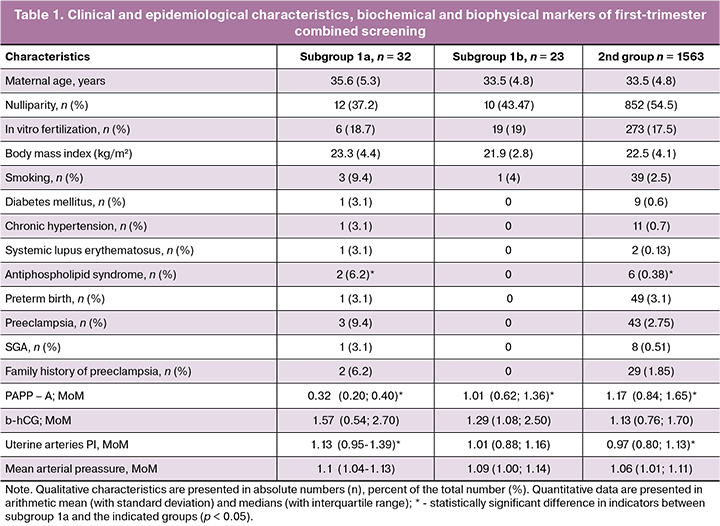
Among the markers of combined screening in patients of subgroup 1a, there was a statistically significant decrease in the level of PAPP-A in comparison with the other two groups (p < 0.0001) and an increase in blood flow resistance in the uterine arteries in comparison with the control group (p = 0.0003) (Table 1).
The markers of subgroup 1b and group 2 did not statistically differ.
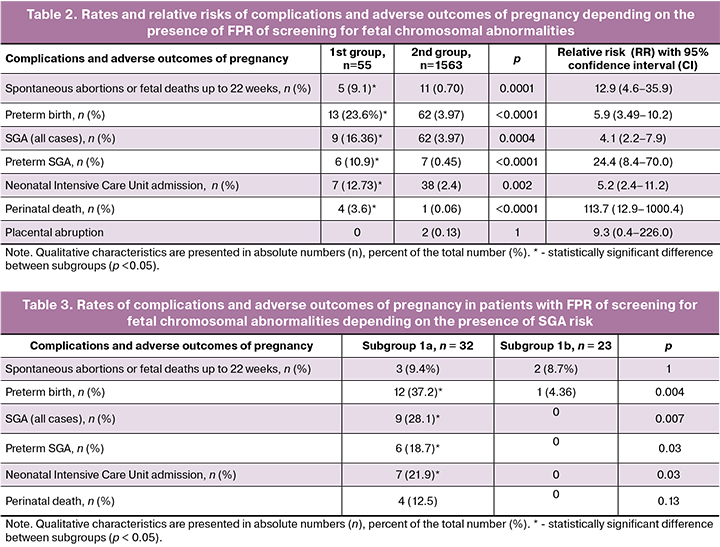
The analysis of the subsequent course of pregnancy revealed a statistically significant increase in the frequency of all the investigated complications and adverse outcomes, with the exception of placental abruption, in patients with FPR of screening for fetal chromosomal abnormalities (Table 2).
When comparing pregnancy outcomes between patients of subgroups 1a and 1b (Table 3), it was found that all cases of SGA (9 cases) and perinatal death (4) were only in subgroup 1a. In addition, statistically significant differences were detected in the preterm birth rate, which was many times higher in subgroup 1a than in subgroup 1b. The number of spontaneous abortions or fetal deaths up to 22 weeks of gestation was almost the same in the groups.
Discussion
The results of this study, conducted for the first time in the Russian Federation, confirmed the data of foreign publications [6–11] on the increase in the frequency of numerous complications in pregnant women with FPR of screening for fetal chromosomal abnormalities.
In our study, a more significant increase in the risk (RR 4.1) of SGA, especially in the premature SGA (RR 24.4) in this group of patients, in which 46.2% (6/13 cases) of newborns in preterm birth were SGA. For comparison, in the control group, the rate of preterm SGA was 11.2% (7/62 cases).
A high probability of spontaneous termination of pregnancy up to 22 weeks (RR 12.9) may be associated with the presence of a fetal pathology that cannot be detected by ultrasound and karyotyping in the first trimester of pregnancy, or with the development of ischemic-cervical insufficiency.
A detailed analysis of outcomes after 22 weeks of gestation showed that the vast majority of complications (preterm delivery, SGA, perinatal death) developed in patients of subgroup 1a with a combination of FPR of screening for fetal chromosomal abnormalities and the birth of a low birthweight infant according to combined screening. Pregnant women of this subgroup have a large number of preconceptional risk factors, statistically significant deviations of biophysical and biochemical markers of combined screening: a marked decrease in PAPP-A (less than 0.4 MoM), an increase in blood flow resistance in the uterine arteries, confirming the prognostic significance of these markers.
An interesting fact is that the study by Baer et al. [9] similarly showed that with a combination of the risks of various pathologies in one fetus, the frequency of subsequent development of pregnancy complications, including perinatal death, increases in dozens or hundreds of times.
The strengths of our study include its prospective design, lack of selectiveness, due to which the general frequency of pregnancy complications corresponded to the population [22], a study of the screening algorithm which has all-Russian application.
The weaknesses of the study are the relatively small number of patients with FPR of screening for fetal chromosomal abnormalities; however, this number reached the required estimated sample size. A retrospective post-hoc analysis determined the statistical strength of the study regarding SGA at 90.4%. This study did not analyze the effect of a high risk for PE and the likelihood of developing this complication in patients with FPR of screening for fetal chromosomal abnormalities, which should be the goal of subsequent studies.
Conclusion
The study showed a significant increase in the likelihood of complications and adverse pregnancy outcomes in cases with FPR of screening for fetal chromosomal abnormalities. After exclusion of chromosomal abnormalities of the fetus, all of the above mentioned patients should be assigned at the high risk group for spontaneous abortion in the period up to 22 weeks and additional ultrasound scan at 16 weeks to assess the anatomy of the fetus and the condition of the cervix should be recommended.
The main predictors of complications after 22 weeks of pregnancy will be a high risk of SGA according to the results of a combined screening; its algorithm takes into account maternal factors, an increase in blood flow resistance in the uterine arteries and a marked decrease in the level of protein A associated with pregnancy (PAPP-A).
An individual plan of treatment and preventive measures and increased surveillance should be developed for these patients until the time of delivery.
In case of an unfavorable outcome of pregnancy, it is advisable to conduct a detailed pathological examination of the fetus and post-mortal chromosomal microarray analysis, as their results can be of great help in preparation for the subsequent pregnancy in these patients.
References
- Audibert F., De Bie I., Johnson J.A., Okun N., Wilson R.D., Armour C., Kim R. No. 348-Joint SOGC-CCMG guideline: update on prenatal screening for fetal aneuploidy, fetal anomalies, and adverse pregnancy outcomes. J Obstet Gynaecol Can. 2017; 39(9): 805–17. https://doi.org/10.1016/j.jogc.2017.01.032
- Committee on Practice Bulletins — Obstetrics, Committee on Genetics, and the Society for Maternal-Fetal Medicine. Practice Bulletin No. 163: Screening for Fetal Aneuploidy. Obstet Gynecol. 2016; 127(5): e123–27. doi: 10.1097/AOG.0000000000001406
- Жученко Л.А., Андреева Е.Н., Одегова Н.О., Степнова С.В., Лагкуева Ф.К., Леонова, В.Ю. Современная концепция и инновационные алгоритмы пренатальной диагностики в рамках нового национального проекта Министерства здравоохранения и социального развития Российской Федерации «Дородовая (пренатальная) диагностика нарушений развития ребенка». Российский вестник акушера-гинеколога. 2011; 11(1), 8–12.3. [Zhuchenko L.A., Andreeva E.N., Odegova N.O., Stepnova S.V., Lagkueva F.K., Leonova V.Yu. Modern concept and innovative algorithms of prenatal diagnosis in the framework of the new national project of the Ministry of Health and Social Development of the Russian Federation “Prenatal (prenatal) diagnosis of impaired child development”. Russian Bulletin of the obstetrician-gynecologist, 2011; 11 (1), 8–12.3 (in Russian)]
- Santorum M., Wright D., Syngelaki A., Karagioti N., Nicolaides, K.H. Accuracy of first‐trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet Gynecol. 2017; 49(6): 714–20. https://doi.org/10.1002/uog.17283
- Жученко Л.А., Андреева Е.Н., Голошубов П.А., Калашникова Е.А., Одегова Н.О., Юдина Е.В. Анализ результатов раннего пренатального скрининга в Российской Федерации АУДИТ-2018. Информационно-справочные материалы. М.: 2018. 111 с. [Zhuchenko L.A., Andreeva E.N., Goloshubov P.A., Kalashnikova E.A., Odegova N.O., Yudina E.V. Analysis of the results of early prenatal screening in the Russian Federation AUDIT-2018. Information and reference materials. M.: 2018. 111 p.(in Russian)]
- Pergament E., Stein A.K., Fiddler M., Cho N.H., Kupferminc M.J. Adverse pregnancy outcome after a false-positive screen for Down syndrome using multiple markers. Obstet Gynecol. 1995; 86(2): 255–8.
- Rodrigues L.C. False positive results of trisomy 21 prenatal screening as a surrogate marker for adverse pregnancy outcome. Diagnóstico Prenat. 2013; 24(4): 135–40. https://doi.org/10.1016/j.diapre.2013.06.004
- Godbole K., Kulkarni A., Kanade A., Kulkarni S., Godbole G., Wakankar A. Maternal Serum Aneuploidy Screen and Adverse Pregnancy Outcomes. J Obstet Gynaecol India. 2016; 66(Suppl 1): 141–8. doi:10.1007/s13224-015-0826-2
- Baer R.J., Currier R.J., Norton M.E., Flessel M.C., Goldman S., Towner D., Jelliffe-Pawlowski L.L. Obstetric, perinatal, and fetal outcomes in pregnancies with false-positive integrated screening results. Obstet Gynecol. 2014; 123(3): 603–9. doi: 10.1097/AOG.0000000000000145
- Yazdani S., Rouholahnejad R., Asnafi N., Sharbatdaran M., Zakershob M., Bouzari Z. Correlation of pregnancy outcome with quadruple screening test at second trimester. Med J Islam Repub Iran. 2015; 29: 281. PMCID: PMC4764288
- Pylypjuk C., Espino J.M. Are false-positive maternal serum screens for fetal aneuploidy associated with adverse outcomes amongst singleton pregnancies globally? A systematic review and meta-analysis. J Obstet Gynaecol Can. 2019; 41(5): 731. https://doi.org/10.1016/j.jogc.2019.02.227
- Dugoff L., Hobbins J.C., Malone F.D., Porter T.F., Luthy D., Comstock C.H., Hankins G., Berkowitz R.L., Merkatz I, Craigo S.D., Timor-Tritsch I.E. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004; 191(4): 1446–51. https://doi.org/10.1016/j.ajog.2004.06.052
- Kaijomaa M., Ulander V.M., Hämäläinen E., Alfthan H., Markkanen H., Heinonen S., Stefanovic V. The risk of adverse pregnancy outcome among pregnancies with extremely low maternal PAPP‐A. Prenatal diagnosis. 2016; 36(12): 1115–20. https://doi.org/10.1002/pd.4946
- Стрижаков А.Н., Мирющенко М.М., Игнатко И.В., Попова Н.Г., Флорова В.С., Кузнецов А.С. Прогнозирование синдрома задержки роста плода у беременных высокого риска. Акушерство и гинекология. 2017; 7: 3
- 4–44. [Strizhakov A.N., Mirushchenko M.M., Ignatko I.V., Popova N.G., Florova V.S., Kuznetsov A.S. Prediction of fetal growth retardation syndrome in high-risk pregnant women. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2017; 7: 34–44. (in Russian)] http://dx.doi.org/10.18565/aig.2017.7.34-44
- Холин А.М., Ходжаева З.С., Гус А.И. Патологическая плацентация и прогнозирование преэклампсии и задержки роста плода в первом триместре. Акушерство и гинекология. 2018; 5: 12–9. [Kholin A.M., Khodzhaeva Z.S., Gus A.I. Pathological placentation and prediction of pre-eclampsia and fetal growth retardation in the first trimester. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2018; 5: 12–9. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.5.12-19
- RCOG Green Top Guidline No.31. The Investigation and Management of the Small-for-Gestational Age Fetus. 2014.
- Zhong Y., Fufan Zhu F., Dinget Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: systematic review and meta-analysis. BMC Pregnancy and Childbirth. 2015; 15: 191. doi: 10.1186/s12884-015-0608-y
- Poon L.C., Syngelaki A., Akolekar R., Lai J., Nicolaides K.H. Combined screening for preeclampsia and small for gestational age at 11–13 weeks. Fetal Diagn Ther. 2013; 33: 16–27. https://doi.org/10.1159/000341712
- Ярыгина Т.А., Батаева Р.С. Методика проведения скринингового исследования в первом триместре беременности с расчетом риска развития преэклампсии и задержки роста плода по алгоритму Фонда медицины плода (Fetal Medicine Foundation). Ультразвуковая и функциональная диагностика. 2018; 4: 77–88. [Yarygina T.A., Batayeva R.S. Methods of screening in the first trimester of pregnancy with the calculation of the risk of preeclampsia and fetal growth retardation according to the algorithm of the Fetal Medicine Foundation. Ultrasound and functional diagnostics. 2018; 4: 77–88. (in Russian)]
- Акушерско-гинекологическая база данных Astraia. Версия 1.23. Руководство пользователя. 2012; 38 с. [Astraia Software obstetric and gynaecological database application. Version 1.23. 2012; 38 р.(in Russian)]
- Fenton T.R., Kim J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics. 2013; 13(1): 59. https://doi.org/10.1186/1471-2431-13-59
- Основные показатели здоровья матери и ребенка, деятельность службы охраны детства и родовспоможения в Российской Федерации за 2017 г. М.: Министерство здравоохранения Российской Федерации, 2018. 171 с. [The main indicators of maternal and child health, the activities of the children’s and maternity facilities in the Russian Federation for 2017. M.: Ministry of Health of the Russian Federation, 2018. 171 p. (in Russian)]
Received 14.06.2019
Accepted 21.06.2019
About the Authors
Tamara A. Yarygina, M.D., Ultrasound and Functional Diagnostics Department, Radiology Division, Research Center for Obstetrics, Gynecology, and Perinatology, Moscow. Phone: +7(495)531–44–44. E-mail: t_yarygina@oparina4.ru; https://orcid.org/0000-0001-6140-1930117997, ul. Oparina 4, Moscow, Russia.
Roza S. Bataeva, M.D., Ph.D., Associate Professor, Division of Diagnostic Ultrasound, Russian Medical Academy of Postgraduate Education; Medical Director and Consultant, Fetal Medicine Centre, Moscow. Phone: +7 (495) 215-12-15. E-mail: drbataeva@gmail.com
101000, ul. Myasnitskaya 4, Moscow, Russia.
Alexandr I. Gus, M.D., Doctor of Medicine, Professor, the head of Ultrasound and Functional Diagnostics Department, Radiology Division, Research Center for Obstetrics, Gynecology, and Perinatology, Moscow. Phone: +7(495)531- 4444. E-mail: a_gus@oparina4.ru; https://orcid.org/0000-0003-1377-3128
117997, ul. Oparina 4, Moscow, Russia.
For citation: Yarygina T.A., Bataeva R.S., Gus A.I. Improvement of management tactics for pregnancy in patients at false-positive risk for fetal chromosomal abnormalities.
Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2020; 1: 71-7. (In Russian).
https://dx.doi.org/10.18565/aig.2020.1.71-77



